Abstract
Piezoceramic membranes have emerged as a prominent solution for membrane fouling control. However, the prevalent use of toxic lead and limitations of vibration-based anti-fouling mechanism impede their wider adoption in water treatment. This study introduces a Mn/BaTiO3 piezoceramic membrane, demonstrating a promising in-situ anti-fouling efficacy and mechanism insights. When applied to an Alternating Current at a resonant frequency of 20 V, 265 kHz, the membrane achieves optimal vibration, effectively mitigating various foulants such as high-concentration oil (2500 ppm, including real industrial oil wastewater), bacteria and different charged inorganic colloidal particles, showing advantages over other reported piezoceramic membranes. Importantly, our findings suggest that the built-in ultrasonic vibration of piezoceramic membranes can generate reactive oxygen species. This offers profound insights into the distinct anti-fouling processes for organic and inorganic wastewater, supplementing and unifying the traditional singular vibrational anti-fouling mechanism of piezoceramic membranes, and potentially propelling the development of piezoelectric catalytic membranes.
Subject terms: Environmental chemistry, Pollution remediation
Piezoceramic membranes in water treatment have been limited by the single vibration-based anti-fouling mechanism. The authors break this limitation using Mn/BaTiO3 membranes to generate reactive oxygen species via piezoelectric vibration, offering insights for developing piezoelectric anti-fouling and catalytic membranes.
Introduction
The escalating global need for efficient wastewater treatment and access to purified drinking water has driven significant advancements in water treatment technologies1,2. Membrane-based separation, renowned for its energy-saving and efficacy, plays a pivotal role in meeting these requirements3,4. However, membrane fouling, characterized by organic and inorganic micro-nano particles commonly recognized as the Achilles’ heel of membrane separation technology, poses a major challenge by adversely affecting membrane flux, energy efficiency, and membrane lifespan3–5. Consequently, considerable efforts have been directed towards devising effective anti-fouling strategies, such as pre-treatment of raw water, operational condition optimization, chemical cleaning, and modifying the membrane surface with static charges to eliminate or repel foulants from adhering to the membrane6,7. Regrettably, these traditional anti-fouling methods often necessitate extra processing steps or brief operational pauses and are generally limited to removing foulants, which can also degrade the structural integrity of membranes6,8. To overcome the limitations of traditional passive anti-fouling methods, active or dynamic anti-fouling strategies have emerged as a viable approach3,6,9. Active anti-fouling strategies, especially those utilizing continuous ultrasonic vibration or photocatalytic activities, have demonstrated notable effectiveness10–12. However, issues like membrane deterioration, the necessity for cavitation regulation, and high energy consumption still limit their practical applications8,11. Fortunately, these drawbacks of conventional ultrasonic approaches can be overcome through the use of self-vibrating piezoelectric membranes that harness the inverse piezoelectric effect13,14. The inverse piezoelectric effect, which converts Alternating Current (AC) stimulation into built-in membrane ultrasonic vibrations, has been conclusively shown to mitigate fouling, thus improving membrane flux and extending its operational life13,14.
Organic piezoelectric response membranes, notably polyvinylidene fluoride (PVDF) types, have been recognized for their hydrophilicity, cost-effectiveness, and efficient anti-fouling via built-in membrane ultrasonic vibration15. Their disadvantage lies in the typically low piezoelectric coefficient (d33 ≤ 30 pC N−1), limited durability to stubborn foulants like oil and bacteria16,17. These limitations highlight the need for alternative piezo-materials that offer enhanced anti-fouling performances with durability14,18. Ceramic membranes offer superior chemical stability and mechanical strength. Typically, the piezoceramics could self-clean by in-situ vibration under AC stimulation14,17. However, the environmental and health risks about lead leakage from commonly used lead zirconate titanate (PZT) limit the use of piezoceramic membranes in anti-fouling19,20. Recent progress in lead-free piezoceramics, including quartz-based materials, presents possibilities for fouling control. However, these are limited by their lower piezoelectric coefficients (commonly ≤ 10 pC N−1) and the requirement for high driving voltages21. Additionally, the basic physical vibration anti-fouling mechanism in piezoelectric membranes may not fully address organic fouling, especially for adhesive organic contaminants, typically necessitating chemical approaches like oxidation3,22. Therefore, there is an urgent need to develop lead-free piezoceramic membranes that deliver effective piezoelectric responses for comprehensive anti-fouling applications and to conduct thorough investigations into the mechanisms of anti-fouling18,23. Barium titanate (BaTiO3) emerges as a leading candidate in lead-free piezoceramics, exhibiting superior piezoelectric properties (d33 ≥ 100 pC N−1)3,16. Enhanced further by strategic elemental doping, its piezoelectric capabilities and mechanical robustness can be significantly improved, paving the way for more effective anti-fouling applications3,24. Importantly, BaTiO3 is safe and has been reported for use in personal care and medical fields such as dental cleaning and cell tissue culture25–27. In prior research, we harnessed the inherent hydraulic pressure of hydraulically driven membrane processes to elicit the direct piezoelectric effect in the Mn-doped BaTiO3 (Mn/BaTiO3) piezoceramic membrane, thereby controlling fouling3. This piezoceramic membrane transforms periodic pressure fluctuations into electrical pulses and rapid voltage changes, thus generating reactive oxygen species (ROS) and dielectrophoretic (DEP) forces at the membrane surface. ROS degrades foulants via oxidation or severs their attachment to the membrane surface, followed by DEP force driving the foulants away from the membrane surface. Since DEP force is independent of the type of foulants, this mechanism offers a universal approach to fouling control3. Notably, the inverse piezoelectric effect, corresponding to the direct piezoelectric effect, is also crucial in anti-fouling applications via piezoelectric ultrasonic vibration. Since at least 1992, there have been reports of using piezoelectric ultrasonic vibration for membrane fouling mitigation. Despite decades of research, this remains the sole mechanism identified, lacking further breakthroughs23,28,29. Actually, from an ultrasonic perspective, the core component of an ultrasonic generator is the piezoelectric ceramic or piezoelectric crystal, and the built-in ultrasonic resonant frequency of piezoceramic membranes usually falls in the high-frequency ultrasonic range (>100 kHz). This high-frequency can generate significant ROS for advanced oxidation processes in water treatment, which previous studies overlooked but is potentially crucial for fouling control in piezoceramic membranes3,14,30,31.
In this study, through the Mn/BaTiO3 piezoceramic membrane, we comprehensively discussed the anti-fouling performances and potential mechanism insights of piezoceramic membranes via the inverse piezoelectric effect, as illustrated in Fig. 1a, b. An in-depth analysis was conducted to determine the optimal operating conditions for the membrane. Additionally, the study investigated the essential anti-fouling mechanisms of membranes, focusing on built-in piezoelectric ultrasonic vibration and ROS synergy, and combined theoretical simulations with experiments across diverse wastewater scenarios.
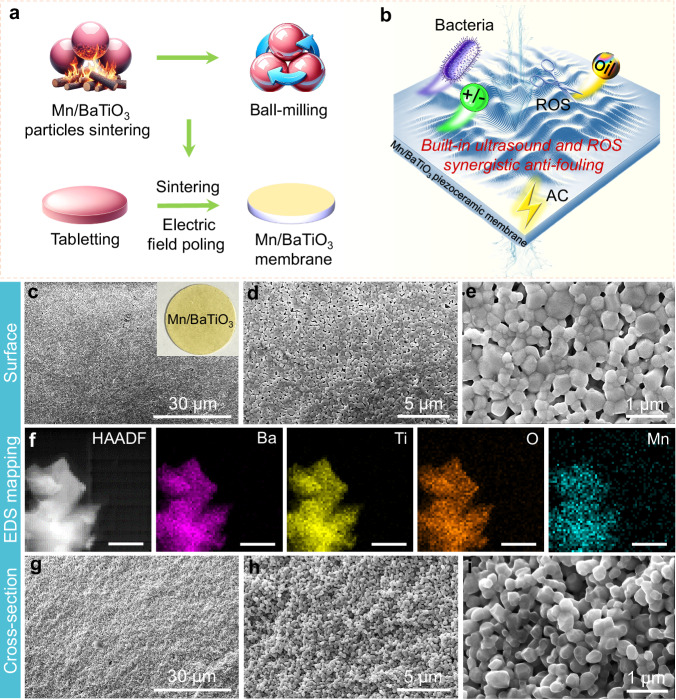
Fig. 1. Fabrication, characterization and anti-fouling mechanism insights of the Mn/BaTiO3 piezoelectric membrane.
a Membrane fabrication process. b Anti-fouling mechanism insights. c–e ESEM images of the surface (with an optical image insert in (c) and g–i cross-section of the membrane. f STEM-HAADF image with EDS elemental mapping of Mn/BaTiO3 grains, scale bar, 100 nm.
Results
Membrane characterization
The Mn/BaTiO3 piezoceramic membrane, depicted in Fig. 1c (insert), was fabricated using a sintering method (Fig. 1a)3,32. This membrane is characterized by its porous surface and detailed cross-sectional microstructure, as shown in the Environmental Scanning Electron Microscopy (ESEM) images, Fig. 1c–e, g–i and Supplementary Fig. 1a. It possesses an average pore size of 210 nm (Supplementary Fig. 1b), a bulk porosity of 22.43%, and measures 30 mm in diameter with a thickness ranging from 2 to 2.2 mm. This membrane exhibits hydrophilic properties, as indicated by an underwater oil angle exceeding 140° (Supplementary Fig. 1c, d) and a pure water permeability of ~91 LMH bar1, which correlates with membrane thickness.
High-Angle Annular Dark-Field (HAADF) imaging and Energy-Dispersive X-ray Spectroscopy (EDS) mapping of the sintered grains (Fig. 1f) and membrane surface (Supplementary Fig. 1a) confirm the composition of Ba, Ti, O, and Mn, derived from the base material of BaTiO3 and the Mn2O3 sintering additive. In addition, as evidenced by the XRD patterns (Supplementary Fig. 1e), the addition of trace amounts of Mn2O3 resulted in only a negligible alteration of the host BaTiO3 crystal structures, with the exception of an enhanced split between the peaks of (002) and (200) at 2θ around 45°. This distinct split signifies the presence of the piezoelectric tetragonal phase in the solid-state sintered BaTiO3 (Fig. 1c, insert), a distinctive feature of the piezoelectric properties of barium titanate32,33.
Self-cleaning performance
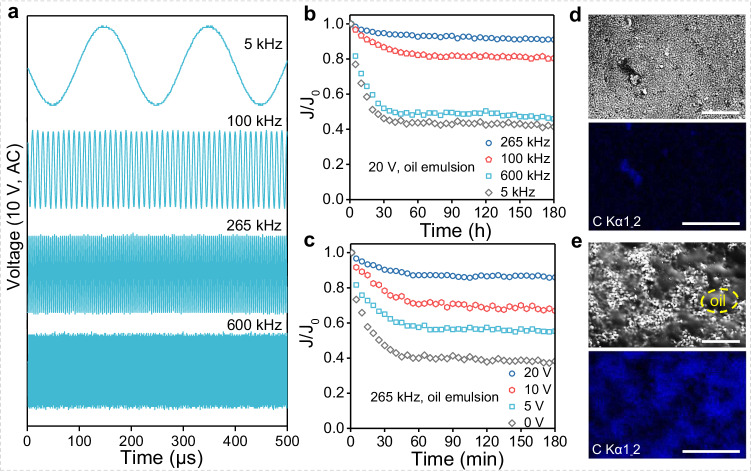
We assessed the optimal self-cleaning conditions for the piezoceramic Mn/BaTiO3 membrane in a cross-flow filtration system, applying different AC settings (as depicted in Fig. 2a and Supplementary Fig. 2), and using a typical oily wastewater O/W (Oil-in-Water, <d > = 0.97 μm, see Fig. 3a, insert) emulsion as the feed solution34. The AC frequency and voltage significantly influenced the normalized membrane flux, as illustrated in Fig. 2b, c. The Mn/BaTiO3 piezoelectric membrane, when operated at a frequency of 265 kHz, maintained a remarkably stable water permeance, retaining 85.9 ~ 91.0% of its initial value during oil emulsion treatment. In stark contrast, membranes vibrated with AC frequencies of 100, 600, and 5 kHz, maintaining only 41.4 ~ 80.4% efficiency after 180 min of operation. It was observed that within a certain range of the tested AC frequencies, membrane anti-fouling effectiveness did not proportionally increase with higher AC frequencies14. This suggests that the AC frequency of 265 kHz is optimal for generating substantial piezoelectric vibration in the Mn/BaTiO3 membrane, leading to consistently high oil anti-fouling efficiency. Furthermore, increasing the intensity of AC, particularly at a resonance frequency of 265 kHz, amplified the vibrational amplitude of membrane. As a result, when the AC voltage was raised from 0 to 20 V, there was a corresponding enhancement in anti-fouling performance, ranging from 38.3% to 91.0% (Fig. 2b, c).
Fig. 2. Oil fouling control of the Mn/BaTiO3 piezoelectric membranes.
a AC frequency variation (5–600 kHz, example at 10 V). b, c Time-based normalized membrane flux under different AC conditions for oil emulsion treatment. Comparative ESEM and the corresponding EDS elemental mapping images of oil-fouled Mn/BaTiO3 piezoelectric membranes surface at 20 V, 265 kHz (d) and 0 V (e), scale bar: 5 μm.
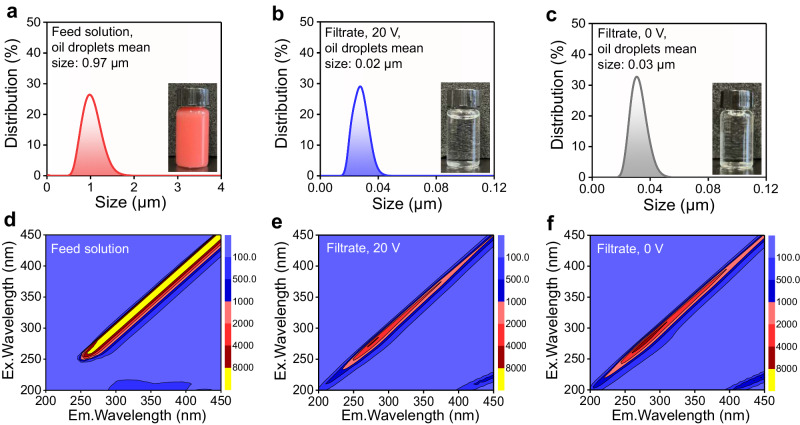
Fig. 3. Membrane retention results.
Particle size distributions of the colored oil emulsion before (a, 2500 ppm) and after (b, c) Mn/BaTiO3 piezoelectric membrane treatment (optical images of water samples, inset). FEEMs analysis of feed (d) and filtrates (e, f, filtration duration of 180 min) across different filtration processes.
Post-filtration analysis using ESEM and elemental carbon mapping revealed significant oily deposits on the surface and internal cross-section of the Mn/BaTiO3 piezoelectric membrane when no AC voltage was applied (0 V), in stark contrast to the condition with 20 V AC voltage (Fig. 2d, e, Supplementary Fig. 3). Regarding membrane fouling, reversible fouling, usually resulting from sparse oil deposits on the membrane surface, can often be mitigated with methods such as flushing to restore flux. Conversely, fouling that penetrates the membrane surface and its pores, causing irreversible contamination, significantly compromises membrane performance8,35. Although AC stimulation did not completely eliminate membrane fouling over the within the 180 min test period, it significantly enhanced the flux recovery ratios (FRR), achieving 99.1% at 20 V (reversible fouling ratio, Rr: 9.9%; irreversible fouling ratio, Rir: 0.9%) and 43.4% at 0 V (reversible fouling ratio, Rr: 6.2%; irreversible fouling ratio, Rir: 56.6%). Compared with other reported membranes for oil fouling control (Table 1, Supplementary Table 1), the as-prepared Mn/BaTiO3 membrane exhibits enhanced anti-fouling performance.
Table 1.
Comparing Mn/BaTiO3 membrane with traditional lead-based and other advanced lead-free piezoelectric water treatment membranes (taking typical oil fouling as an example)
| Piezoelectric Membrane | Lead-containing | Applied voltage (V) | Flux (L m−2h−1) | Oil rejection (%)/ concentration (ppm) | Anti-fouling efficiency (%)a | Single running time (min) | Reference |
|---|---|---|---|---|---|---|---|
| PZT | √ | 20 | ~85 | −, 500 | ~85 | 180 | 14 |
| α-Al2O3/PZT | √ | 40 | 150 | 99.7, 2000 | 59 | 120 | 52 |
| PZT/Ti | √ | 60 | ~210 | ~95, 500 | 90–95 | ~120 | 53 |
| BaTiO3/PVDF | × | 20 | ~58 | ~95, 397.3 | 85.9 | 180 | 54 |
| SiO2-Al2O3-MgO | × | 60 | 272 | 99.7, 500 | ~85 | ~120 | 55 |
| ZrO2-SiO2 | × | 100 | ~200 | 97.5, 500 | ~75–85 | ~120 | 56 |
| Al2O3/α-quartz | × | 100 | 190 | 97.9, 500 | ~55 | 120 | 31 |
| Mn/BaTiO3 | × | 20 | ~91 | 98.4, 2500 | 91 | 180 | This work |
| 75.7 | 900 |
aOnly single-run anti-fouling test results are considered, excluding scenarios where membranes are cleaned and retested after fouling.
Moreover, to further validate the long-term self-cleaning effect of the membrane on typical oil fouling, the test duration was extended to 900 min. Even when challenged with a high-concentration emulsion of 2500 ppm, the membrane still maintained an anti-fouling efficiency of 75.7% (Supplementary Fig. 4). Considering factors such as membrane lifespan, anti-fouling efficiency, retention rate, and the value of the applied voltage, the Mn/BaTiO3 piezoelectric membrane demonstrates a clear superiority over other reported piezoelectric or advanced membranes (Table 1, Supplementary Table 1). In this study, the Mn/BaTiO3 piezoelectric membrane demonstrates sustained anti-fouling performance at an optimal 20 V, surpassing traditional piezoelectric membranes that require 40 ~ 100 V for operation (Table 1). Given operational safety and energy saving concerns, an applied voltage of 20 V is considered practically appropriate.
To assess membrane performance in real wastewater treatment, anti-fouling tests were conducted on oily emulsion wastewater from the food industry. As illustrated in Supplementary Fig. 5, the average oil particle size in this real wastewater was 1.27 μm, with a Total Organic Carbon (TOC) content of 862 ppm, which is lower than 2500 ppm of oil emulsion prepared for testing. Under an AC stimulation of 20 V, 265 kHz, the membrane demonstrated a 10.9% reduction in flux over an extended 900 min test time, with an oil particle retention rate exceeding 99.9%. The Mn/BaTiO3 piezoelectric membrane exhibited competitive practical application value (Table 1, Supplementary Table 1).
We utilized an O/W emulsion stained with the lipid-soluble Oil Red O dye to visually demonstrate the oil retention performance of the Mn/BaTiO3 piezoelectric membranes. The results, as depicted in Fig. 3a–c, showed that the filtrates were remarkably clear and transparent. Further analysis through the Fluorescence Excitation-Emission Matrices (FEEMs, Fig. 3d, f) revealed that the membrane pore physical size sieving effect effectively removed a majority of oil droplets during various filtration processes. Additionally, the TOC removal rates, which remained consistently above 98.4% after 180 min of filtration is exemplary, especially when compared to other membranes reported in the literature (Table 1, Supplementary Table 1), highlighting the superior performance of the Mn/BaTiO3 piezoelectric membrane.
Against various foulants
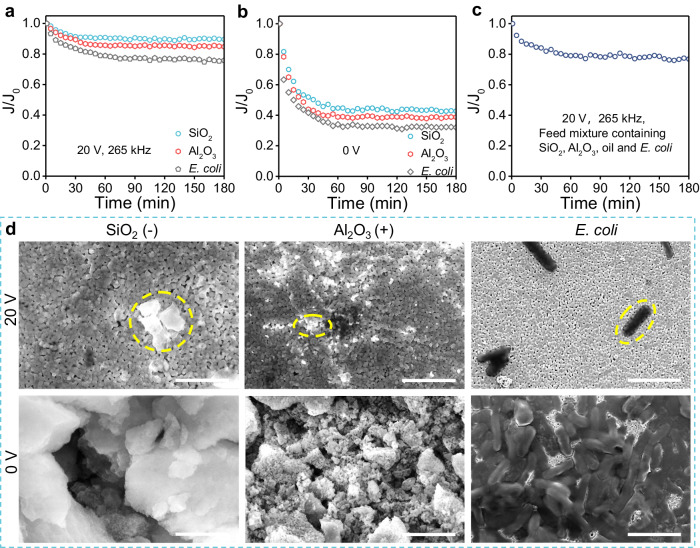
Foulants in water include both organic and inorganic substances, with oil being just one typical example of organic foulants. The Mn/BaTiO3 piezoelectric membrane not only excels in oil fouling control, outperforming other advanced anti-fouling membranes (as listed in Table 1, Supplementary Table 1), but also demonstrates remarkable versatility. To assess its anti-fouling capabilities for various foulants, the membrane was challenged with a series of representative micro-nanoscale foulants (Supplementary Fig. 6), including microbes such as Escherichia coli (E. coli, <d > = 1.69 μm, ζ = −16.1 mV), and inorganic colloidal particulate matter in wastewater (2500 ppm) with different charges (Al2O3 <d > = 0.42 μm, ζ = +39.2 mV) and SiO2 (<d > = 0.56 μm, ζ = −23.5 mV). In all cases, the Mn/BaTiO3 membrane, under a stimulation of 20 V at 265 kHz, maintained over 75% of its initial flux after 180 min: 89.6% for SiO2, 84.8% for Al2O3, and 75.8% for E. coli (Fig. 4a). This performance starkly contrasts with the control group without the piezoelectric effect (0 V), which showed significantly lower anti-fouling efficiencies of 32.0% for E. coli, 38.8% for Al2O3, and 42.7% for SiO2 in the cross-flow filtration system (Fig. 4b). Furthermore, when tested with a mixed solution of these foulants (combined in equal volumetric ratios) to mimic complex wastewater conditions, the membrane showed only about a 20% flux reduction over 180 min (Fig. 4c). Electron microscopy results (Fig. 4d, Supplementary Figs. 7–9) and the elemental mapping of the post-filtration membrane surfaces (Figs. 2d, e, 5a) clearly illustrate the versatile anti-fouling effects (organic/inorganic) of the membranes under AC stimulation of 20 V, 265 kHz. Typically, the membrane maintained high anti-fouling efficiency even against microorganisms such as E. coli, which can strongly adhere to membrane surface by their viscous extracellular polymers, as demonstrated in Fig. 4d and Supplementary Fig. 936,37.
Fig. 4. Effects of the Mn/BaTiO3 piezoelectric membrane against different foulants.
a–c Normalized membrane flux trends with various AC conditions for typical membrane foulants. d ESEM images of foulant-impacted membranes at different AC voltages (265 kHz), scale bar, 5 μm, representative foulants are delineated with yellow dashed lines for clarity.
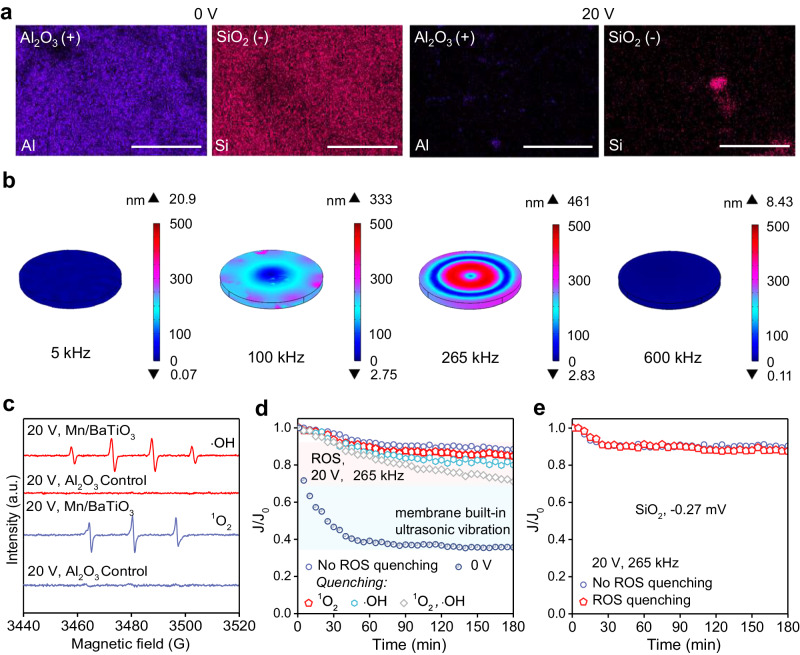
Fig. 5. Insights into the anti-fouling mechanism of Mn/BaTiO3 piezoelectric membrane: synergistic effects of membrane built-in ultrasonic vibration and in-situ ROS generation.
a Elemental mapping of SiO2 and Al2O3 on the surface of the Mn/BaTiO3 membrane post-filtration. b Finite element simulations of membrane displacement at 20 V across different AC frequencies. c ROS detection during membrane processes, 20 V, 265 kHz. d Distinct contributions of ROS and membrane built-in piezoelectric ultrasonic vibration to anti-organic (oil) fouling. e The impact of ROS generated by Mn/BaTiO3 piezoelectric membrane on anti-inorganic (near-neutral charge SiO2 particles) fouling.
The Mn/BaTiO3 piezoelectric membrane, when stimulated with AC voltage, demonstrates a remarkably versatile anti-fouling capabilities, effectively repelling a wide range of foulants irrespective of their type or surface characteristics. However, it remains to be elucidated whether these versatile anti-fouling effects are exclusively attributed to the membrane built-in ultrasonic vibrations. Therefore, a comprehensive investigation into the anti-fouling mechanisms responsible for various foulants of Mn/BaTiO3 piezoelectric membrane is crucial and merits an in-depth discussion.
Anti-fouling mechanism insights
Membrane fouling is usually related to the physicochemical characteristics of both the membranes and the foulants, encompassing aspects such as surface hydrophobicity, electrostatic potential, and the size of pores and particles38–40. When stimulated with AC, the Mn/BaTiO3 piezoelectric membrane demonstrated a remarkable ability to mitigate the deposition of colloids with opposite charges (Figs. 4a, 5a, Supplementary Figs. 7–8). This suggests that despite the presence of electrostatic interactions, the piezoelectric vibration predominantly drives the membrane’s self-cleaning efficacy15,22,41. To verify the anti-fouling mechanism hypothesis, finite element method simulations were conducted, elucidating the correlation between membrane microscale displacement and applied AC conditions.
Finite element simulations reveal and quantify how varying AC frequencies and voltages trigger the membrane’s microscale piezoelectric vibrations, illuminating the understanding of the anti-fouling mechanism42,43. As depicted in Fig. 5b and Supplementary Fig. 10, the application of AC ranging from 5 ~ 20 V at frequencies of 5 ~ 600 kHz initiates vibration of the Mn/BaTiO3 piezoelectric membrane through the inverse piezoelectric effect. Typically, piezoelectric materials exhibit optimal vibration performance at their specific resonant frequencies44,45. In this case, the Mn/BaTiO3 membrane, known for its high piezoelectric properties, demonstrated its optimal resonant frequency around 265 kHz within the tested range. Notably, the membrane’s total spatial displacement increases with rising AC voltage, peaking at 461 nm at 20 V8. These simulation results align closely with the anti-fouling experiments (Figs. 2, 4), indicating that the built-in piezoelectric vibration on the membrane surface and within its pores plays a crucial role in actively preventing the adherence and accumulation of foulants, thereby ensuring effective and continuous anti-fouling performance.
It has been reported that high-frequency ultrasound over 100 kHz could trigger acoustic cavitation, leading to the creation of collapsing microbubbles in water30,46,47. This phenomenon results in exceptionally high temperatures and pressures in supercritical areas, and the collapse of these microbubbles produces ROS with potent oxidative capabilities30,46,47. Significantly, and often neglected in prior research on piezoelectric water treatment ceramic membranes, is the capability of membrane built-in piezoelectric ultrasonic vibration to in-situ generate ROS, and the subsequent role of ROS in mitigating membrane fouling3,14,18. A potential anti-fouling mechanism involves ROS-mediated oxidative disruption of the adhesion between colloidal foulants and the membrane surface, particularly relevant for organic foulants such as oil3. As shown in Fig. 5b, c and Supplementary Fig. 11, upon stimulation at the optimal resonant high frequency (265 kHz, 20 V), significant ROS signals of ·OH, 1O2 and H2O2 were detected via the membrane built-in ultrasonic vibration. These ROS have redox potentials of 1.9 ~ 2.7 V for ·OH and 2.2 V for 1O2 versus the standard hydrogen electrode, sufficient to oxidize the organic foulants used in this study, thereby confirming the aforementioned hypothesis48. Additionally, under the same conditions, no such phenomenon was observed in the control group of common Al2O3 ceramic membrane without piezoelectric effect, eliminating the main possibility of ROS generation by AC alone (Fig. 5c).
The revelation that built-in piezoelectric ultrasonic vibration of the membrane leads to ROS generation, thereby facilitating the oxidation of organic foulants and contributing to their fouling control, represents a relatively novel phenomenon. The ROS quenching results presented in Fig. 5d indicate that the individual contributions of 1O2 and ·OH to membrane fouling control in the oil fouling system are relatively minimal. Within the 180 min test duration, they account for only 4% and 9.6% of the overall fouling control, respectively. However, a significant decline in membrane flux is observed when both types of ROS are simultaneously quenched, showing a 17.5% of flux decrease compared to the group without ROS quenching, accounting for 19.8% of the total anti-fouling effect. This highlights the synergistic contribution of different ROS oxidation in anti-organic fouling. In comparison to the 0 V control group, which is devoid of membrane piezoelectric ultrasonic vibration and piezoelectric ROS generation, the removal of ROS in this system indicates that the contribution of membrane built-in piezoelectric ultrasonic vibration to anti-fouling is significant, accounting for 49.9%. Notably, the quantitative results detailing the contributions of each factor to anti-organic fouling should be viewed as referential, given that the synergy between the built-in piezoelectric ultrasonic vibration of the membrane and the ROS oxidation processes (Figs. 5d, 1b) enhances their collective efficacy. However, for the inorganic fouling, near-electrically neutral colloidal particles (SiO2-hexadecyl trimethyl ammonium bromide, ζ = −0.27 mV, Fig.5e) were used here to eliminate the influence of surface charge on the particles. It was observed that the ROS oxidative anti-fouling effect for inorganic SiO2 is limited, which primarily relies on the piezoelectric vibration of the Mn/BaTiO3 membrane (Fig. 5e). Inorganic colloidal foulants, lacking the adhesive properties of organic foulants such as oil and microbes, merely deposit on membrane surface. Such deposits can be effectively removed through continuous membrane in-situ ultrasonic vibration, without requiring ROS oxidation for detachment3,8,43. These results could provide universal guidance for enhancing the understanding of the anti-fouling properties of piezoceramic membranes, which rely on membrane built-in piezoelectric ultrasonic vibration.
Discussion
The development and application of the Mn/BaTiO3 piezoceramic membrane, as detailed in this study, present significant environmental implications. When activated by AC, this membrane exhibits a significant enhancement in oil anti-fouling efficiency, with its performance increasing from 38.3% to 91.0% as the AC voltage is raised from 0 to 20 V. Furthermore, it delivers versatile anti-fouling performance regardless of the foulants’ properties. Its self-cleaning property reduces the reliance on chemical cleaning agents, which can be environmentally harmful. Additionally, its enhanced water treatment efficiency, combined with a competitive cost compared to other reported piezoelectric or advanced membranes, is vital for sustainable development (Table 1, Supplementary Table 1)49,50. Finite element simulations not only optimize the operational parameters of the membrane but also elucidate the anti-fouling mechanisms at the micro-scale level of the membrane. More importantly, considering this piezoceramic membrane as a case study, supplements and unifies the traditional anti-fouling mechanism, which previously relied solely on the built-in ultrasonic vibration of piezoceramic membranes15,18. It reveals that the membranes in-situ ultrasonic vibration could generate previously neglected ROS around the membranes, thereby offering potential synergistic benefits to the anti-fouling process30,46,47. This study offers a comprehensive self-cleaning solution and universal mechanism insights among piezoceramic membranes. Future studies could explore fabricating lead-free barium titanate piezoceramic ultrafiltration and nanofiltration ceramic membranes. This approach includes developing barium titanate piezoelectric thin layers on macro-porous supports to improve membrane flux, thereby increasing their applicability and scalability in water treatment. Additionally, by co-sintering functional catalysts with piezoelectric ceramics to form piezoelectric catalytic membranes, this approach not only combats membrane fouling but also advances catalytic membrane technology for wastewater treatment, with a particular focus on the treatment of persistent organic wastewaters.
Methods
Materials
Titanium dioxide (TiO2, 99%), barium carbonate (BaCO3, 99%), polyvinyl alcohol (PVA, alcoholysis degree, 98 ~ 99%), sodium dodecyl sulfate (SDS, C12H25SO4Na, 99%), hexadecyl trimethyl ammonium bromide (CTAB, C19H42BrN, 99%), 1,2-dichloroethane (C2H4Cl, 99%), soybean oil (reagent grade), petroleum ether (water free), Oil Red O (C26H24N4O, for biochemical research), aluminum oxide (Al2O3, 99.9%), 2,2,6,6-tetramethyl-4-piperidinol (TEMP), tertbutyl alcohol (TBA, C4H10O, 99%), β-carotene (C40H56, 96%), acetone and manganese oxide (Mn2O3, 99.9%) were obtained from Aladdin. 5,5-dimethyl-1-pyrolin-N-oxide (DMPO) was purchased from Dojindo Laboratories. The commercial H2O2 test kit was purchased from Solarbio. Silicon dioxide (SiO2, 99.9%) was purchased from Mackin. Luria-Bertani (LB) was procured from Hopebiol. Escherichia coli (E. coli, ATCC® 23716™) was selected as the experimental strain. The real oily wastewater was obtained from local food service industry. All experimental solutions were prepared with deionized water (18.2 MΩ•cm).
Membrane fabrication
Synthesis of BaTiO3 powders: Initially, BaCO3 and TiO2 were in an equimolar ratio and were mixed in alcohol, then ball-milled at 550 rpm for 16 h, followed by drying at room temperature. The mixed powders were then calcined in air at 1180 °C for 4 h, maintaining a steady heating rate of 5 °C min−1. After calcination, the powders were ball-milled using the previously described method to obtain the final BaTiO3 powders32.
Sintering of the membranes: Powders of BaTiO3 with 0.1% Mn2O3 were milled with ethanol by ball milling in a planetary ball mill (XGB-04, Boyuntong Instrument Technology, China) at 550 rpm for 12 h. This was followed by drying at 70 °C for 8 h, and then the mixture was sintered at 1000 °C for 4 h. The resulting sintered powders were milled and dried under the aforementioned conditions, after which an aqueous solution of PVA (8% w/w) was added, and the mixture was uniaxially compressed at 15 MPa for 30 s to shape circular membranes. Upon drying at room temperature overnight, these green-pressed membranes were then sintered in a high-temperature muffle furnace at a heating rate of 5 °C min−1 and kept at 1180 °C for 4 h. Finally, the membranes were allowed to naturally cool to room temperature.
Poling of the membranes: The sintered membranes, equipped with copper electrodes on both sides, were submerged in a paraffin bath heated to 110 °C. A poling treatment, applying a direct-current (DC) voltage of 3 kV mm−1, was conducted for 1 h. After poling, the membranes were immersed in ethanol at 70 °C for an hour and rinsed three times with ultrapure water to eliminate all traces of paraffin and ethanol, and ultimately obtained the piezoelectric Mn/BaTiO3 membranes.
Characterization
The surface and cross-sectional morphology of the membranes were examined using Environmental Scanning Electron Microscopy (ESEM, Quanta FEG-250), complemented by energy dispersive X-ray Spectroscopy (EDS) for detailed elemental analysis. Additionally, Scanning Transmission Electron Microscopy (STEM) and High-Angle Annular Dark Field (HAADF) imaging, paired with EDS mapping, were employed to assess the elemental distribution of the Mn/BaTiO3 grains using FEI, TF20. X-ray Diffraction (XRD) patterns were captured using a Bruker D8 Advance diffractometer, employing Cu Kα radiation to scan a 2θ angle range from 20° to 80° with a step size of 0.05°. Fluorescence Excitation-Emission Matrices (FEEMs) of the samples were recorded with a Hitachi F7000 fluorescence spectrophotometer. The Total Organic Carbon (TOC) content in the feed solution and filtrate was analyzed using a Shimadzu TOC-L analyzer. Particle size distribution, mean particle size, and zeta potentials were determined using a Malvern Zeta-sizer Nano ZS90 laser scattering analyzer. The pore size distribution of the sintered membrane was calculated using Nano Measurer 1.2.0 software, based on the ESEM image of Fig. 1b (surface, scale bar 5 μm). Membrane porosity was calculated using the gravimetric method defined as: porosity (%) = 100 × (G3 − G1)/(G3 − G2), where G3 represents the weight of the thoroughly water-wetted membrane, G1 is the dry weight of the membranes, and G2 is the submerged weight determined by water buoyancy. The underwater oil contact angle for a 10 μL droplet of 1,2-dichloroethane on the membrane surface was measured using a DSA100 video-based contact angle measuring device from Kruss Scientific. The total spatial displacement of the piezoelectric membranes under various AC conditions was simulated using COMSOL Multiphysics® 5.6, establishing the physical field of the membranes in a three-dimensional domain.
Preparation of the O/W emulsion wastewater
An O/W emulsion was prepared by adding the appropriate weight of soybean oil, sodium dodecyl sulfate, and Oil Red O dye to deionized water. This mixture was subjected to ultrasonic dispersion at room temperature for 1 h, followed by mechanical agitation for 12 h.
Preparation of the E. coli suspension
25 g of Luria-Bertani medium was dissolved in 0.95 L of deionized water, and the solution was then autoclaved at 121 °C for 20 min to sterilize. After cooling, 1 mL of culture was added to the sterilized medium and incubated at 37 °C, 160 rpm for 48 h. The E. coli feed solution was prepared by diluting the liquid culture medium tenfold to simulate the real microbial wastewater, which typically contains a mixture of nutrients and microorganisms.
Detection of ROS
The Electron Paramagnetic Resonance (EPR) spectra of 5,5-dimethylpyrroline N-oxide (DMPO)-·OH and 2,2,6,6-tetramethylpiperidine (TEMP)-1O2 were recorded using an EMX-10/12 (Bruker) to detect the generation of ROS induced by the built-in ultrasonic vibration of different membranes, under the application of a 20 V AC voltage. 200 mL of deionized water with 22 mL of 1 mmol L−1 DMPO or 200 mL of deionized water with 50 mL of 0.5 mmol L−1 TEMP for ·OH and 1O2 detection, respectively. Different radical scavengers were introduced into the reaction system, including TBA (scavenger for ·OH) and β-carotene (scavenger for 1O2, acetone as solvent)51. The Mn/BaTiO3 piezoelectric membrane was submerged in 100 mL of deionized water. After applying various AC voltages to its surface for 5 min, water samples were collected for further analysis to detect generated H2O2. Detection was performed using a commercial test kit, according to the procedures specified in the manual.
Membrane performances
The filtration and anti-fouling capabilities of the membranes were assessed using a lab-scale cross-flow filtration system operated at a pressure of 1 bar. The system featured a cross-flow membrane module, outfitted with two porous steel electrodes for applying AC signals (UTG9003C, UNIT Inc. China) to the Mn/BaTiO3 piezoelectric membranes. During the separation process, constant stirring was maintained to ensure the stability of the feed solution. The filtrate volume was monitored and recorded at 5 min intervals, and the permeate was collected in a reservoir situated on an electronic balance. The membrane flux (J) was calculated over time using the specified equation Eq. (1):
| 1 |
where V (L), A (m2) and Δt (h) denote the volume of permeation, the effective membrane area, and the testing duration, respectively. Membrane fouling was assessed by tracking the normalized membrane flux ( J/J0) over time, where J0 represents the initial membrane flux. The oil rejection RTOC (%) was calculated according to Eq. (2):
| 2 |
where Cf and Cp represent the TOC concentrations of the feed and permeate, respectively.
To evaluate the synergy between the built-in piezoelectric ultrasonic vibration and in-situ ROS on membrane fouling, tests were conducted on membranes both with and without the application of AC voltage. The flux recovery ratio (FRR), reversible fouling ratio (Rr), and irreversible fouling ratio (Rir) were utilized to assess the anti-fouling performance and fouling resistance as follows8:
| 3 |
| 4 |
| 5 |
Where q0 is the pure water flux, q1 is the flux of O/W emulsion of 2500 ppm, and q2 is the pure water flux the membrane after cleaning (membranes were cleaned by rinsing with pure water for 20 min, L m−2 h−1 bar−1).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This work is supported by the National Natural Science Foundation of China (52371346 (Z.Y.), 22306026 (Z.Y.)), Young Elite Scientists Sponsorship Program by China Association for Science and Technology (2023QNRC001 (Z.Y.)), Ecological Society of China (STQT2023C07 (Z.Y.)), the Fundamental Research Funds for the Central Universities (2242024K40007 (Z.Y.)), and the Start-up Research Fund of Southeast University (RF1028623141 (Z.Y.)).
Author contributions
Y.Z. conceived the idea, carried out the experiments and finite element simulation, and wrote the paper. F.Y., H.J., and G.D.G. helped with data analysis and manuscript polishing. All the authors discussed results and provided comments during the manuscript preparation.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data supporting the findings of the study are included in the main text and supplementary information files. Raw data can be obtained from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-49266-1.
References
- 1.Schwarzenbach RP, et al. The challenge of micropollutants in aquatic systems. Science. 2006;313:1072–1077. doi: 10.1126/science.1127291. [DOI] [PubMed] [Google Scholar]
- 2.Loo SL, Fane AG, Krantz WB, Lim TT. Emergency water supply: a review of potential technologies and selection criteria. Water Res. 2012;46:3125–3151. doi: 10.1016/j.watres.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, et al. Pulsed hydraulic-pressure-responsive self-cleaning membrane. Nature. 2022;608:69–73. doi: 10.1038/s41586-022-04942-4. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Lin W, Chou S, Dai P, Huang X. Patterned membranes for improving hydrodynamic properties and mitigating membrane fouling in water treatment: a review. Water Res. 2023;236:119943. doi: 10.1016/j.watres.2023.119943. [DOI] [PubMed] [Google Scholar]
- 5.Dong D, et al. Double‐defense design of super‐anti‐fouling membranes for oil/water emulsion separation. Adv. Funct. Mater. 2022;32:2113247. doi: 10.1002/adfm.202113247. [DOI] [Google Scholar]
- 6.Zhang R, et al. Antifouling membranes for sustainable water purification: strategies and mechanisms. Chem. Soc. Rev. 2016;45:5888–5924. doi: 10.1039/C5CS00579E. [DOI] [PubMed] [Google Scholar]
- 7.Shao J, Hou J, Song H. Comparison of humic acid rejection and flux decline during filtration with negatively charged and uncharged ultrafiltration membranes. Water Res. 2011;45:473–482. doi: 10.1016/j.watres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Pu L, et al. Membrane cleaning strategy via in situ oscillation driven by piezoelectricity. J. Membr. Sci. 2021;638:119722. doi: 10.1016/j.memsci.2021.119722. [DOI] [Google Scholar]
- 9.Qi L, Hu Y, Liu Z, An X, Bar-Zeev E. Improved anti-biofouling performance of thin -film composite forward-osmosis membranes containing passive and active moieties. Environ. Sci. Technol. 2018;52:9684–9693. doi: 10.1021/acs.est.7b06382. [DOI] [PubMed] [Google Scholar]
- 10.Chen D, Weavers L, Walker H, Lenhart J. Ultrasonic control of ceramic membrane fouling caused by natural organic matter and silica particles. J. Membr. Sci. 2006;276:135–144. doi: 10.1016/j.memsci.2005.09.039. [DOI] [Google Scholar]
- 11.Chen D, Weavers LK, Walker HW. Ultrasonic control of ceramic membrane fouling: effect of particle characteristics. Water Res. 2006;40:840–850. doi: 10.1016/j.watres.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Wan Y, Luo J, Darling SB. Drawing on membrane photocatalysis for fouling mitigation. ACS Appl. Mater. Interfaces. 2021;13:14844–14865. doi: 10.1021/acsami.1c01131. [DOI] [PubMed] [Google Scholar]
- 13.Darestani MT, Coster HGL, Chilcott TC. Piezoelectric membranes for separation processes: operating conditions and filtration performance. J. Membr. Sci. 2013;435:226–232. doi: 10.1016/j.memsci.2013.02.024. [DOI] [Google Scholar]
- 14.Mao H, et al. Self-cleaning piezoelectric membrane for oil-in-water separation. ACS Appl. Mater. Interfaces. 2018;10:18093–18103. doi: 10.1021/acsami.8b03951. [DOI] [PubMed] [Google Scholar]
- 15.Zou D, Lee YM. Design strategy of poly(vinylidene fluoride) membranes for water treatment. Prog. Polym. Sci. 2022;128:101535. doi: 10.1016/j.progpolymsci.2022.101535. [DOI] [Google Scholar]
- 16.You YM, et al. An organic-inorganic perovskite ferroelectric with large piezoelectric response. Science. 2017;357:306–309. doi: 10.1126/science.aai8535. [DOI] [PubMed] [Google Scholar]
- 17.Mestre S, Gozalbo A, Lorente-Ayza MM, Sánchez E. Low-cost ceramic membranes: a research opportunity for industrial application. J. Eur. Ceram. Soc. 2019;39:3392–3407. doi: 10.1016/j.jeurceramsoc.2019.03.054. [DOI] [Google Scholar]
- 18.Krinks JK, et al. Piezoceramic membrane with built-in ultrasonic defouling. J. Membr. Sci. 2015;494:130–135. doi: 10.1016/j.memsci.2015.07.058. [DOI] [Google Scholar]
- 19.Santucci RJ, Jr., Scully JR. The pervasive threat of lead (Pb) in drinking water: unmasking and pursuing scientific factors that govern lead release. Proc. Natl. Acad. Sci. USA. 2020;117:23211–23218. doi: 10.1073/pnas.1913749117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito Y, et al. Lead-free piezoceramics. Nature. 2004;432:84–87. doi: 10.1038/nature03028. [DOI] [PubMed] [Google Scholar]
- 21.Mao H, et al. Application of piezoelectric quartz for self-cleaning membrane preparation. Ceram. Int. 2022;48:16599–16610. doi: 10.1016/j.ceramint.2022.02.204. [DOI] [Google Scholar]
- 22.Liu Y, Gao G, Vecitis CD. Prospects of an electroactive carbon nanotube membrane toward environmental applications. Acc. Chem. Res. 2020;53:2892–2902. doi: 10.1021/acs.accounts.0c00544. [DOI] [PubMed] [Google Scholar]
- 23.Zou D, Mao H, Zhong Z. Construction strategies of self-cleaning ceramic composite membranes for water treatment. Ceram. Int. 2022;48:7362–7373. doi: 10.1016/j.ceramint.2021.12.086. [DOI] [Google Scholar]
- 24.Damamme R, et al. 3D printing of doped barium-titanate using robocasting—toward new generation lead-free piezoceramic transducers. J. Eur. Ceram. Soc. 2023;43:3297–3306. doi: 10.1016/j.jeurceramsoc.2023.02.054. [DOI] [Google Scholar]
- 25.Wang P, et al. Ultrasmall barium titanate nanoparticles for highly efficient hypoxic tumor therapy via ultrasound triggered piezocatalysis and water splitting. ACS Nano. 2021;15:11326–11340. doi: 10.1021/acsnano.1c00616. [DOI] [PubMed] [Google Scholar]
- 26.Kapat K, Shubhra QTH, Zhou M, Leeuwenburgh S. Piezoelectric nano-biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 2020;30:1909045. doi: 10.1002/adfm.201909045. [DOI] [Google Scholar]
- 27.Wang Y, et al. Piezo-catalysis for nondestructive tooth whitening. Nat. Commun. 2020;11:1328. doi: 10.1038/s41467-020-15015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narang, S. C., Sharma, S. K., Ventura, S., Roberts, D., Ahner, N. Research and development to overcome fouling of membranes. First annual report, October 1, 1989-October 31, 1990) (SRI International, 1992).
- 29.Ahner N, et al. Piezoelectrically assisted ultrafiltration. Sep. Sci. Technol. 1993;28:895–908. doi: 10.1080/01496399308019526. [DOI] [Google Scholar]
- 30.Matafonova G, Batoev V. Review on low- and high-frequency sonolytic, sonophotolytic and sonophotochemical processes for inactivating pathogenic microorganisms in aqueous media. Water Res. 2019;166:115085. doi: 10.1016/j.watres.2019.115085. [DOI] [PubMed] [Google Scholar]
- 31.Gao J, Qiu M, Chen X, Verweij H, Fan Y. One-step sintering for anti-fouling piezoelectric α-quartz and thin layer of alumina membrane. J. Membr. Sci. 2023;667:121188. doi: 10.1016/j.memsci.2022.121188. [DOI] [Google Scholar]
- 32.Zhao Y, Gu Y, Gao G. Piezoelectricity induced by pulsed hydraulic pressure enables in situ membrane demulsification and oil/water separation. Water Res. 2022;215:118245. doi: 10.1016/j.watres.2022.118245. [DOI] [PubMed] [Google Scholar]
- 33.Xiong C, et al. Active silicon integrated nanophotonics: ferroelectric BaTiO3 devices. Nano Lett. 2014;14:1419–1425. doi: 10.1021/nl404513p. [DOI] [PubMed] [Google Scholar]
- 34.Tanudjaja HJ, Hejase CA, Tarabara VV, Fane AG, Chew JW. Membrane-based separation for oily wastewater: a practical perspective. Water Res. 2019;156:347–365. doi: 10.1016/j.watres.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Mao H, et al. PZT/Ti composite piezoceramic membranes for liquid filtration: fabrication and self-cleaning properties. J. Membr. Sci. 2019;581:28–37. doi: 10.1016/j.memsci.2019.03.022. [DOI] [Google Scholar]
- 36.Sun J, et al. Maintaining antibacterial activity against biofouling using a quaternary ammonium membrane coupling with electrorepulsion. Environ. Sci. Technol. 2023;57:1520–1528. doi: 10.1021/acs.est.2c08707. [DOI] [PubMed] [Google Scholar]
- 37.Cheng H, Hong PY. Removal of antibiotic-resistant bacteria and antibiotic resistance genes affected by varying degrees of fouling on anaerobic microfiltration membranes. Environ. Sci. Technol. 2017;51:12200–12209. doi: 10.1021/acs.est.7b03798. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, et al. The roles of particles in enhancing membrane filtration: a review. J. Membr. Sci. 2020;595:117570. doi: 10.1016/j.memsci.2019.117570. [DOI] [Google Scholar]
- 39.Ronen A, Walker SL, Jassby D. Electroconductive and electroresponsive membranes for water treatment. Rev. Chem. Eng. 2016;32:533–550. doi: 10.1515/revce-2015-0060. [DOI] [Google Scholar]
- 40.Belfort G. Membrane filtration with liquids: a global approach with prior successes, new developments and unresolved challenges. Angew. Chem. Int. Ed. Engl. 2019;58:1892–1902. doi: 10.1002/anie.201809548. [DOI] [PubMed] [Google Scholar]
- 41.Bao X, She Q, Long W, Wu Q. Ammonium ultra-selective membranes for wastewater treatment and nutrient enrichment: Interplay of surface charge and hydrophilicity on fouling propensity and ammonium rejection. Water Res. 2021;190:116678. doi: 10.1016/j.watres.2020.116678. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q, et al. Interlaced CNT electrodes for bacterial fouling reduction of microfiltration membranes. Environ. Sci. Technol. 2017;51:9176–9183. doi: 10.1021/acs.est.7b00966. [DOI] [PubMed] [Google Scholar]
- 43.Liu B, Xia Q, Zhao Y, Gao G. Dielectrophoresis-based universal membrane antifouling strategy toward colloidal foulants. Environ. Sci. Technol. 2022;56:10997–11005. doi: 10.1021/acs.est.2c03900. [DOI] [PubMed] [Google Scholar]
- 44.Zhu J, et al. Frequency scaling, elastic transition, and broad-range frequency tuning in WSe2 nanomechanical resonators. Nano Lett. 2022;22:5107–5113. doi: 10.1021/acs.nanolett.2c00494. [DOI] [PubMed] [Google Scholar]
- 45.Han JH, et al. Basilar membrane-inspired self-powered acoustic sensor enabled by highly sensitive multi tunable frequency band. Nano Energy. 2018;53:198–205. doi: 10.1016/j.nanoen.2018.08.053. [DOI] [Google Scholar]
- 46.Dehghani MH, et al. Recent trends in the applications of sonochemical reactors as an advanced oxidation process for the remediation of microbial hazards associated with water and wastewater: a critical review. Ultrason. Sonochem. 2023;94:106302. doi: 10.1016/j.ultsonch.2023.106302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng J, Vecitis CD, Park H, Mader BT, Hoffmann MR. Sonochemical degradation of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in groundwater: kinetic effects of matrix inorganics. Environ. Sci. Technol. 2010;44:445–450. doi: 10.1021/es902651g. [DOI] [PubMed] [Google Scholar]
- 48.Yi Q, et al. Singlet oxygen triggered by superoxide radicals in a molybdenum cocatalytic fenton reaction with enhanced REDOX activity in the environment. Environ. Sci. Technol. 2019;53:9725–9733. doi: 10.1021/acs.est.9b01676. [DOI] [PubMed] [Google Scholar]
- 49.Qu X, Brame J, Li Q, Alvarez PJ. Nanotechnology for a safe and sustainable water supply: enabling integrated water treatment and reuse. Acc. Chem. Res. 2013;46:834–843. doi: 10.1021/ar300029v. [DOI] [PubMed] [Google Scholar]
- 50.Yusuf A, et al. A review of emerging trends in membrane science and technology for sustainable water treatment. J. Clean. Prod. 2020;266:121867. doi: 10.1016/j.jclepro.2020.121867. [DOI] [Google Scholar]
- 51.Luo R, et al. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition. Water Res. 2019;148:416–424. doi: 10.1016/j.watres.2018.10.087. [DOI] [PubMed] [Google Scholar]
- 52.Mao HY, et al. High-performance self-cleaning piezoelectric membrane integrated with in-situ ultrasound for wastewater treatment. J. Eur. Ceram. Soc. 2020;40:3632–3641. doi: 10.1016/j.jeurceramsoc.2020.04.003. [DOI] [Google Scholar]
- 53.Mao HY, et al. PZT/Ti composite piezoceramic membranes for liquid filtration: fabrication and self-cleaning properties. J. Membr. Sci. 2019;581:28–37. doi: 10.1016/j.memsci.2019.03.022. [DOI] [Google Scholar]
- 54.Tan Z, et al. Antifouling BaTiO3/PVDF piezoelectric membrane for ultrafiltration of oily bilge water. Water Sci. Technol. 2022;85:2980–2992. doi: 10.2166/wst.2022.154. [DOI] [PubMed] [Google Scholar]
- 55.Mao HY, et al. Piezoceramic membrane equipped with superwetting interface and in-situ ultrasound performance for efficient oil/water emulsion separation. Desalination. 2023;555:116545. doi: 10.1016/j.desal.2023.116545. [DOI] [Google Scholar]
- 56.Mao HY, et al. Application of piezoelectric quartz for self-cleaning membrane preparation. Ceram. Int. 2022;48:16599–16610. doi: 10.1016/j.ceramint.2022.02.204. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of the study are included in the main text and supplementary information files. Raw data can be obtained from the corresponding author upon request.