Abstract
Measles virus infection induces a profound immunosuppression that can lead to serious secondary infections. Here we demonstrate that measles virus induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mRNA and protein expression in human monocyte-derived dendritic cells. Moreover, measles virus-infected dendritic cells are shown to be cytotoxic via the TRAIL pathway.
Secondary infections due to a marked immunosuppression have long been recognized as a major cause of the high morbidity and mortality rates associated with measles virus (MV) infection. The mechanisms underlying the inhibition of cell-mediated immunity following MV infection are not clearly understood, but dysfunction of MV-infected monocytes (7, 9, 10) and dendritic cells (DCs) as antigen-presenting cells (3, 5, 12) has been described. In previous work, we demonstrated induction of T-cell apoptosis by MV-infected DCs (3). To account for this killer activity of MV-infected DCs, we investigated tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) expression in human monocyte-derived DCs after MV infection. TRAIL is a type II transmembrane protein that was initially identified based on the homology of its extracellular domain with those of TNF family members (15). TRAIL does not seem to be cytotoxic toward normal cells but induces apoptosis of a wide variety of transformed cell lines (6, 14). Moreover, T cells from human immunodeficiency virus (HIV) type 1-infected patients, which were previously shown to exhibit increased Fas sensitivity, are even more susceptible to TRAIL-induced cell death (6, 8). These data suggest that TRAIL may participate in apoptosis of lymphoid cells and that it may be involved in dysregulated apoptosis following infection by immunosuppressive viruses such as HIV type 1. Here we demonstrate for the first time that functional TRAIL is produced by MV-infected DCs and mediates their cytotoxic activity.
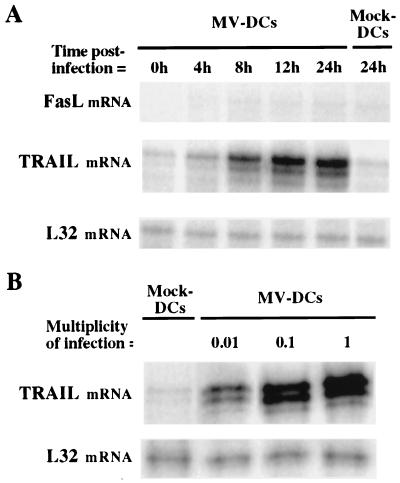
DCs were obtained from purified peripheral blood monocytes cultured for 6 days in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4 as previously described (3). DCs were infected with 1 PFU of Vero cell-derived MV (Edmonston strain) per cell for 3 h at 37°C, then washed and placed in culture. Cells were harvested 4, 8, 12, and 24 h after infection, and total RNA was extracted. TRAIL mRNA expression was quantified by an RNase protection assay (Riboquant hApo3) in accordance with the manufacturer's (PharMingen, San Diego, Calif.) specifications. As shown in Fig. 1A, TRAIL mRNA was weakly expressed in uninfected DCs and strongly up-regulated following MV infection. Supernatant from uninfected Vero cells did not up-regulate TRAIL mRNA expression in DCs. In contrast, Fas ligand (FasL) mRNA was not expressed in uninfected DCs and not induced by MV infection. DCs were then infected with different doses of MV (0.01, 0.1, or 1 PFU/cell), placed in culture, and harvested 24 h later for use in the RNase protection assay. As shown in Fig. 1B, up-regulation of TRAIL mRNA expression correlated with increasing multiplicities of infection. Thus, TRAIL mRNA up-regulation was dependent on the MV dose used for the infection. TRAIL mRNA expression was also induced by MV in monocytes, but not in a thymic epithelial cell line (13) (data not shown). Thus, MV-infected DCs express TRAIL but not FasL mRNA, and induction of TRAIL mRNA expression by MV seems to be dependent on the cell type infected.
FIG. 1.
Analysis of TRAIL and FasL mRNA expression in MV-infected or mock-treated DCs. TRAIL and FasL mRNA expression was quantified by performing an RNase protection assay (Riboquant hApo3) on total RNA extracts in accordance with the manufacturer's (PharMingen) specifications. (A) DCs were either mock treated (Mock-DCs) or infected with MV at 1 PFU/cell (MV-DCs), then cultured for 0, 4, 8, 12, or 24 h. (B) DCs were either mock treated or infected with MV at 0.01, 0.1, or 1 PFU/cell as described above, then placed in culture and harvested 24 h later. For both panels, L32 mRNA, encoding a constitutively expressed ribosomal protein, was used as a control probe. Data shown are representative of three experiments; standard deviations were always below 10%.
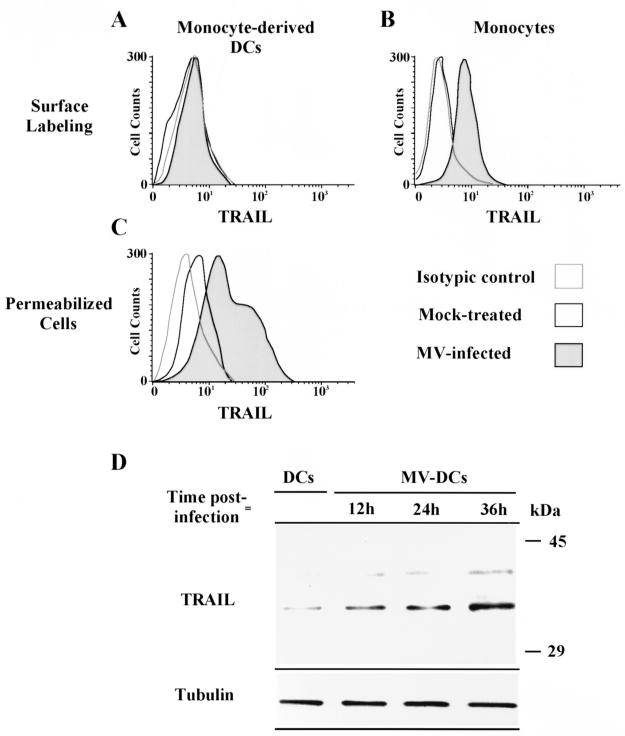
TRAIL protein expression was studied by flow cytometry analysis. DCs and monocytes were either mock treated or infected with MV at 1 PFU/cell, then cultured for 24 h. Cells were labeled with a biotin-conjugated anti-TRAIL antibody or an isotypic control (R&D Systems, Minneapolis, Minn.). Immunolabeling was revealed by using phycoerythrin-conjugated streptavidin (Caltag Laboratories, South San Francisco, Calif.). TRAIL could be detected on the surface of MV-infected but not mock-treated monocytes (Fig. 2B). MV infection also induced surface TRAIL expression in CD3-activated T cells (data not shown). In contrast, TRAIL was never detected on the surface of DCs (Fig. 2A). However, after permeabilization of the cells with 0.33% saponin, TRAIL protein was detected on DCs (Fig. 2C). TRAIL was weakly expressed in mock-treated DCs but strongly expressed in DCs infected with as little as 0.1 PFU of MV/cell. These data were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Fig. 2D). DCs were MV infected, washed, and placed in culture. DCs were harvested at 12, 24, and 36 h. Protein extracts were separated by SDS–10% PAGE, transferred to a polyvinylidene difluoride membrane, and blotted with a specific monoclonal anti-TRAIL antibody (Clone B35-1; PharMingen) or an anti-β-tubulin antibody (Amersham Life Science, Little Chalfont, United Kingdom) as a protein loading control. Immunoreactive bands were visualized by using secondary horseradish peroxidase-conjugated antibodies (Promega, Madison, Wis.) and chemiluminescence (NEN Life Science, Boston, Mass.). As shown in Fig. 2D, TRAIL is weakly expressed in uninfected DCs and up-regulated by MV infection. According to Mariani and Krammer (11), the ca. 33-kDa form corresponds to the full-length monomeric TRAIL and the ca. 40-kDa form corresponds to the cleaved dimeric shorter form of TRAIL.
FIG. 2.
Determination of TRAIL expression in MV-infected or mock-treated DCs or monocytes by flow cytometry and Western blot analysis. DCs and monocytes were either mock treated or infected with MV at 1 PFU/cell, then placed in culture for 24 h. (A and B) DCs (A) and monocytes (B) were stained with an anti-TRAIL antibody after mock treatment (open histograms) or MV infection (gray histograms). Dotted histograms represent staining with an isotypic control. (C) Similar staining was also performed after permeabilization of DCs infected with 0.1 PFU/cell. (D) TRAIL expression in uninfected (DCs) or MV-infected (MV-DCs) DCs cultured for 12, 24, or 36 h was analyzed by Western blotting. Protein extracts were separated by SDS–10% PAGE, transferred to a membrane, and blotted with either a specific monoclonal anti-TRAIL antibody or an anti-β-tubulin antibody (as a protein loading control). The positions of molecular mass markers are shown on the right. Data shown are representative of three experiments; standard deviations were always below 10%.
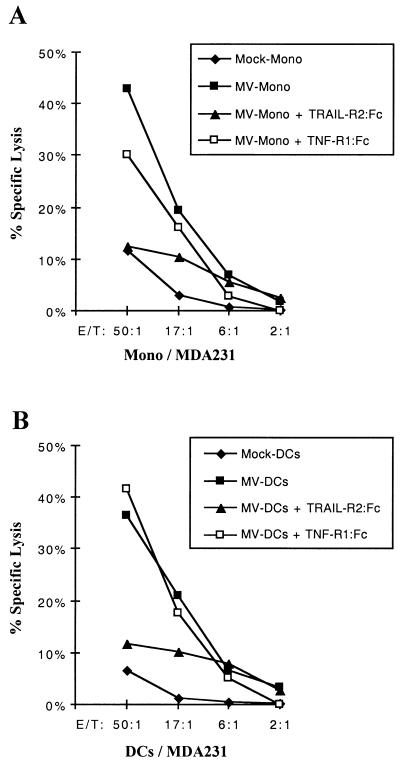
To demonstrate that functional TRAIL is produced by MV-infected DCs but is not expressed on their surface, a cytotoxicity assay was performed. DCs or monocytes were infected with MV at 1 PFU/cell or mock treated, then placed in culture for 12 h. TRAIL-sensitive MDA-231 cells (4) were labeled with 100 μCi of 51Cr for 1 h at 37°C, washed with complete medium (RPMI 1640 with 10% fetal calf serum) three times, and resuspended in complete medium. To determine TRAIL-induced cell death, 51Cr-labeled MDA-231 cells (104/well) were incubated with various numbers of DCs or monocyte effector cells for 8 h. MV-infected monocytes induced 43% specific target cell lysis (Fig. 3A). Similar levels of target cell lysis were obtained with MV-infected DCs in spite of the absence of TRAIL on the surface of these cells (Fig. 3B). Mock-treated monocytes and DCs did not induced target cell lysis (Fig. 3). Supernatant of MV-infected DCs or monocytes did not induced MDA-231 cell lysis (data not shown). The presence of a TRAIL-R2:Fc chimera (R&D Systems) at 20 μg/ml inhibited cell lysis induced by both MV-infected monocytes (Fig. 3A) and DCs (Fig. 3B), while a TNF-R1:Fc chimera (R&D Systems) either did not inhibit or poorly inhibited target cell lysis. Therefore, cytotoxic activity of MV-infected DCs and monocytes is mainly TRAIL mediated in this system.
FIG. 3.
Role of TRAIL in the cytotoxic activity of MV-infected DCs and monocytes. DCs and monocytes were either mock treated (Mock-DCs and Mock-Mono, respectively) or MV infected (MV-DCs and MV-Mono, respectively), then placed in culture for 12 h. Harvested monocytes (A) and DCs (B) were then cultured with 51Cr-labeled MDA-231 target cells at the indicated effector-to-target cell (E/T) ratios. TRAIL-R2:Fc or TNF-R1:Fc chimeras (20 μg/ml) were added or not added to the assay to inhibit TRAIL or TNF-α-induced cell death. 51Cr release was measured 8 h later. Results are means of data from three experiments; in all cases, standard deviations were below 8%.
Together, these results demonstrate that MV infection induces the synthesis of functional TRAIL in human DCs. When DCs were infected with MV at 0.1 PFU/cell, 20% of the cells stained positive for MV nucleoprotein while TRAIL up-regulation was observed in more than 55% of the cells (Fig. 2C). This supports the hypothesis that a soluble factor is involved in TRAIL up-regulation. MV was found to induce alpha/beta interferon expression in monocytes (2), and alpha interferon treatment was shown to induce functional TRAIL expression in monocytes (4). Similarly, alpha interferon treatment also up-regulated intracellular TRAIL in DCs (data not shown). Therefore, this cytokine might be responsible for TRAIL up-regulation following MV infection of DCs and monocytes.
We have previously shown that apoptosis of activated T lymphocytes is induced by MV-infected DCs (3). By using MDA-231 cells as targets, we demonstrated here that MV-induced cytotoxic activity of DCs is TRAIL mediated. Killer activity of DCs is also induced by HIV infection. Indeed, HIV infection of human DCs leads to the release of an as-yet-unidentified soluble factor(s) which induces apoptosis of thymocytes and activated peripheral blood mononuclear cells (1). TRAIL involvement in this killer activity of HIV-infected DCs has not been tested yet. However, increased sensitivity of T cells from HIV patients to TRAIL-induced apoptosis has been described (6, 8). Cytotoxic activity of either MV- or HIV-infected DCs results in lymphocyte apoptosis and may be TRAIL mediated. Depending on the context, either of two different functions could therefore be assigned to human DCs in vivo: an antigen-presenting cell function, generating effector T cells, or cytotoxic activity upon infection with immunosuppressive viruses.
Acknowledgments
This work was supported by institutional grants from Institut National de la Santé et de la Recherche Médicale and from Ministère de l'Education Nationale, de l'Enseignement supérieur et de la Recherche, and by Association pour la Recherche sur le Cancer (CRC 6108), Ligue Nationale Contre le Cancer (CRC), Programme PRFMMIP, and Région Rhône-Alpes.
We thank Hélène Valentin and Jacqueline Marvel for helpful comments. We thank Alain Puisieux, who kindly provided the MDA-231 cell line.
REFERENCES
- 1.Beaulieu S, Lafontaine M, Richer M, Courchesne I, Cohen E A, Bergeron D. Characterization of the cytotoxic factor(s) released from thymic dendritic cells upon human immunodeficiency virus type 1 infection. Virology. 1998;241:285–297. doi: 10.1006/viro.1997.8977. [DOI] [PubMed] [Google Scholar]
- 2.Dhib-Jalbut S S, Cowan E P. Direct evidence that interferon-beta mediates enhanced HLA-class I expression in measles virus-infected cells. J Immunol. 1993;151:6248–6258. [PubMed] [Google Scholar]
- 3.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan M C, Liu Y J, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith T S, Wiley S R, Kubin M Z, Sedger L M, Maliszewski C R, Fanger N A. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosjean I, Caux C, Bella C, Berger I, Wild F, Banchereau J, Kaiserlian D. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J Exp Med. 1997;186:801–812. doi: 10.1084/jem.186.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeremias I, Herr I, Boehler T, Debatin K M. TRAIL/Apo-2-ligand-induced apoptosis in human T cells. Eur J Immunol. 1998;28:143–152. doi: 10.1002/(SICI)1521-4141(199801)28:01<143::AID-IMMU143>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 7.Karp C L, Wysocka M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 8.Katsikis P D, Garcia-Ojeda M E, Torres-Roca J F, Tijoe I M, Smith C A, Herzenberg L A, Herzenberg L A. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J Exp Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leopardi R, Ilonen J, Mattila L, Salmi A A. Effect of measles virus infection on MHC class II expression and antigen presentation in human monocytes. Cell Immunol. 1993;147:388–396. doi: 10.1006/cimm.1993.1078. [DOI] [PubMed] [Google Scholar]
- 10.Leopardi R, Vainionpaa R, Hurme M, Siljander P, Salmi A A. Measles virus infection enhances IL-1 beta but reduces tumor necrosis factor-alpha expression in human monocytes. J Immunol. 1992;149:2397–2401. [PubMed] [Google Scholar]
- 11.Mariani S M, Krammer P H. Differential regulation of TRAIL and CD95 ligand in transformed cells of the T and B lymphocyte lineage. Eur J Immunol. 1998;28:973–982. doi: 10.1002/(SICI)1521-4141(199803)28:03<973::AID-IMMU973>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 12.Schnorr J J, Xanthakos S, Keikavoussi P, Kampgen E, ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci USA. 1997;94:5326–5331. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valentin H, Azocar O, Horvat B, Williems R, Garrone R, Evlashev A, Toribio M L, Rabourdin-Combe C. Measles virus infection induces terminal differentiation of human thymic epithelial cells. J Virol. 1999;73:2212–2221. doi: 10.1128/jvi.73.3.2212-2221.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walczak H, Miller R E, Ariail K, Gliniak B, Griffith T S, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin R G, Rauch C T, Schuh J C, Lynch D H. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 15.Wiley S R, Schooley K, Smolak P J, Din W S, Huang C P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]