Abstract
Scedosporium/Lomentospora infections are rare and are associated with a high mortality rate in immunocompromised patients. A 69-year-old man with nontuberculous mycobacteria (NTM) died during induction chemotherapy for acute myeloid leukemia because of multiple organ failure due to pneumonia. During an autopsy, Lomentospora prolificans was detected using a fungal gene analysis of the blood, lungs, spleen, kidneys, and intestines, and Scedosporium aurantiacum was detected in the lungs. NTM disease may predispose patients to Scedosporium/Lomentospora infections. Physicians should consider Scedosporium/Lomentospora spp. as an invasive fungal infection that occurs during myelosuppression, particularly when NTM is a complication.
Keywords: Lomentospora prolificans, Scedosporium aurantiacum, acute myeloid leukemia, nontuberculous mycobacteria
Introduction
Scedosporium/Lomentospora species are saprophytic fungi that are widely distributed in soil, marshes, and sludge. Scedosporium/Lomentospora cause disseminated infections, pneumonia, osteomyelitis, and arthritis in immunocompromised patients with malignancies, cystic fibrosis, solid organ transplantation, or acquired immunodeficiency syndrome (1-3). Previously, Lomentospora prolificans was included among Scedosporium species, although it is now classified as an independent species (4).
We herein report a patient who died of disseminated coinfection with L. prolificans and Scedosporium aurantiacum during induction therapy for acute myeloid leukemia (AML).
Case Report
A 69-year-old man was diagnosed with AML with maturation (World Health Organization 2017) and AML M2 (French-American-British classification), accompanied by pancytopenia. His medical history included hypertension and nontuberculous mycobacteria (NTM). Mycobacterium intracellulare and Mycobacterium avium were detected in sputum cultures; however, the patient had not previously received any agents against NTM. He was not a smoker. On admission, the patient was conscious and his vital signs and physical examination results were normal.
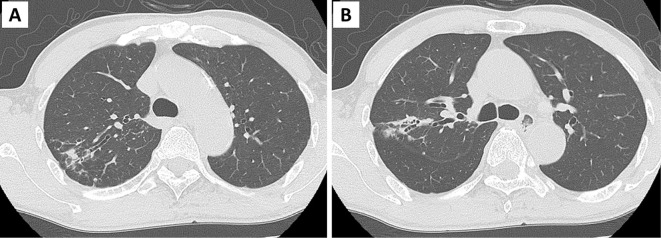
Blood test results revealed pancytopenia (Table). Biochemical and coagulation tests revealed no abnormalities. Peripheral blood blast cells and bone marrow myeloblasts comprised 1% and 81.4% of the population, respectively, with a 100% myeloperoxidase positivity rate. Flow cytometry revealed that the blasts in the peripheral blood and bone marrow were CD13+, CD33+, CD34-, CD56+, and HLA-DR-. The G-band analysis revealed a normal karyotype. Real-time polymerase chain reaction screening was not performed. Chest computed tomography (CT) revealed bronchiectasis and scattered surrounding shadows in the upper and middle lobes of the right lung (Fig. 1).
Table.
Laboratory Data on Admission.
| Blood cell count | Biochemistry | Bone marrow | ||||||||
| WBC | 1,740 | /µL | AST | 17 | IU/L | NCC | 114,000 | /µL | ||
| Blast | 1 | % | ALT | 12 | IU/L | MgK | 6 | /µL | ||
| Neutrophil | 42 | % | LDH | 192 | IU/L | M/E | 8.5 | |||
| Eosinophil | 0 | % | ALP | 206 | IU/L | |||||
| Basophil | 0 | % | T-bil | 0.7 | mg/dL | Myeloblast | 81.4 | % | ||
| Monocyte | 3 | % | TP | 7.7 | g/dL | Promyelocyte | 0.2 | % | ||
| Lymphocyte | 54 | % | Alb | 4.5 | g/dL | Myelocyte | 0.6 | % | ||
| RBC | 362 | ×104/µL | BUN | 19.2 | mg/dL | Metamyelocyte | 0.2 | % | ||
| Hemoglobin | 12.5 | g/dL | Cre | 0.85 | mg/dL | Band | 0.4 | % | ||
| Hematocrit | 37.4 | % | UA | 5.9 | mg/dL | Segment | 2.4 | % | ||
| Platelets | 4.9 | ×104/µL | Na | 142 | mEq/L | Monocyte | 0.8 | % | ||
| Reticulocytes | 4 | ×104/µL | K | 4.4 | mEq/L | Lymphocyte | 3.8 | % | ||
| Ca | 9.4 | mg/dL | Plasma cell | 0.2 | % | |||||
| Coagulation | BS | 89 | mg/dL | Erythroblast | 10 | % | ||||
| PT-INR | 1.06 | CRP | 0.03 | mg/dL | ||||||
| APTT | 30.9 | s | Ferritin | 143 | ng/mL | FCM | ||||
| Fibrinogen | 214 | mg/dL | CD13 | (+) | ||||||
| FDP | 3 | µg/mL | Immunochemistry | CD33 | (+) | |||||
| IgG | 1,621 | mg/dL | CD56 | (+) | ||||||
| IgA | 475 | mg/dL | CD34 | (-) | ||||||
| IgM | 52 | mg/dL | HLA-DR | (-) | ||||||
| Karyotype | ||||||||||
| 46, XY [20/20] | ||||||||||
WBC: white blood cell, RBC: red blood cell, PT-INR: prothrombin time international normalized ratio, APTT: activated partial thromboplastin time, FDP: fibrinogen degradation product, AST: aspartate transaminase, ALT: alanine transaminase, LDH: lactic acid dehydrogenase, ALP: alkaline phosphatase, T-bil: total bilirubin, TP: total protein, Alb: albumin, BUN: blood urea nitrogen, Cre: creatinine, UA: uric acid, Na: sodium, K: pottasium, Ca: calcium, BS: blood sugar, CRP: C-reactive protein, NCC: nuclear cell count, MgK: megakaryocyte, M/E: myeloid/erythroid, FCM: flow cytometry
Figure 1.
Chest computed tomography (CT) findings at admission. (A, B) Chest CT revealed bronchiectasis and surrounding scatter shadows in the upper and middle lobe tongues of the right lung.
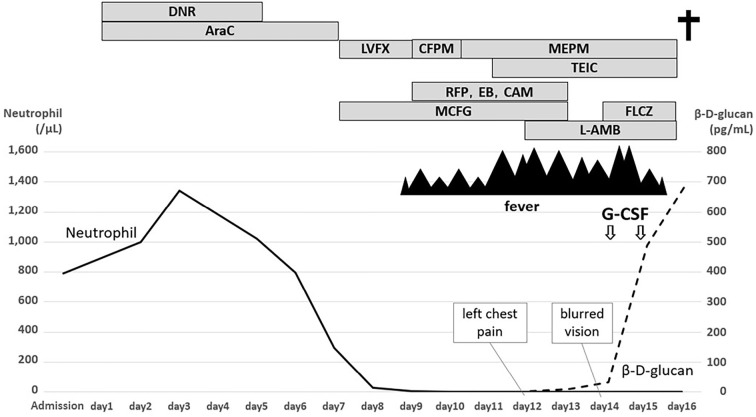
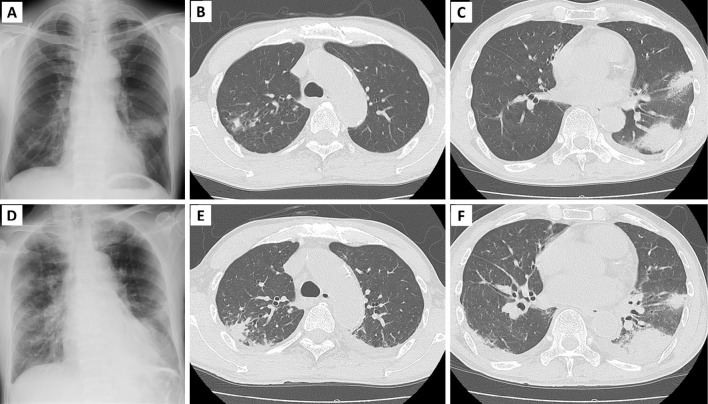
The clinical course of the patient is summarized in Fig. 2. Treatment with daunorubicin (40 mg/m2, 5 days) and cytarabine (80 mg/m2, 7 days) was initiated as induction therapy. On day 7 of induction therapy, 50 mg micafungin (MCFG) was initiated as prophylaxis for fungal infection. Rifampicin (RFP), ethambutol, and clarithromycin were administered to treat NTM on day 9. However, the patient developed a fever on the same day when the neutrophil count was 9 cells/μL. Blood culture results were negative, and the β-D-glucan level was <2.14 pg/mL. No new abnormal shadows were detected on chest radiography. Cefepime (CFPM) was initiated because of febrile neutropenia; however, the fever persisted, and the patient presented with cough. On day 10, CFPM was replaced with meropenem, the central venous catheter was removed, and teicoplanin was added. The following day, the β-D-glucan level increased slightly to 3.28 pg/mL, and chest radiography indicated infiltration of the left lower lung field (Fig. 3A). CT revealed infiltration shadows with ground-glass shadows around the lower left lobe and tongue. Left pleural effusion was also observed (Fig. 3B, C). Based on the CT findings, infectious diseases, including fungal diseases and exacerbation of NTM, were suspected.
Figure 2.
Summary of the clinical course of the patient. DNR: daunorubicin, AraC: cytarabine, LVFX: levofloxacin, CFPM: cefepime, MEPM: meropenem, TEIC: teicoplanin, RFP: rifampicin, EB: ethambutol, CAM: clarithromycin, MCFG: micafungin, L-AMB: liposomal amphotericin B, FLCZ: fluconazole, G-CSF: granulocyte colony-stimulating factor, CRP: C-reactive protein
Figure 3.
Chest radiography and computed tomography (CT) obtained on day 12 (A-C) and day 15 (D-F) after induction therapy. Chest radiography on day 12 revealed infiltration in the lower left lung, and CT revealed infiltration shadows with ground glass shadows around the lower left lobe and tongue. Chest radiography and CT on day 15 revealed exacerbation of the infiltrative shadow on the periphery of the upper lobe of the right lung and worsening of the infiltrative shadow on the left lung.
Aspergillus and Cryptococcus antigens in the blood were negative, and Candida antigen was weakly positive. Although the MCFG dose was increased from 50 mg to 150 mg, his general condition deteriorated, and liposomal amphotericin B (L-AMB) was initiated at a dose of 2.5 mg/kg/day. However, the fever and chills persisted, and the patient experienced left-sided chest pain. On day 13, the β-D-glucan level increased to 8.14 pg/mL. The L-AMB dose was increased to 5.0 mg/kg/day on the same day. However, he had blurred vision, and the fundus findings suggested fungal endophthalmitis, including vitreous hemorrhaging, retinal hemorrhaging, and choroiditis. Since disseminated candidemia was suspected, fluconazole was added to L-AMB, and granulocyte colony-stimulating factor (G-CSF) was administered.
The patient presented with liver and renal dysfunction, and the β-D-glucan level increased to 488.70 pg/mL. CT revealed exacerbation of the infiltrative shadow on the periphery of the upper lobe of the right lung and worsening of the infiltrative shadow in the left lung (Fig. 3D-F). In the evening, the patient presented with impaired consciousness and developed septic shock. He was started on noradrenaline but died on day 16. On the same day, filamentous fungi were detected in blood cultures that had been collected on day 11.
Autopsy findings
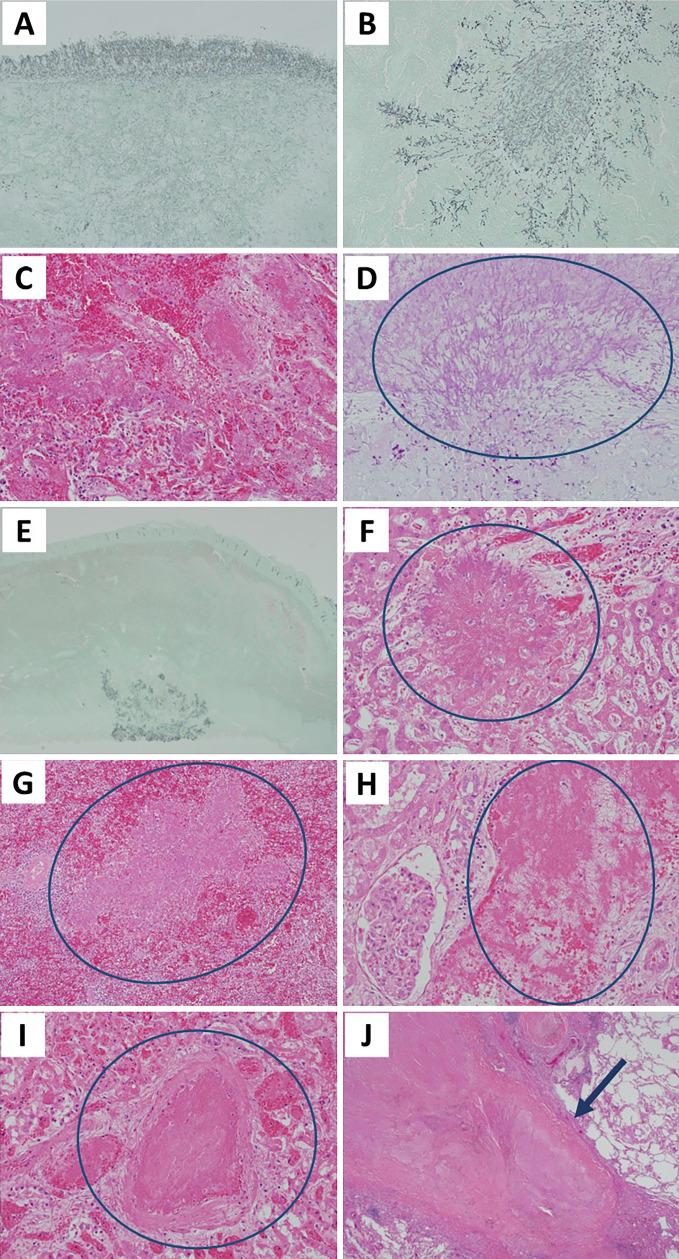
Microscopically, bacterial growth was observed on the surface of the upper and lower lobes of the left lung, as well as on the upper lobe of the right lung, mainly in and around the arteries (Fig. 4A-C). Furthermore, hyphal growth accompanied by hemorrhaging and necrosis was observed in the left ventricular endocardium, small intestine, liver, spleen, and both kidneys (Fig. 4D-I). Multiple caseous necroses corresponding to NTM were observed in the upper lobe of the right lung (Fig. 4J). A fungal gene analysis using polymerase chain reaction was performed to detect L. prolificans in the blood, lower lobe of the left lung, spleen, right kidney, and circumflex valve and S. aurantiacum in the upper lobe of the left lung. A brain autopsy was not performed. No residual myeloblasts, which were aplastic, were observed in the bone marrow.
Figure 4.
Histological findings of the autopsy. Fungal hyphae were observed in the upper lobe of the left lung (Grocott staining, ×40) (A), the lower lobe of the left lung (Grocott staining, ×100) (B), the upper lobe of the right lung [Hematoxylin and Eosin (H&E) staining, ×400] (C), the left ventricular endocardium (periodic acid-Schiff staining, ×200) (D), the small intestine (Grocott staining, ×40) (E), the liver (H&E staining, ×200) (F), the spleen (H&E staining, ×100) (G), the kidney (H&E staining, ×200) (H, I). Multiple caseous necroses corresponding to nontuberculous mycobacteria are observed in the upper lobe of the right lung (arrow) (H&E staining, ×20) (J).
Discussion
Scedosporium/Lomentospora infections are extremely rare opportunistic infections with 5.7 cases per 10,000 patients diagnosed with acute leukemia (5). Furthermore, they can be fatal in immunocompromised patients. The fatality rate of L. prolificans infection is approximately 46.9%, which increases to 87.5% in cases of dissemination (3). A retrospective multicenter study focusing on Scedosporium/Lomentospora infections identified 5 cases among 8,622 patients with acute leukemia. All of the patients developed myelosuppression and eventually died (5). Furthermore, the average duration from neutropenia to disseminated infection (positive blood culture) was 9 days, and the average duration from disseminated infection to death was 17 days (5). However, our patient exhibited rapid progression, with disseminated infection occurring within five days of neutropenia and death within five days thereafter.
In the present study, Scedosporium and Lomentospora spp. were detected. Delhaes et al. reported that 47 (38.2%) of 123 patients had disseminated infections, including 75 with L. prolificans, 25 with Scedosporium apiospermum (S. apiospermum), and 23 with S. aurantiacum. The number of cases of disseminated infection caused by L. prolificans was 39/47 (82.9%), which was significantly higher than that caused by other species (8 isolates, 17.0%) (6). L. prolificans infection is associated with a higher frequency of fungemia than S. apiospermum infection in patients who receive transplantation, and more severe toxicity has been reported (7). In our case, co-infection with L. prolificans and S. aurantiacum was observed; however, S. aurantiacum was detected in the lung lesion, whereas L. prolificans induced a disseminated infection. Therefore, the invasive capacities of these two fungal species may differ.
L. prolificans and S. aurantiacum may have colonized the bronchodilation site via the NTM and progressed to invasive mycosis during myelosuppression in our case. CT conducted before induction therapy indicated bronchiectasis equivalent to NTM in the upper lobe of the right lung, and autopsy findings revealed multiple caseous necroses at the same site and mycelia in the same lung area. The incidence of pulmonary aspergillosis is significantly higher in patients with NTM (33.3%, 10/30 cases) than in those without NTM (9.8%, 6/61 cases) (8). Furthermore, the incidence of pulmonary aspergillosis is high in patients with NTM, as local immunity decreases due to the destruction of normal lung structures (8). In a previous study, all seven patients with Scedosporium/Lomentospora infections in the lungs after lung transplantation demonstrated structural changes in the airways (9). Murayama et al. reported a case of co-infection with M. avium and S. apiospermum in the bronchi and highlighted that bronchodilation by M. avium may decrease local immunity, colonization, and proliferation of fungi (10).
In general, the risk of invasive fungal infections (IFIs) is caused by complex factors, such as the presence of an underlying malignancy, therapies, immune status, and patient-specific and environmental factors (11). Previous studies have identified several pretreatment risk factors for invasive mold infections, such as Aspergillus spp. or Scedosporium/Lomentospora spp., in patients who received induction chemotherapy for AML, including a performance status ≥2 according to the Eastern Cooperative Oncology Group scale, recent house renovation, construction work, farming, gardening, and forestry work, and chronic obstructive pulmonary disease (11). However, our patient did not present with any of these risk factors. In our case, complications of NTM disease, neutropenia due to AML, and induction therapy were considered risk factors. No underlying immunocompromised condition was identified. The lymphocyte count before treatment was slightly lower than the normal range (940 cells/μL), and the immunoglobulin levels were within the normal range.
Patients with AML who are ineligible for intensive chemotherapy due to advanced age or comorbidities can be treated with a venetoclax-azacitidine combination regimen (ven/aza) (12). In a retrospective multicenter cohort study of 235 patients with AML receiving ven/aza, the incidence of IFI in possible and probable/confirmed cases was 7.7% and 5.1%, respectively (13). In contrast, in a prospective multicenter study, the incidence of IFI among 881 patients receiving intensive chemotherapy was 15.6% and 8.7% in possible and probable/confirmed cases, respectively (11). The proportions of patients prescribed antifungal agents were 67.2% and 80.8% for ven/aza and intensive chemotherapy, respectively (11,13). The ven/aza regimen may be more effective than intensive chemotherapy in reducing the incidence of IFIs. One reason for this is that the ven/aza regimen minimizes neutropenia by reducing the duration of venetoclax therapy or administration of G-CSF, thereby decreasing the risk of IFIs (13). Recovery from neutropenia is independently associated with a reduced mortality risk from Scedosporium/Lomentospora infection in patients with hematological malignancies (3). In our case, selection of the ven/aza regimen may have been better for induction therapy. However, as no studies have reported the incidence of Scedosporium/Lomentospora infections in patients treated with ven/aza, further investigation is required.
Patients with prolonged neutropenia (<500 cells/μL and >7 days), such as those undergoing myelosuppressive chemotherapy or hematopoietic stem cell transplantation, are at high risk of IFIs. The clinical benefits of antifungal prophylaxis have been demonstrated in this population (14,15). A network meta-analysis and the Infectious Diseases Working Party of the German Society for Hematology and Medical Oncology guidelines strongly recommend posaconazole for AML patients (14,15). However, evidence regarding the efficacy of voriconazole (VRCZ) prophylaxis for IFI prevention remains limited (15). The European Federation of Medical Mycology recommends VRCZ as a first-line therapy for Scedosporium spp. infections and strongly supports first-line VRCZ-based combination antifungal therapy, particularly VRCZ and terbinafine, for treating L. prolificans infections (16). Scedosporium/Lomentospora spp. are naturally resistant to L-AMB and fluconazole and less sensitive to echinocandins (17-19). L. prolificans is much more resistant to the currently available antifungals than Scedosporium spp. (7,20). Furthermore, in some cases, L. prolificans is resistant to VRCZ monotherapy (8,17,19,20). VRCZ and terbinafine have demonstrated in vitro synergism and have been successfully used to treat lomentosporiosis in several case reports (16). However, the synergistic effects of terbinafine remain controversial (17,20). According to a registry study, the overall survival was not significantly different between patients with lomentosporiosis receiving combination therapy with VRCZ and terbinafine and those receiving therapy with VRCZ alone (20). In addition, routine drug susceptibility testing for this fungus is not indicated in clinical practice as in the present study. Therefore, determining the best treatment for Scedosporium/Lomentospora infections is difficult.
At present, VRCZ is not administered because of its interaction with RFP. When administered according to the manufacturer's instructions, RFP decreased the maximum blood concentration and area under the blood concentration-time curve of VRCZ by 93% and 96%, respectively. In addition, RFP affected the pharmacokinetics of concomitant drugs for approximately one to two weeks after discontinuation (21). A randomized study that excluded patients with malignancies and those receiving immunosuppressant therapy demonstrated that a two-drug regimen (clarithromycin and ethambutol) for treating M. avium complex lung disease was not inferior to a three-drug regimen (clarithromycin, ethambutol, and RFP) (22). Nevertheless, avoiding RFP to select an optimal antifungal agent and ensuring drug blood concentration when neutropenia is prolonged might have to be considered, as in the induction therapy for AML.
We encountered a case of disseminated L. prolificans infection and pneumonia caused by S. aurantiacum during induction therapy in a patient with AML and NTM infection. NTM may serve as a gateway for IFIs and is an important risk factor for Scedosporium/Lomentospora infection. Physicians should consider possible Scedosporium/Lomentospora infections when fungal infections are suspected, such as in instances of β-D-glucan elevation during myelosuppression, especially in patients with NTM. VRCZ or combination therapy with VRCZ and terbinafine can induce a treatment response.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Cortez KJ, Roilides E, Quiroz-Telles F, et al. Infections caused by Scedosporium spp. Clin Microbiol Rev 21: 157-197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooley L, Spelman D, Thursky K, Slavin M. Infection with Scedosporium apiospermum and S. prolificans, Australia. Emerg Infect Dis 13: 1170-1177, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodriguez-Tudela JL, Berenguer J, Guarro J, et al. Epidemiology and outcome of Scedosporium prolificans infection, a review of 162 cases. Med Mycol 47: 359-370, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Lackner M, de Hoog GS, Yang L, et al. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Divers 67: 1-10, 2014. [Google Scholar]
- 5.Caira M, Girmenia C, Valentini CG, et al. Scedosporiosis in patients with acute leukemia: a retrospective multicenter report. Haematologica 93: 104-110, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Delhaes L, Harun A, Chen SC, et al. Molecular typing of Australian Scedosporium isolates showing genetic variability and numerous S. aurantiacum. Emerg Infect Dis 14: 282-290, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husain S, Muñoz P, Forrest G, et al. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin Infect Dis 40: 89-99, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Kunst H, Wickremasinghe M, Wells A, Wilson R. Nontuberculous mycobacterial disease and Aspergillus-related lung disease in bronchiectasis. Eur Respir J 28: 352-357, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Tamm M, Malouf M, Glanville A. Pulmonary scedosporium infection following lung transplantation. Transpl Infect Dis 3: 189-194, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Murayama T, Amitani R, Tsuyuguchi K, et al. Polypoid bronchial lesions due to Scedosporium apiospermum in a patient with Mycobacterium avium complex pulmonary disease. Eur Respir J 12: 745-747, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Caira M, Candoni A, Verga L, et al.; the SEIFEM Group. Pre-chemotherapy risk factors for invasive fungal diseases: prospective analysis of 1,192 patients with newly diagnosed acute myeloid leukemia (SEIFEM 2010-a multicenter study). Haematologica 100: 284-292, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 383: 617-629, 2020. [DOI] [PubMed] [Google Scholar]
- 13.On S, Rath CG, Lan M, et al. Characterisation of infections in patients with acute myeloid leukaemia receiving venetoclax and a hypomethylating agent. Br J Haematol 197: 63-70, 2022. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Zhou M, Xu JY, Zhou RF, Chen B, Wan Y. Comparison of antifungal prophylaxis drugs in patients with hematological disease or undergoing hematopoietic stem cell transplantation: a systematic review and network meta-analysis. JAMA Netw Open 3: e2017652, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellinghoff SC, Panse J, Alakel N, et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann Hematol 97: 197-207, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoenigl M, Salmanton-García J, Walsh TJ, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis 21: e246-e257, 2021. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy MW, Katragkou A, Iosifidis E, Roilides E, Walsh TJ. Recent advances in the treatment of scedosporiosis and fusariosis. J Fungi (Basel) 4: 73, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troke P, Aguirrebengoa K, Arteaga C, et al.; the Global Scedosporium Study Group. Treatment of scedosporiosis with voriconazole: clinical experience with 107 patients. Antimicrob Agents Chemother 52: 1743-1750, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Grossman N, Totten M, et al. Antifungal susceptibility profiles and drug resistance mechanisms of clinical Lomentospora prolificans isolates. Antimicrob Agents Chemother 64: e00318-e00320, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidel D, Meißner A, Lackner M, et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScopeⓇ. Crit Rev Microbiol 45: 1-21, 2019. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita D, Wada K, Yoshimuta T, et al. Drug interaction between voriconazole and rifampicin after discontinuation of rifampicin: a case report. Iryou Yakugaku (Jpn J Pharm Health Care Sci) 36: 323-327, 2010. (in Japanese, Abstract in English). [Google Scholar]
- 22.Miwa S, Shirai M, Toyoshima M, et al. Efficacy of clarithromycin and ethambutol for Mycobacterium avium complex pulmonary disease. A preliminary study. Ann Am Thorac Soc 11: 23-29, 2014. [DOI] [PubMed] [Google Scholar]