Abstract
Objective
The daily step count is associated with mortality in idiopathic pulmonary fibrosis (IPF). However, the factors associated with this phenomenon are not yet fully understood. We therefore clarified its association with clinical parameters.
Methods
Fifty-nine patients with IPF with available data for daily step counts; 6-minute walk distance (6MWD); chest, abdominal, and pelvic computed tomography (CT); pulmonary function; psychological evaluations; and sarcopenia assessments were prospectively enrolled. The daily step count was measured continuously for seven consecutive days. The cross-sectional areas of the erector spinae muscles at the level of the 12th vertebra (ESMCSA) and psoas major muscle volume (PMV) obtained by CT were assessed.
Results
The average age of the patients was 73.3±8.1 years old, and the percent predicted forced vital capacity was 81.6%±15.8%. The median daily step count was 4,258 (2,155-6,991) steps. The average 6MWD, ESMCSA, and PMV were 413±97 m, 25.5±6.7 cm2, and 270±75.6 cm3, respectively. A linear regression analysis for daily step count showed that the ESMCSA and 6MWD were independent factors for the daily step count, whereas the PMV and skeletal muscle index were not. The daily step count, ESMCSA, and 6MWD were lower in patients with sarcopenia than in those without sarcopenia.
Conclusion
A lower daily step count was associated with a smaller erector spinae muscle area and sarcopenia in patients with IPF. Further studies are warranted to confirm the importance of physical therapy for muscle strengthening in patients with IPF.
Keywords: idiopathic pulmonary fibrosis, erector spinae muscle, computed tomography, 6-minute walk test, daily step count, sarcopenia
Introduction
Idiopathic pulmonary fibrosis (IPF) is a fibrotic and progressive pulmonary disease that can lead to death in most patients (1,2). As the disease progresses, most IPF patients experience exercise intolerance, physical inactivity, and an impaired health-related quality of life (3).
A meta-analysis showed that taking more steps per day was associated with a progressively lower risk of all-cause mortality, according to appropriate levels that vary with age (4). Daily activity is associated with mortality in IPF (5). The cutoff value of the daily step count for predicting the 1-year survival in IPF was 3,473 steps/day (6). However, the factors associated with a low daily step count in patients with IPF are not fully understood. Therefore, a solution is needed to address the problem of low daily step count in IPF patients.
The 6-minute walk test (6MWT) is commonly used for the objective assessment of the functional exercise capacity in the management of patients with IPF. The distance walked during the 6MWT [6-minute walk distance (6MWD)] was reported to be an independent predictor of mortality in patients with IPF (7). The 6MWD is correlated with the daily step count in patients with chronic obstructive respiratory disease (COPD) (8). However, to our knowledge, no correlation between the daily step count and 6MWD has been reported.
Skeletal muscle mass can be evaluated by the cross-sectional area (CSA) of muscles on computed tomography (CT) because the CSA of muscles correlates well with the total body muscle mass in healthy participants (9). Numerous studies have reported that the CSA of the psoas major muscles at the level of the 3rd or 4th lumbar vertebra (PMCSA) is a surrogate marker of skeletal muscle loss in different patient populations (10-14). The PMCSA is reportedly a predictor of major surgical complications (11) and is associated with mortality in patients with both benign (12) and malignant diseases (13,14). However, there are no reports on psoas major muscle measurements in IPF, as abdominal-pelvic CT is not usually performed in these patients. Further studies are warranted to confirm the clinical importance of psoas major muscle measurement in patients with IPF.
A correlation was reported between the paraspinous muscle area at the level of the 12th thoracic vertebra and the total psoas muscle area at the level of the 4th lumbar vertebra (r=0.72, p<0.001), both of which were associated with lower mortality rates after surgery (15). The CSA of the erector spinae muscle at the level of the 12th thoracic vertebra (ESMCSA) has been reported in patients with IPF (16-18). The baseline ESMCSA (16) and an early decline in the ESMCSA (17) have been reported to be associated with mortality.
Sarcopenia is an age-related condition characterized by a progressive and generalized loss of the skeletal muscle mass and function and is correlated with physical disability, a poor quality of life, and death (19,20). Sarcopenia was identified in 32.1% of patients with interstitial lung disease (21) and 22.9-39.3% of patients with IPF (22,23). The 6MWD is an independent factor associated with sarcopenia (23). Thus, sarcopenia may be associated with a low daily step count. Sarcopenia management involves physical therapy for muscle strengthening and gait training (24).
We hypothesized that the daily step count would be associated with the ESMCSA, psoas major muscle volume (PMV), and sarcopenia in patients with IPF. In this prospective cross-sectional study, we assessed the correlations between the daily step count and the ESMCSA, PMV, pulmonary function tests, 6MWD, psychological scores, and sarcopenia.
Materials and Methods
Ethics
This single-center, cross-sectional pilot study was conducted in accordance with the amended Declaration of Helsinki (as revised in 2013) and approved by the Ethics Review Board of Nagoya City University Hospital (approval number: 60-20-0190, approval date: February 24, 2021). Written informed consent was obtained from all participants. The authors had access to information that could identify individual participants during data collection.
Participants
IPF was diagnosed through multidisciplinary discussions according to the 2018 international guidelines (25). The members who participated in multidisciplinary discussions were HO, ANa, KF, YO, and TM. Between April 2021 and June 2022, outpatients with stable IPF were screened at Nagoya City University Hospital (Nagoya, Japan). The inclusion criteria were written informed consent obtained for this study and the ability to perform the 6MWT. The following exclusion criteria were applied: long-term oxygen treatment at rest and active cancer. To reduce missing data, we excluded patients who required supplemental oxygen therapy at rest, given their difficulty in performing the 6MWT, pulmonary function tests, and daily step count or completing the time-consuming questionnaires.
CT
All patients underwent chest, abdominal, and pelvic CT using commercially available CT scanners with a high-frequency algorithm. High-resolution CT (HRCT) images were obtained without intravenous contrast and with the patient in the supine position at full inspiration. HRCT images with 1-mm-thick slices at 1-mm intervals were used for the analyses.
Derivation of the ESMCSA using an imaging analysis software program
The SYNAPSE VINCENT (Fujifilm Medical Systems, Tokyo, Japan) CT imaging analysis software program was used to derive the ESMCSA, which was calculated manually according to previously published methods (17,18). In brief, ESMCSA was measured on a single-slice axial CT image at the level of the spinous process of the 12th thoracic vertebra. For the quantitative analysis of the ESMs, chest HRCT images were reconstructed using mediastinal window settings (window level, 40 HU; window width, 300 HU). The left and right ESMs were identified and manually shaded, and the ESM area is reported as the sum of the right and left ESMs.
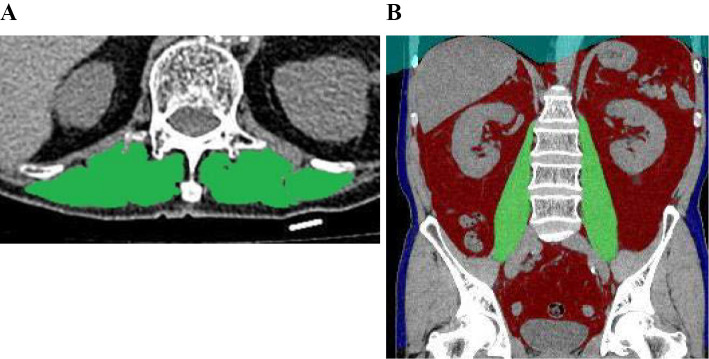
All CT analyses were independently performed by trained individuals (KF and KI) blinded to the patients' clinical information. The average ESMCSA values obtained by KF and KI were analyzed. Fig. 1A shows ESMCSA images of a patient with IPF.
Figure 1.
Cross-sectional area of the erector spinae muscles and psoas major muscle volume. The cross-sectional areas of the erector spinae muscles are shown in green (A). The automatically extracted psoas major muscles are shown in green (B).
Derivation of the PMV using an imaging analysis software program
The SYNAPSE VINCENT (Fujifilm Medical Systems) CT imaging analysis software program was also used to derive and automatically compute the PMV for all patients. Fig. 1B shows automatically extracted psoas major muscle images of a patient with IPF. The PMV index was defined as the PMV divided by the cube of the body height. The PMCSA at the 3rd lumbar vertebra level was also computed using the above CT imaging analysis software program.
Pulmonary function tests and the 6MWT
All patients completed pulmonary function tests using spirometry (CHESTAC-8900; CHEST, Tokyo, Japan) according to the American Thoracic Society/European Respiratory Society criteria (26). The diffusion capacity of carbon monoxide (DLCO) was also measured (CHESTAC-8900). In addition, the percentage of predicted forced vital capacity (%FVC), percentage of predicted forced expiratory volume in 1.0 s (FEV1), and percentage of predicted DLCO (%DLCO) were calculated based on the patient height, age, and sex, according to Japanese standardized methods (27). The 6MWT was performed according to the American Thoracic Society guidelines (28). Each patient walked around a flat straight course of 30 m. They were instructed to walk the longest distance possible in 6 min and to rest as often as 6 min during the test. The examiner did not accompany the patient while walking, and the voice conveyed fixed content every minute. In the 6MWT, oxygen therapy and walking aids were not used. The heart rate, dyspnea, fatigue, and percutaneous arterial oxygen saturation were measured before and after the test. The 6MWT recording was supported by pulse oximetry using a dedicated software program (Anypal walk, ATP-W03; Fukuda Denshi, Tokyo, Japan).
Daily step count
The daily step count was assessed using a tri-axis accelerometer (FB-732-BK; Tanita, Tokyo, Japan). This device is water-resistant, lightweight (30 g), and small (74×165×20 mm3) and has a data storage capacity of 14 days. Participants were instructed to wear the device in a pocket of a shirt or pants or to hang it around their neck with a strap continuously for seven consecutive days, except while bathing and sleeping. The median value of the daily step counts for seven consecutive days for each patient was used for the analyses.
Psychological evaluations
Psychological evaluations were performed using the Hospital Anxiety and Depression Scales (HADS) (29). The HADS comprises 14 items, including 7 items each for the anxiety and depression subscales. Each item is rated on a scale of 0-3. Accordingly, the total subscale score ranged from 0 (no distress) to 21 (maximum distress), with higher scores indicating more severe distress.
The sarcopenia diagnosis
Sarcopenia was defined based on the algorithm and criteria of the Asian Working Group for Sarcopenia 2019 (20). Accordingly, sarcopenia was diagnosed if the patient had a low muscle mass, muscle strength, and/or physical performance; the presence of all three indicated severe sarcopenia. The appendicular skeletal mass index (height squared-adjusted, kg/m2) was calculated using a multifrequency bioelectrical impedance analyzer (InBody 720; InBody Japan, Tokyo, Japan). The cutoff criteria for a low muscle mass were <7.0 kg/m2 for men and <5.7 kg/m2 for women. Handgrip strength measurement is the recommended method for detecting low muscle strength. Handgrip strength was measured in the standing position with full elbow extension using an electronic dynamometer (HG-251; N-Force, CORVETTE, Higashimuro, Japan). Measurements were performed twice for each hand, and the largest grip strength value was used for the analysis. The cut-off criteria for low muscle strength were defined as <28.0 kg for men and <18.0 kg for women. Physical performance was evaluated using the usual gait speed, which was calculated by measuring the time taken to walk down a 10-m corridor at the usual speed. The cut-off criterion for low physical performance was defined as <1.0 m/s for both sexes.
Gender, age, and physiology (GAP) staging
IPF staging was conducted according to the GAP staging system (30).
Statistical analyses
Continuous variables were tested for normality using the Shapiro-Wilk test, with normally and non-normally distributed values presented as mean±standard deviation and median (interquartile range), respectively. The correlation and agreement of the ESMCSA between the two trained individuals were analyzed using Pearson's correlation coefficient test and a Bland-Altman analysis, respectively. Univariate linear regression analyses were performed to investigate the predictive utility of the ESMCSA, PMV, and clinical parameters for the daily step count. A multiple stepwise linear regression analysis was also conducted. The relationship between the daily step count and ESMCSA, PMV, skeletal muscle index, %FVC, and %DLCO was evaluated using Spearman's rank correlation coefficients. Differences between patients with and without sarcopenia were analyzed using the Mann-Whitney U test or Student's t-test, as appropriate.
Statistical significance was set at p<0.05. We included factors with p<0.05 in the univariate analysis for the multiple stepwise analyses. All statistical analyses were performed using the SPSS software program, version 28 (IBM, Armonk, USA).
Results
Patient characteristics
Among the outpatients screened at our hospital, 76 patients with stable IPF were identified. Among them, we excluded four patients who underwent long-term oxygen therapy at rest, one who was unable to undergo the 6MWT because of unstable angina, three who were unable to perform the 6MWT because they typically used wheelchairs, five who declined to participate in this study, and four who did not submit records of daily step counts. Ultimately, 59 outpatients with IPF (mean age 73.3±8.1 years old, mean %FVC 81.6±15.8%) were enrolled in the study.
Patient characteristics are presented in Table 1. The median daily step count was 4,258 (2,155-6,991) steps. The mean 6MWD was 413±97 m. The mean ESMCSA and PMV were 25.5±6.7 cm2, and 270±75.6 cm3, respectively. The correlation of the ESMCSA values between trained individuals was r=0.985 (p<0.001). The Bland-Altman analysis revealed that the agreement between the two individuals was sufficient.
Table 1.
Characteristics of Patients with IPF Included in the Study (n=59).
| Age, years | 73.3±8.1 |
| Sex, female, n (%) | 6 (10.2%) |
| Body mass index, kg/m2 | 22.6±3.3 |
| Histological diagnosis, n (%) | 19 (32.2%) |
| GAP stage (I/II/III), n (%) | 35 (59%)/23 (39%)/1 (2%) |
| FVC, % predicted | 81.6±15.8 |
| FEV1, % predicted | 83.4±14.7 |
| FEV1/FVC, % | 81.9±7.81 |
| DLCO, % predicted | 66.6±19.7 |
| Distance walked during 6MWT, m | 413±97 |
| Lowest SpO2 during 6MWT, % | 89 [85-92] |
| ESMCSA, cm2 | 25.5±6.7 |
| PMCSA, cm2 | 13.3 [9.9-15.3] |
| PMV, cm3 | 270.2±9.8 |
| PMV index, cm3/m3 | 62.1±1.9 |
| Skeletal muscle index, kg/m2 | 6.8±0.9 |
| Handgrip strength, kg | 32.0±9.8 |
| Usual gait speed, m/s | 1.1±0.3 |
| Daily step count | 4,258 [2,155-6,991] |
| HADS anxiety score | 3.5 [1-7] |
| HADS depression score | 6 [3-8.25] |
| Sarcopenia, n (%) | 22 (31.9%) |
| Severe sarcopenia, n (%) | 8 (13.6%) |
GAP: gender, age, and physiology, FVC: forced vital capacity, FEV1: forced expiratory volume in 1.0 s, DLCO: diffusion capacity of the lung for carbon monoxide, 6MWT: 6-minute walk test, SpO2: oxygen saturation by pulse oximetry, ESMCSA: cross-sectional area of erector spinae muscles, PMV: psoas major muscle volume, HADS: Hospital Anxiety and Depression Scale
Data are presented as the mean (±standard deviation), median [interquartile range], or number (%)
Linear regression analyses of the daily step count
Table 2 presents the results of the linear regression analyses of daily step counts. Univariate linear regression analyses showed that the body mass index (standardized β 0.27, p=0.048), 6MWD (standardized β 0.35, p=0.009), lowest oxygen saturation during the 6MWT (standardized β 2.08, p=0.042), and ESMCSA (standardized β 0.36, p=0.007) were significant factors for the daily step count, whereas the PMV, skeletal muscle index, %FVC, and %DLCO were not. A multiple stepwise linear regression analysis showed that the 6MWD (standardized β 0.35, p=0.010) and ESMCSA (standardized β 0.38, p=0.009) were independent factors influencing the daily step count.
Table 2.
Linear Regression Analyses of the Daily Step Count.
| B | 95%CI | Standardized β | p value | |
|---|---|---|---|---|
| Univariate | ||||
| Age, years | -87.2 | -218.7 to 44.2 | -0.18 | 0.189 |
| Body mass index, kg/m2 | 325.6 | 2.6 to 648.5 | 0.27 | 0,048 |
| FVC, percent predicted, % | 38.1 | -29.4 to 105.6 | 0.15 | 0.263 |
| DLCO, percent predicted, % | 42.4 | -13.3 to 97.8 | 0.20 | 0.133 |
| 6MWD, m | 14.5 | 3.9 to 25.1 | 0.35 | 0.009 |
| 6MWT, lowest SpO2, % | 173.6 | 6.3 to 340.8 | 0.28 | 0.042 |
| ESMCSA, cm2 | 2.1 | 0.6 to 3.7 | 0.36 | 0.007 |
| PMCSA, cm2 | 126.9 | -153.1 to 407.0 | 0.12 | 0.368 |
| PMV, cm3 | 8.4 | -5.7 to 22.4 | 0.16 | 0.236 |
| PMV index, cm3/m3 | 32.2 | -40.2 to 104.7 | 0.12 | 0.376 |
| Skeletal muscle index, kg/m2 | 866 | -257 to 1,990 | 0.26 | 0.128 |
| Handgrip strength, kg | 60.1 | -48.7 to 168.8 | 0.15 | 0.273 |
| Usual gait speed, m/s | 2,772 | -1,422 to 6,967 | 0.17 | 0.191 |
| HADS anxiety score | 93.8 | -194 to 382 | 0.09 | 0.517 |
| HADS depression score | -52.9 | -362 to 257 | -0.05 | 0.734 |
| Multiple | ||||
| 6MWD, m | 14.5 | 3.8 to 25.1 | 0.35 | 0.010 |
| ESMCSA, cm2 | 2.2 | 0.6 to 3.8 | 0.38 | 0.009 |
CI: confidence interval, FVC: forced vital capacity, DLCO: diffusion capacity of the lung for carbon monoxide, 6MWD: 6-minute walk distance, 6MWT: 6-minute walk test, SpO2: oxygen saturation by pulse oximetry, ESMCSA: cross-sectional area of erector spinae muscles, PMCSA: cross-sectional area of psoas major muscles, PMV: psoas major muscle volume, HADS: Hospital Anxiety and Depression Scale
Correlations of the daily step count with clinical parameters
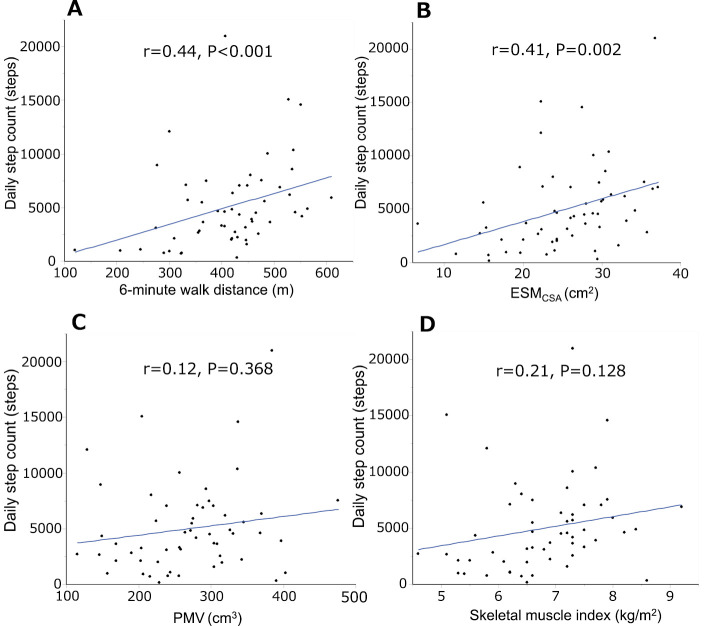
Fig. 2 shows the correlations between the daily step count and the 6MWD, ESMCSA, PMV, and skeletal muscle index. The daily step count was significantly correlated with the 6MWD (r=0.44, p<0.001) (Fig. 2A) and ESMCSA (r=0.41, p=0.002) (Fig. 2B) but not with the PMV (r=0.12, p=0.368) or skeletal muscle index (r=0.21, p=0.128). The daily step count was weakly correlated with the %FVC (r=0.27, p=0.042) and %DLCO (r=0.31, p=0.017).
Figure 2.
Correlations of the 6-minute walk distance and ESMCSA with the daily step count. The daily step counts were significantly correlated with the 6-minute walk distance (r=0.44, p<0.001) (A) and ESMCSA (r=0.41, p=0.002) (B) but not with the PMV (r=0.12, p=0.368) (C) or skeletal muscle index (r=0.21, p=0.128) (D). ESMCSA: cross-sectional area of the erector spinae muscles at the level of the 12th thoracic vertebra, PMV: psoas major muscle volume
Comparisons of the daily step count, ESMCSA, and 6MWD between patients with and without sarcopenia
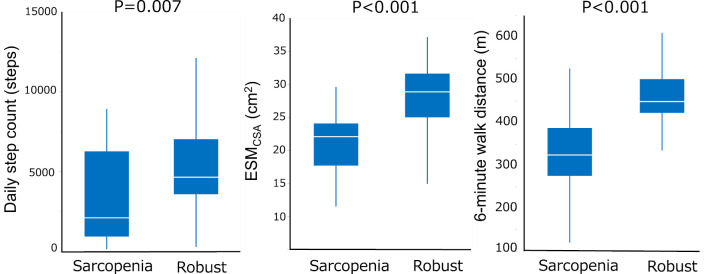
Comparisons of the daily step count, ESMCSA, and 6MWD between patients with sarcopenia and robust IPF are shown in Fig. 3. The daily step count, ESMCSA, and 6MWD were lower in patients with sarcopenia than in those without it.
Figure 3.
Comparisons of the daily step count, ESMCSA, and 6MWD between patients with and without sarcopenia. Comparisons of the daily step count, ESMCSA, and 6MWD between patients with sarcopenia and robust patients are shown. The daily step count, ESMCSA, and 6MWD were significantly lower in patients with sarcopenia than in robust patients. 6MWD: 6-minute walk distance, ESMCSA: the cross-sectional area of the erector spinae muscles at the level of the 12th thoracic vertebra
Discussion
A stepwise multiple linear regression analysis indicated that the 6MWD and ESMCSA were independent factors for the daily step count, while the age and body mass index were not. In addition, we observed that many patients with low daily step counts had a small ESMCSA and a short 6MWD. Since the daily step count, 6MWD, and ESMCSA are known prognostic factors for patients with IPF, they may be confoundingly related to mortality.
According to the criteria of the Asian Working Group for Sarcopenia 2019 (20), sarcopenia is diagnosed in patients with a low muscle mass, low muscle strength assessed by handgrip strength, and/or low physical performance assessed by usual gait speed. The muscle mass should be assessed using the appendicular skeletal mass index (height squared-adjusted, kg/m2), which is calculated using dual-energy X-ray absorptiometry or a multifrequency bioelectrical impedance analyzer. However, the diagnosis of sarcopenia is not always easy to make in medical facilities, especially if dual-energy X-ray absorptiometry or a multifrequency bioelectrical impedance analyzer is not available. In the present study, we demonstrated that the daily step count, ESMCSA, and 6MWD were lower in patients with sarcopenia than in those without it. These parameters may help predict sarcopenia in patients with IPF.
Numerous studies have reported that measuring the size of the psoas major muscle is useful for evaluating skeletal mass loss in various diseases (10-14). However, we found that the ESMCSA, but not the PMV, was associated with the daily step count. Although the reason for this finding in patients with IPF is unknown, it could be because the size of the psoas muscle is related to hard exercise, such as running (31) and sexual activity (32). It is also unknown whether or not there is a correlation between the daily step count and PMV in healthy individuals. Considering the radiation exposure of patients with IPF, where chest CT examinations are performed at the time of the diagnosis and at follow-up evaluations, it is not significant to assess the PMV in IPF patients.
The daily step count was weakly correlated with the %FVC (r=0.27, p=0.042) and %DLCO (r=0.31, p=0.017). In patients with COPD (33), the daily step count was strongly correlated with the %FVC (r=0.54, p<0.001), %FEV1 (r=0.66, p<0.001), and %DLCO (r=0.51, p<0.001) (33). Why the daily step count was weakly correlated with the pulmonary function in the present study is unclear. However, one possible reason is that the previous study included patients with advanced-stage COPD [Global Initiative for Chronic Obstructive Lung Disease (GOLD) I, 25%; GOLD II, 37%; GOLD III, 22%, GOLD IV, 15%] (33), while few patients with IPF in this study had advanced disease (GAP stage I, 59%; GAP stage II, 39%; GAP stage III, 2%). Second, depression is reported to be associated with the daily step count in moderate-to-severe COPD (34), so differences in the number of patients with depression may explain our findings. The HADS depression score was not associated with the daily step count in the present study. Although 6 patients (10.2%) had depression symptoms according to the HADS depression score, no patients with severe depression were included in our study.
The skeletal mass index was calculated using a multifrequency bioelectrical impedance analyzer in the present study. This index is associated with the total skeletal muscle volume of the upper and lower limbs and is an officially recognized parameter for diagnosing sarcopenia (20). However, this index was not a significant factor influencing the daily step count in the present study. Sarcopenia is a condition characterized by a progressive loss of the skeletal muscle mass and function and is correlated with physical disability. Further research may be warranted to clarify which is better, the skeletal mass index or ESMCSA, for evaluating future outcomes in patients with IPF.
Several limitations associated with the present study warrant mention. First, the results were obtained by analyzing only the data of Japanese patients from a single center with a small sample size. In addition, a few patients with IPF in this study had advanced disease (GAP stage III; n=1). Second, this was a cross-sectional study. A longitudinal survey will help clarify the clinical significance of an increase or decrease in the ESMCSA. Third, whether the results of this study are specific to patients with IPF or are applicable to all human beings is unknown. Finally, the ESMCSA was measured manually, while the PMV was computed automatically. The development of a software program that measures the ESMCSA automatically is needed.
Conclusion
The present study showed a strong relationship between a low daily step count, small ESMCSA, and short 6MWD in patients with IPF. These parameters may help identify sarcopenia in patients with IPF. Further studies are warranted to clarify the importance of physical therapy for muscle strengthening to improve these parameters and the survival in patients with IPF.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med 378: 1811-1823, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 205: e18-e47, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furukawa T, Taniguchi H, Ando M, et al. The St. George's Respiratory Questionnaire as a prognostic factor in IPF. Respir Res 18: 18, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paluch AE, Bajpai S, Bassett DR, et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health 7: e219-e228, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishiyama O, Yamazaki R, Sano H, et al. Physical activity in daily life in patients with idiopathic pulmonary fibrosis. Respir Investig 56: 57-63, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Shingai K, Matsuda T, Kondoh Y, et al. Cutoff points for step count to predict 1-year all-cause mortality in patients with idiopathic pulmonary fibrosis. Respiration 100: 1151-1157, 2021. [DOI] [PubMed] [Google Scholar]
- 7.du Bois RM, Albera C, Bradford WZ, et al. 6-minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J 43: 1421-1429, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Moy ML, Danilack VA, Weston NA, Garshick E. Daily step counts in a US cohort with COPD. Respir Med 106: 962-969, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YS, Kim EY, Kang SM, Ahn HK, Kim HS. Single cross-sectional area of pectoralis muscle by computed tomography - correlation with bioelectrical impedance based skeletal muscle mass in healthy subjects. Clin Physiol Funct Imaging 37: 507-511, 2017. [DOI] [PubMed] [Google Scholar]
- 10.Murata Y, Nakamura E, Tsukamoto M, et al. Longitudinal study of risk factors for decreased cross-sectional area of psoas major and paraspinal muscle in 1849 individuals. Sci Rep 11: 16986, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis 17: O20-O26, 2015. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs CR, Scali ST, Filiberto A, et al. Psoas muscle area as a prognostic factor for survival in patients undergoing endovascular aneurysm repair conversion. Ann Vasc Surg 87: 1-12, 2022. [DOI] [PubMed] [Google Scholar]
- 13.Matsui K, Kawakubo H, Hirata Y, et al. Relationship between early postoperative change in total psoas muscle area and long-term prognosis in esophagectomy for patients with esophageal cancer. Ann Surg Oncol 28: 6378-6387, 2021. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto T, Yagyu T, Uchinaka E, et al. The prognostic significance of combined geriatric nutritional risk index and psoas muscle volume in older patients with pancreatic cancer. BMC Cancer 21: 342, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canvasser LD, Mazurek AA, Cron DC, et al. Paraspinous muscle as a predictor of surgical outcome. J Surg Res 192: 76-81, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Yoshimura K, Enomoto Y, et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci Rep 8: 14074, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakano A, Ohkubo H, Taniguchi H, et al. Early decrease in erector spinae muscle area and future risk of mortality in idiopathic pulmonary fibrosis. Sci Rep 10: 2312, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita K, Ohkubo H, Nakano A, et al. Decreased peak expiratory flow rate associated with mortality in idiopathic pulmonary fibrosis: a preliminary report. Chron Respir Dis 19: 14799731221114153, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39: 412-423, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 21: 300-307, 2020. [DOI] [PubMed] [Google Scholar]
- 21.Hanada M, Sakamoto N, Ishimoto H, et al. A comparative study of the sarcopenia screening in older patients with interstitial lung disease. BMC Pulm Med 22: 45, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faverio P, Fumagalli A, Conti S, et al. Sarcopenia in idiopathic pulmonary fibrosis: a prospective study exploring prevalence, associated factors and diagnostic approach. Respir Res 23: 228, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita K, Ohkubo H, Nakano A, et al. Frequency and impact on clinical outcomes of sarcopenia in patients with idiopathic pulmonary fibrosis. Chron Respir Dis 19: 14799731221117298, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhillon RJ, Hasni S. Pathogenesis and management of sarcopenia. Clin Geriatr Med 33: 17-26, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 198: e44-e68, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax 61: 744-746, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kubota M, Kobayashi H, Quanjer PH, Omori H, Tatsumi K, Kanazawa M. Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig 52: 242-250, 2014. [DOI] [PubMed] [Google Scholar]
- 28.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 193: 1185, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Matsudaira T, Igarashi H, Kikuchi H, et al. Factor structure of the Hospital Anxiety and Depression Scale in Japanese psychiatric outpatient and student populations. Health Qual Life Outcomes 7: 42, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Ann Intern Med 156: 684-691, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Tottori N, Kurihara T, Otsuka M, Isaka T. Relationship between lateral differences in the cross-sectional area of the psoas muscle and curve running time. J Physiol Anthropol 35: 3, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kashiwagi E, Imada K, Monji K, et al. Psoas muscle volume is correlated with sexual activity and erectile dysfunction among patients with localised prostate cancer. Andrologia 51: e13354, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida M, Hiramoto T, Moriwaki A, et al. Impact of extrapulmonary comorbidities on physical activity in chronic obstructive pulmonary disease in Japan: a cross-sectional study. PLoS One 17: e0270836, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bamonti PM, Perndorfer C, Robinson SA, Mongiardo MA, Wan ES, Moy ML. Depression symptoms and physical activity in veterans with COPD: insights from a web-based, pedometer-mediated physical activity intervention. Ann Behav Med 57: 855-865, 2023. [DOI] [PubMed] [Google Scholar]