Abstract
Optic neuritis is an inflammatory condition involving the optic nerve causing vision abnormalities ranging from decreased to complete vision loss. We present a 30 years old lady who suffered acute gradual reduced vision, which progressed to complete vision loss in her right eye the next day after receiving one dose of intravenous Metronidazole.
Keywords: Metronidazole, Optic neuritis, Pulse steroids
Introduction
Metronidazole is a bactericidal, amoebicidal, and trichomonacidal agent targeting anaerobic bacteria, certain amoebic and protozoal species. It is a nitroimidazole that inhibits nucleic acid synthesis by disrupting DNA resulting in strand breakage. Its bioavailability is more than 80 % when taken orally. It is a usually well-tolerated antimicrobial but can cause gastrointestinal side effects like a gastric upset in the form of nausea, vomiting, abdominal cramps, diarrhea, or constipation [1]. It has also been associated with other adverse effects like dizziness, darkening of the urine, and joint pain. Disulfiram reaction has been reported, especially in those drinking ethanol. Neuro-ophthalmologic side effects such as peripheral neuropathy, ataxia, encephalopathy, and even seizures have been reported with metronidazole use[2]. Optic neuritis, an inflammation of the optic nerve, is an unrecognized side effect of Metronidazole and has been reported in association with metronidazole administration in a few case reports. The nerve damage induced by Metronidazole can be either temporary or permanent.
Here, we are reporting a 30-year-old lady who presented to the emergency department with a bout of gastroenteritis and was empirically given a dose of IV Metronidazole and one day after which she started noticing decreased right eye vision, which progressed to complete vision loss in her right eye. She was managed with IV Pulse steroids. She showed a slight improvement in her right eyesight.
Case presentation
A 30-year-old Kenyan lady who is medically free presented to the emergency department of our hospital with right eye blurry vision that started one week before admission and gradually progressed over one day to complete right eye vision loss. Her condition was associated with mild right eye pain, which was exacerbated by eye movement. Her left eye was normal. She denied any previous similar episodes; she gave no history of any focal weakness or sensory abnormalities. She had no headache, no dizziness, and no abnormal body movements. She reported no fever. She did not have any history of head trauma, and she denied any urinary or defecation difficulties. On further questioning, the patient elaborated that she had an episode of gastroenteritis a week before admission. She was treated empirically with IV Metronidazole 500 mg (one dose) and discharged on Pantoprazole PO 40 mg daily (which she did not take) and Metoclopramide tablets 10 mg as needed for nausea and vomiting. The day after her discharge, she started complaining of decreased eye vision which progressively worsened to complete vision loss in one day; she did not seek medical advice due to financial issues. Upon admission, her vital signs were normal (Body Temperature: 36.7 Celsius degrees, Heart Rate: 78 beats per minute, Blood Pressure: 117/70 mmHg, SPO2: 98 % on room air). She had unremarkable chest and abdominal examinations. A central nervous system examination showed normal higher mental status. Normal power, tone, reflexes, coordination, and normal sensory function. Her gait was normal. Her cranial nerves examination showed complete loss of vision in her right eye, otherwise unremarkable. Her right eye ophthalmologic examination was significant for right afferent pupillary defect and optic disc oedema with the normal macula with normal left eye examination. Her Complete blood count, serum electrolytes, renal function, liver function tests, thyroid function test, and B12 level were all normal. Her Head Computed Tomography was unremarkable.
Lumbar Puncture and Cerebrospinal fluid (CSF) analysis were done to exclude viral CNS infection and possible other inflammatory conditions and showed WBC of 44/ul (Normal range 1–5/ul), 97 % lymphocytic, RBC 2/ul (Normal range 0–2/ul), Glucose 2.90 mmol/l (Normal range 2.22–3.89 mmol/l) and protein of 0.65 gm/l (Normal range of 0.15–0.45 gm/l). CSF was negative for culture and viral panel as well as oligoclonal bands. Her MRI brain and orbit MRI confirmed right optic neuritis. (Fig. 1 The patient was started on IV Methylprednisolone 1000 mg daily for five doses. She showed a slight improvement in her eyesight from nil to hand movement at a 50 cm distance on discharge. Follow up visits at one week and two months after discharge for the patient showed that her vision stabilized at “close counting fingers” level with no further improvement.
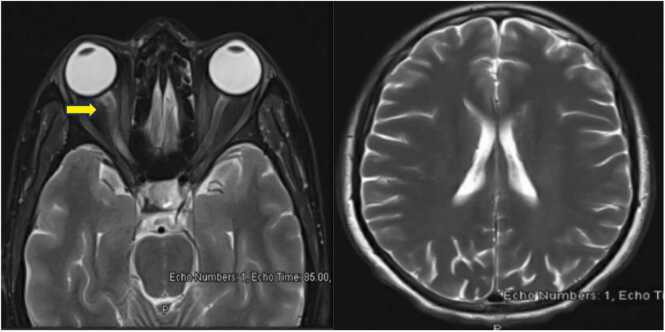
Fig. 1.
MRI Sections of the brain and orbit with contrast. The right optic nerve appears swollen with evidence of abnormal T2WI hyperintensity involving the chiasmatic, canalicular, and proximal to mid-orbital segments with mild blurring of the retro-orbital fat.(Arrowed).
Flowchart of the case presentation:
 |
Discussion
Metronidazole is an effective antimicrobial that was first used against trichomonas infections in the late 1950s and then was found to have effects on anaerobic bacteria and protozoal species. It is frequently used and usually has gastrointestinal side effects of gastric upset and metallic taste feeling. Its neurological adverse effects have been infrequent and only reported in case reports; these include dizziness, ataxia, encephalopathy, and seizure [2]. Ophthalmic side effects have been rarely reported and only seen in case reports and are not frequently recognized by healthcare providers. Here we described a case report of 30-year-old lady who was medically free who developed right optic neuritis after one dose of IV metronidazole given empirically for a bout of gastroenteritis with no significant recovery of her vision after pulse steroid is commenced for 5 days.
An extensive literature review was done, and we found 10 case reports and 1 case report with a literature review discussing the neuro-ophthalmic profile of Metronidazole. The age of patients ranged from 6 to 67 years, 8 were males, and 9 were females. All of the cases we could extract data for were administered Metronidazole orally. The dose ranged between 250 mg to 1500 mg per day, and the duration of drug administration to symptom development was from one dose of 250 mg up to chronic use of 2 years. 15 patients developed optic neuritis, three patients developed aseptic meningitis, one patient developed myelitis, and two patients had cerebellar involvement. All the patients were managed with the withdrawal of Metronidazole, except one patient with aseptic meningitis who was treated with a 3rd generation intravenous cephalosporin for one week, and another patient with optic neuritis who was initiated on coenzyme Q10 for 3 months in addition to cessation of metronidazole. Most patients fully recovered their vision, but one whose damage was irreversible, and seven other patients had 71 % reversibility of their sight (Please see Table 1 for the summary and comparison of literature review and our case).
Table 1.
Comparison of our case and summary of literature review.
| Case | Age | Gender | Route of administration | Dose | Duration | Outcome | Treatment | Reversibility |
|---|---|---|---|---|---|---|---|---|
| Our Case | 30 years old | Female | IV | 500 mg | One dose | Right optic neuritis and aseptic neuritis | IV Pulse steroid 500 mg IV Methylprednisolone for 5 days | Some reversibility |
| A.Viharika et al.[3] | 49 years old | Male | Oral | 400 mg BID | One week | Aseptic meningitis | Cefotaxime 500 mg BID IV for one week | Yes |
| Nicole M McGrath et al.[4] | 67 years old | Female | Oral | 400 mg BID | 8 months | Bilateral optic neuritis | Stopping Metronidazole | Yes |
| Pai-Huei-Peng et al.[5] | 57 years old | Female | Oral | 1000 mg OD | 1 week | Bilateral optic neuritis and meylitis | Stopping Metronidazole | No |
| Natalie Anwyll et al.[6] | 36 years old | Male | NA | NA | Two years | Optic neuritis | Stopping Metronidazole | Yes |
| Audrey P et al.[7] | 20 years old | Female | Oral | 250 mg | One dose | Aseptic meningitis | Stopping Metronidazole | Yes |
| Erin Nuro et al.[8] | 58 years old | Female | oral | 500 mg TID | For two years | Optic neuropathy and cerebellar toxicity | Stopping Metronidazole | Yes |
| Bouraoui R et al.[9] | 6 years and 8 years old | Female | Oral | NA | Two weeks | Bilateral optic neuritis | Stopping Metronidazole | Yes |
| Sujoy Khan et a[10] | 42 years old | Male | Oral | 400 mg | One dose | Aseptic meningitis | Stopping Metronidazole | Yes |
| Putnam et al.[11] | 7 patients from 26-53 years old | 4 females and 3 males | Oral | 750-1000 mg daily | 7-365 days | Optic neuritis | Stopping Metronidazole | Some reversibility |
| Kim M Cecil et al.[12] | 17 years old | Male | Oral | 500 mg TID | Over 6 months | Optic neuropathy and cerebellar toxicity | Stopping Metronidazole Starting coenzyme Q10 | Yes |
| De Bleecker et al.[13] | 20 years old | Male | Oral | 1500 mg daily | 2 years | Optic neuropathy | Stopping Metronidazole | Yes |
Possible limitation of this paper is that it is only reporting a case, although, rare case reports have been found about a potential similar serious side effect of metronidazole but the finding in our case can still be a coincidence and our patient will be having long term follow ups to monitor for the potential development of a demyelinating disease in the future like multiple sclerosis.
A possible clinical implication in our finding is that clinicians should always have a broad view on each and every case that can not be explained after extensive investigation with a special attention to medications review.
Conclusion
Metronidazole is an effective and widely used antimicrobial commonly associated with gastrointestinal side effects. In addition, its neuro-ophthalmic profile, though rare, can be sight-threatening, resulting in irreversible vision loss. Therefore, clinicians should be attentive to the adverse effect of even one dose of intravenous or oral Metronidazole.
Ethical Approval
Ethical approval was obtained from Hamad Medical Research Center (MRC) on 6th of September,2022 under MRC number of 04–22-541.
Consent Statement
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Funding
We thank Qatar National Library (QNL) for funding the publication of this work.
CRediT authorship contribution statement
Haidar Hussein Barjas: Data curation, Writing – original draft, Writing – review & editing. Mohamed Elshafei: Writing – original draft. Shamin Mahmud: Writing – original draft. Abdel-Nasser Elzouki: Data curation, Supervision.
Declaration of Competing Interest
All authors confirm that there is no personal or financial interest related this case and we agree for the publication of this work as open access.
References
- 1.Löfmark S., Edlund C., Nord C.E. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis. 2010;50(Suppl 1):S16–S23. doi: 10.1086/647939. PMID: 20067388. [DOI] [PubMed] [Google Scholar]
- 2.Kusumi R.K., Plouffe J.F., Wyatt R.H., Fass R.J. Central nervous system toxicity associated with metronidazole therapy. Ann Intern Med. 1980;93(1):59–60. doi: 10.7326/0003-4819-93-1-59. PMID: 7396319. [DOI] [PubMed] [Google Scholar]
- 3.Viharika A., Basheer S., Bharathi G.V., Reddy T.R., Ramana V. Metronidazole Induced Meningtis: A Case Report DOI: 10.9790/0853-1811073739. [DOI]
- 4.McGrath N.M., Kent-Smith B., Sharp D.M. Reversible optic neuropathy due to metronidazole. Clin Exp Ophthalmol. 2007;35(6):585–586. doi: 10.1111/j.1442-9071.2007.01537.x. PMID: 17760644. [DOI] [PubMed] [Google Scholar]
- 5.Peng P.H., Wu T.E., Lin T.Y. Metronidazole-Induced Irreversible Optic Neuropathy. Case Rep Ophthalmol. 2021;12(2):392–395. doi: 10.1159/000512625. PMID: 34054490; PMCID: PMC8136306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anwyll N., Gerry P., Gormley J. Reversible optic neuropathy secondary to metronidazole. BMJ Case Rep. 2020;13(12) doi: 10.1136/bcr-2020-237141. PMID: 33334752; PMCID: PMC7747533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corson A.P., Chretien J.H. Metronidazole-associated aseptic meningitis. Clin Infect Dis. 1994;19(5):974. doi: 10.1093/clinids/19.5.974. PMID: 7893894. [DOI] [PubMed] [Google Scholar]
- 8.Nuro E., Bursztyn L.L., Mendonça D.A., Pelz D.M., Budhram A. Neuroimaging Evidence of Optic Tract Involvement in Metronidazole-Induced Optic Neuropathy. Can J Neurol Sci. 2023;50(2):290–291. doi: 10.1017/cjn.2022.21. Epub 2022 Feb 22. PMID: 35190000. [DOI] [PubMed] [Google Scholar]
- 9.Bouraoui R., Limaiem R., Bouladi M., Mghaieth F., El Matri L. Effets secondaires neuro-ophtalmologiques du traitement par métronidazole chez l′enfant: à propos de deux cas [Neuro-ophthalmic adverse effects of metronidazole treatment in children: Two case studies] Arch Pediatr. 2016;23(2):167–170. doi: 10.1016/j.arcped.2015.11.003. (. French) [DOI] [PubMed] [Google Scholar]
- 10.Khan S., Sharrack B., Sewell W.A. Metronidazole-induced aseptic meningitis during Helicobacter pylori eradication therapy. Ann Intern Med. 2007;146(5):395–396. doi: 10.7326/0003-4819-146-5-200703060-00017. PMID: 17339628. [DOI] [PubMed] [Google Scholar]
- 11.Putnam D., Fraunfelder F.T., Dreis M. Metronidazole and optic neuritis. Am J Ophthalmol. 1991;112(6):737. doi: 10.1016/s0002-9394(14)77290-3. PMID: 1957918. [DOI] [PubMed] [Google Scholar]
- 12.Cecil K.M., Halsted M.J., Schapiro M., Dinopoulos A., Jones B.V. Reversible MR imaging and MR spectroscopy abnormalities in association with metronidazole therapy. J Comput Assist Tomogr. 2002;26(6):948–951. doi: 10.1097/00004728-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 13.De Bleecker J.L., Leroy B.P., Meire V.I. Reversible visual deficit and Corpus callosum lesions due to metronidazole toxicity. Eur Neurol. 2005;53(2):93–95. doi: 10.1159/000085506. [DOI] [PubMed] [Google Scholar]