Abstract
Human MxA protein accumulates in the cytoplasm of interferon-treated cells and inhibits the multiplication of several RNA viruses, including Thogoto virus (THOV), a tick-borne orthomyxovirus that transcribes and replicates its genome in the cell nucleus. The antiviral mechanism of MxA was investigated by using two alternative minireplicon systems in which recombinant viral ribonucleoprotein complexes (vRNPs) of THOV were reconstituted from cloned cDNAs. A chloramphenicol acetyltransferase reporter minigenome RNA was expressed either by T7 RNA polymerase in the cytoplasm of transfected cells or, alternatively, by RNA polymerase I in the nucleus. The inhibitory effect of MxA was studied in both cellular compartments by coexpressing wild-type MxA or TMxA, an artificial nuclear form of MxA. Our results indicate that both MxA proteins recognize the assembled vRNP rather than the newly synthesized unassembled components. The present findings are consistent with previous data which indicated that cytoplasmic MxA prevents transport of vRNPs into the nucleus, whereas nuclear MxA directly inhibits the viral polymerase activity in the nucleus.
Viral infections induce the production of alpha/beta interferons, which, in turn, establish an antiviral state in surrounding cells through the synthesis of proteins with antiviral activity (16, 18). One of these effector molecules is the human MxA protein, a large GTPase (Mr of 76,000) that accumulates in the cytoplasm of interferon-treated cells (1). MxA inhibits the multiplication of several RNA viruses, including Thogoto virus (THOV), a member of the Orthomyxoviridae family (5). In MxA-expressing cells, THOV transcripts and viral proteins are not detectable, demonstrating that cytoplasmic MxA mediates an early and efficient block in virus multiplication (3, 6, 12). GTP-bound MxA is able to bind to nucleocapsids of THOV, as demonstrated by cosedimentation experiments (9). An efficient interaction between MxA and viral nucleocapsids also seems to take place in the cytoplasm of living cells, because MxA blocks the transport of microinjected THOV nucleocapsids into the nucleus (8). TMxA, a nuclear form of MxA that contains the nuclear localization signal of the simian virus 40 large T antigen, also inhibits the multiplication of influenza A virus and THOV, indicating that MxA can be antivirally active within the nucleus (3, 22). In contrast, mutant MxA(T103A), which has a threonine-to-alanine substitution at position 103, lacks GTPase and antiviral activity (15).
We have investigated the antiviral mechanism of MxA using a recently established minireplicon system in which recombinant viral ribonucleoprotein complexes (vRNPs) of THOV are reconstituted from cloned cDNAs (21). In this system, expression of a model minigenome RNA containing the chloramphenicol acetyltransferase (CAT) gene in the negative-sense orientation flanked by the conserved 5′- and 3′-terminal sequences of the THOV nucleoprotein (NP) gene segment, together with the three polymerase subunits (PA, PB1, and PB2) and the viral NP, leads to the formation of transcriptionally active vRNPs. Here, we show that MxA interferes with the activity of these reconstituted viral transcription units, provided that MxA and the artificial vRNPs are located in the same subcellular compartment.
MxA inhibits polymerase activity of reconstituted vRNPs.
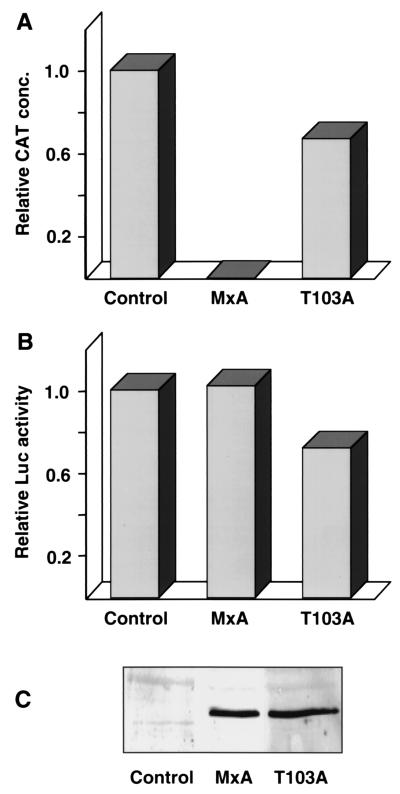
COS-1 cells were transfected with cDNA constructs coding for the three THOV polymerase subunits, with the NP cDNA of THOV, and with a plasmid coding for the CAT minigenome RNA (21). Expression of the four cDNAs and the minigenome RNA was driven by the T7 RNA polymerase provided by the recombinant vaccinia virus vTF7-3 (4). Synthesis of CAT protein could be detected in the transfected cells, indicating that functional vRNPs were reconstituted (Fig. 1A). It should be noted that the CAT minigenome RNA was produced in the negative-sense orientation by the T7 RNA polymerase. Therefore, CAT mRNA was generated exclusively by the viral polymerase complex, and the amount of CAT protein reflected the activity of the reconstituted vRNPs (21).
FIG. 1.
MxA inhibits reporter gene expression in a THOV minireplicon system. COS-1 cells were transfected with T7 promoter constructs coding for the components of the viral polymerase complex PA, PB1, and PB2 (100 ng each), for NP (500 ng), and for a CAT minigenome, pT7ribo-THOV/CAT (100 ng), as previously described (21). In addition, a T7-driven luciferase construct, pBS-T7/Luc (100 ng), was cotransfected. Wild-type MxA or MxA(T103A) was expressed under the control of the T7 promoter using pBS-T7/MxA or pBS-T7/MxA(T103A) expression plasmids (1 μg). Five hours posttransfection, cells were infected with the recombinant vaccinia virus vTF7-3, and 18 h postinfection, cells were harvested. The reporter gene expression levels of experiments without MxA were set to 1 (control). (A) CAT protein concentration (conc.) was determined in the cell lysates by a colorimetric immunoassay (Boehringer Mannheim). (B) Expression of luciferase activity was measured with the cell lysates used for panel A. (C) Expression of MxA protein. Aliquots of the same cell lysates (10 μg of protein per lane) were analyzed by Western blotting with a polyclonal rabbit antiserum directed against MxA.
Coexpression of MxA under the control of the T7 RNA polymerase promoter led to complete inhibition of CAT protein synthesis (Fig. 1A). To demonstrate the specificity of inhibition, the antivirally inactive MxA(T103A) mutant was used. MxA(T103A) did not grossly affect THOV polymerase activity (Fig. 1A), although protein amounts were comparable to amounts of wild-type MxA, as demonstrated by Western blot analyses of cell lysates (Fig. 1C). On transfection of increasing amounts of the wild-type MxA plasmid, a linear correlation between MxA expression levels and the degree of inhibition was observed (data not shown). Transfection of 250 ng of MxA plasmid resulted in a half-maximal reduction of CAT synthesis, whereas 1 μg of MxA plasmid led to complete inhibition. We concluded that MxA specifically inhibited viral polymerase activity in the minireplicon system and that the degree of inhibition is directly proportional to the amount of MxA protein present.
To monitor possible nonspecific effects of MxA on T7-mediated gene expression, a T7-luciferase construct was added to the plasmid mixture as a control. Comparable amounts of luciferase activity were present in all three experiments, demonstrating that MxA did not affect the T7 expression system (Fig. 1B). The slight decrease of CAT synthesis in MxA(T103A)-expressing cells is paralleled by a decrease of luciferase activity and therefore reflects a variation in transfection efficiency. Thus, luciferase activity was used to normalize CAT expression obtained in different transfection experiments, and the ratio of CAT protein concentration to luciferase activity (CAT/Luc ratio) was calculated in later experiments.
The antiviral activity of MxA is not affected by overexpression of viral proteins.
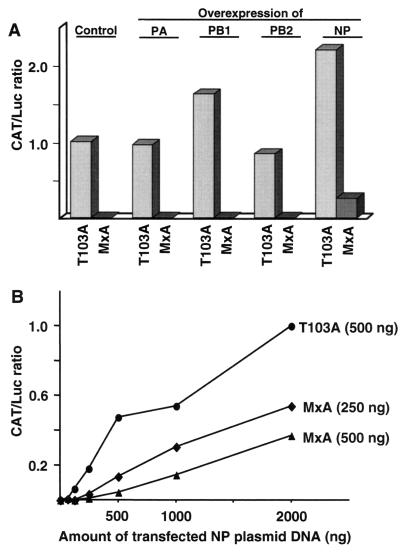
In the THOV minireplicon system, five viral components could serve as targets of MxA; the model RNA minigenome, the NP, and the three subunits of the viral RNA polymerase. Since MxA has no RNA-binding activity (G. Kochs and M. Schwemmle, unpublished results), the model vRNA was excluded as a candidate target structure. To test the remaining components, we overexpressed each viral protein individually in the reconstituted system. In separate experiments, the amounts of PA, PB1, PB2, and NP plasmids were each increased to 1 μg in the basic plasmid mixture. In the presence of MxA(T103A), CAT protein synthesis was enhanced by overexpression of the PB1 subunit and NP, whereas elevated expression levels of PB2 and PA had no profound effect (Fig. 2A). When the wild-type MxA expression plasmid was cotransfected instead of the MxA(T103A) plasmid, none of the three polymerase subunits was able to neutralize the MxA-mediated block (Fig. 2A). In contrast, CAT production was restored to a certain extent when an excess of NP expression plasmid was provided. To further investigate the effect of NP overexpression, increasing amounts of NP plasmid were added to fixed amounts of the other viral expression plasmids, and the effects of mutant and wild-type MxA cDNAs were analyzed. In the presence of MxA(T103A), increasing amounts of NP plasmid resulted in a proportional increase in viral polymerase activity (Fig. 2B). The same was true when wild-type MxA plasmid was transfected, although the amount of CAT protein production was consistently lower. In the presence of 250 ng of MxA, roughly twice the amount of NP plasmid was needed to reach CAT protein levels comparable to those obtained in the presence of 500 ng of MxA(T103A). When the amount of MxA plasmid was raised to 500 ng, fourfold more NP cDNA was necessary to obtain control levels. We concluded that higher NP expression levels led to a general stimulation of viral polymerase activity which was efficiently reversed by an increase in MxA protein levels. The alternative view would be that an increase in NP concentrations specifically interfered with the inhibitory effect of MxA. However, this is rather unlikely given the nonspecific stimulatory effect of NP in the control experiment with MxA(T103A).
FIG. 2.
Overexpression of the nucleocapsid components. (A) COS-1 cells were transfected with plasmids encoding the basic THOV minireplicon system described in the legend to Fig. 1 in the presence of MxA(T103A) or wild-type MxA expression plasmids (1 μg). The amounts of the indicated expression plasmids were increased to 1μg. The CAT/Luc ratio for the control experiment was set to 1 (control). (B) Constant amounts of expression plasmids were cotransfected in the THOV reconstitution system together with increasing amounts of NP plasmid. To maintain constant total DNA concentrations, pBSK(+) vector DNA was added to the mixtures. The CAT/Luc ratio with 2 μg of NP plasmid and 500 ng of MxA(T103A) plasmid was taken as 1. Amounts of NP plasmid above 2 μg were inhibitory for the system.
MxA acts in the subcellular compartment of vRNP assembly.
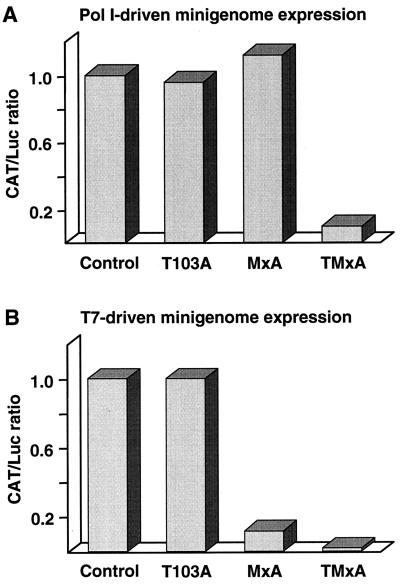
To study whether newly synthesized viral proteins or assembled vRNPs are the targets of MxA, we used a modified THOV minireplicon system in which the CAT reporter minigenome RNA is produced in the cell nucleus. In all previous experiments, the CAT minigenome RNA was expressed by the T7 RNA polymerase and accumulated in the cytoplasm. Presumably, the model vRNA and the newly synthesized viral proteins formed vRNPs that were transported into the nucleus. In the modified minireplicon system, synthesis of the CAT minigenome RNA was localized to the nucleus by the cellular RNA polymerase I (14). This restricts vRNP assembly to the nuclear compartment. In this system, CAT protein production was as efficient as in the previous experiments (data not shown). Surprisingly however, when wild-type MxA was coexpressed, CAT minigenome expression was not affected and CAT protein production was as high as in the presence of the inactive mutant MxA(T103A) (Fig. 3A). These results demonstrate that cytoplasmic MxA has no effect on viral protein synthesis or the transport of newly synthesized proteins into the nucleus. In contrast, MxA was inhibitory in the presence of the cytoplasmic T7-expressed minigenome and caused a strong reduction in CAT protein synthesis, as expected (Fig. 3B). We reasoned that the failure of MxA to inhibit CAT expression in the modified minireplicon system was due to the different subcellular location of the putative interacting partners, with MxA accumulating in the cytoplasm and vRNPs being formed in the nucleus. We therefore investigated whether TMxA, a nuclear form of MxA (22), would be able to block reporter gene expression mediated by the THOV polymerase in the RNA polymerase I-driven system. Indeed, expression of TMxA inhibited CAT production when the minigenome was synthesized by the RNA polymerase I (Fig. 3A). Likewise, TMxA blocked reporter gene expression when the minigenome was produced in the cytoplasm by the T7-RNA polymerase (Fig. 3B), indicating that TMxA was also capable of interfering with preformed vRNPs that were translocated into the nucleus. Taken together, these results indicate that MxA does not recognize viral nucleocapsid proteins as long as they are not complexed with RNA in the form of vRNP structures. This view is supported by the fact that we and others have not succeeded in showing a direct interaction of MxA with either RNA or single viral proteins in various in vitro binding assays (20). Also, interaction studies using the yeast two-hybrid system failed to reveal specific interactions between MxA and viral proteins (M. Trost, Ph.D. thesis, University of Freiburg, Germany). However, recently we demonstrated a physical interaction of MxA with purified THOV nucleocapsids in an in vitro cosedimentation assay (10). Furthermore, artificial RNPs consisting of only synthetic RNA molecules and Escherichia coli-expressed purified recombinant NP were able to cosediment with MxA in glycerol gradients (9). This interaction was independent of the secondary structure of the RNA backbone, indicating that the RNA-bound NP is recognized by MxA.
FIG. 3.
MxA action depends on the site of nucleocapsid assembly. Cells were transfected with the components of the THOV minireplicon system as described in the legend to Fig. 1. The CAT minigenome was transcribed either with the nuclear RNA polymerase I (Pol I) by using pPolI-THOV/CAT (500 ng) (A) or with the cytoplasmic T7 polymerase by using the plasmid pT7ribo-THOV/CAT (100 ng) (B). Plasmid DNA (1 μg) encoding either wild-type MxA, MxA(T103A), or TMxA (a nuclear form of wild-type MxA containing a foreign nuclear localization signal) was added to the plasmid mixtures. The CAT/Luc ratio in the presence of 1 μg of pBSK(+) was set to 1 (control).
How could MxA affect the function of the viral polymerase? We propose that MxA recognizes assembled vRNPs and that this interaction has different functional consequences depending on whether it occurs in the cytoplasm or in the nucleus. When the interaction occurs in the cytoplasm, THOV RNPs are trapped in this compartment and are prevented from entering the nucleus. As a consequence, the virion polymerase has no access to the nuclear environment in which viral transcription has to occur. Indeed, recent microinjection experiments have clearly demonstrated that cytoplasmic MxA blocks THOV nucleocapsid translocation into the nucleus, thereby preventing primary transcription (8). In contrast, TMxA acts after the viral nucleocapsids have entered the nucleus and inactivates the virion polymerase by an as-yet-unknown mechanism. TMxA may directly impair the functional integrity of the THOV polymerase or act indirectly by making host cell factors unavailable for the viral RNA transcriptase (17). A similar course of events may also apply to influenza A virus. TMxA is known to affect primary transcription mediated by the viral polymerase in influenza A virus-infected cells (22). This effect is comparable to the action of the mouse Mx1 protein. Mx1 accumulates in the nucleus of interferon-treated murine cells and inhibits the function of influenza virus nucleocapsids by blocking primary transcription (11, 13, 19). Interestingly, Broni and coworkers have demonstrated that influenza A virion nucleocapsids are efficiently transported into the nuclei of mouse cells expressing high levels of Mx1 protein (2). They concluded that the murine Mx1 protein acts in the nucleus to inhibit viral mRNA synthesis, exactly as proposed here for TMxA. Moreover, Huang et al. have reported that Mx1 inhibits reporter gene expression in an influenza A virus minireplicon system (7) much in the same way as TMxA inhibits reporter gene expression in the present THOV system. In summary, our results suggest that vRNPs are the prime target structures recognized by MxA. It will be interesting to elucidate the structural requirements of the MxA-vRNP interaction and to study the fate of these complexes in the infected cell.
Acknowledgments
We thank Simone Gruber for excellent technical assistance; Othmar Engelhardt and Adolfo Garcia-Sastre for providing plasmid pPolI-SapI-Rib; Anne Bridgen, Ewan Dunn, and Richard M. Elliott for providing plasmid pT7ribo; Peter Staeheli for helpful suggestions; and Michael Frese for critical reading of the manuscript.
This work was supported by grant Ko 1579/1-2 from the Deutsche Forschungsgemeinschaft, by grant ZKF-B1 from the Zentrum für Klinische Forschung I of the University of Freiburg, and by the Forschungsschwerpunktprogramm des Landes Baden-Württemberg.
REFERENCES
- 1.Aebi M, Fäh J, Hurt N, Samuel C E, Thomis D, Bazzigher L, Pavlovic J, Haller O, Staeheli P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broni B, Julkunen I, Condra J H, Davies M-E, Berry M J, Krug R M. Parental influenza virion nucleocapsids are efficiently transported into the nuclei of murine cells expressing the nuclear interferon-induced Mx protein. J Virol. 1990;64:6335–6340. doi: 10.1128/jvi.64.12.6335-6340.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frese M, Kochs G, Meier-Dieter U, Siebler J, Haller O. Human MxA protein inhibits tick-borne Thogoto virus but not Dhori virus. J Virol. 1995;69:3904–3909. doi: 10.1128/jvi.69.6.3904-3909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuerst T R, Niles E G, Studier F W, Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci USA. 1986;83:8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haller O, Frese M, Kochs G. Mx proteins: mediators of innate resistance to RNA viruses. Rev Sci Tech Off Int Epizoot. 1998;17:220–230. doi: 10.20506/rst.17.1.1084. [DOI] [PubMed] [Google Scholar]
- 6.Hefti H P, Frese M, Landis H, Di Paolo C, Aguzzi A, Haller O, Pavlovic J. Human MxA protein protects mice lacking a functional alpha/beta interferon system against La Crosse virus and other lethal viral infections. J Virol. 1999;73:6984–6991. doi: 10.1128/jvi.73.8.6984-6991.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang T, Pavlovic J, Staeheli P, Krystal M. Overexpression of the influenza virus polymerase can titrate out inhibition by the murine Mx1 protein. J Virol. 1992;66:4154–4160. doi: 10.1128/jvi.66.7.4154-4160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochs G, Haller O. Interferon-induced human MxA GTPase blocks nuclear import of Thogoto virus nucleocapsids. Proc Natl Acad Sci USA. 1999;96:2082–2086. doi: 10.1073/pnas.96.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kochs G, Haller O. GTP-bound human MxA protein interacts with the nucleocapsids of Thogoto virus (Orthomyxoviridae) J Biol Chem. 1999;274:4370–4376. doi: 10.1074/jbc.274.7.4370. [DOI] [PubMed] [Google Scholar]
- 10.Kochs G, Trost M, Janzen C, Haller O. MxA GTPase: oligomerization and GTP-dependent interaction with viral RNP target structures. Methods Companion Methods Enzymol. 1998;15:255–263. doi: 10.1006/meth.1998.0629. [DOI] [PubMed] [Google Scholar]
- 11.Krug R M, Shaw M, Broni B, Shapiro G, Haller O. Inhibition of influenza viral mRNA synthesis in cells expressing the interferon-induced Mx gene product. J Virol. 1985;56:201–206. doi: 10.1128/jvi.56.1.201-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlovic J, Arzet H A, Hefti H P, Frese M, Rost D, Ernst B, Kolb E, Staeheli P, Haller O. Enhanced virus resistance of transgenic mice expressing the human MxA protein. J Virol. 1995;69:4506–4510. doi: 10.1128/jvi.69.7.4506-4510.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pavlovic J, Haller O, Staeheli P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J Virol. 1992;66:2564–2569. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pleschka S, Jaskunas S R, Engelhardt O G, Zürcher T, Palese P, García-Sastre A. A plasmid-based reverse genetics system for influenza A virus. J Virol. 1996;70:4188–4192. doi: 10.1128/jvi.70.6.4188-4192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponten A, Sick C, Weeber M, Haller O, Kochs G. Dominant-negative mutants of human MxA protein: domains in the carboxy-terminal moiety are important for oligomerization and antiviral activity. J Virol. 1997;71:2591–2599. doi: 10.1128/jvi.71.4.2591-2599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samuel C E. Antiviral actions of interferon. Interferon-regulated cellular proteins and their surprisingly selective antiviral activities. Virology. 1991;183:1–11. doi: 10.1016/0042-6822(91)90112-o. [DOI] [PubMed] [Google Scholar]
- 17.Schuster A, Johnston I C D, Das T, Banerjee A K, Pavlovic J, ter Meulen V, Schneider-Schaulies S. Expression of the human MxA protein is associated with hyperphosphorylation of VSV P protein in human neural cells. Virology. 1996;220:241–245. doi: 10.1006/viro.1996.0308. [DOI] [PubMed] [Google Scholar]
- 18.Staeheli P. Interferon-induced proteins and the antiviral state. Adv Virus Res. 1990;38:147–200. doi: 10.1016/s0065-3527(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 19.Staeheli P, Haller O, Boll W, Lindenmann J, Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986;44:147–158. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- 20.Stranden A, Staeheli P, Pavlovic J. Function of the mouse Mx1 protein inhibited by overexpression of influenza virus PB2. Virology. 1993;197:642–651. doi: 10.1006/viro.1993.1639. [DOI] [PubMed] [Google Scholar]
- 21.Weber F, Jambrina E, Gonzalez S, Dessens H, Leahy M, Kochs G, Portela A, Nuttall P, Haller O, Ortin J, Zürcher T. In vivo reconstitution of active Thogoto virus polymerase: assays for the compatibility with other orthomyxovirus core proteins and template RNAs. Virus Res. 1998;58:13–20. doi: 10.1016/s0168-1702(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 22.Zürcher T, Pavlovic J, Staeheli P. Mechanism of human MxA protein action: variants with changed antiviral properties. EMBO J. 1992;11:1657–1661. doi: 10.1002/j.1460-2075.1992.tb05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]