Mutations in the epidermal growth factor receptor (EGFR) are major genetic alterations in patients with advanced non-small cell lung cancer (NSCLC). Two decades ago, EGFR-tyrosine kinase inhibitors (TKIs) as first-generation (1G) agents such as gefitinib or erlotinib were administered to patients with pulmonary adenocarcinoma harboring activating EGFR mutation (1). Clinical studies have demonstrated the clinical benefits of second-generation (2G) (afatinib or dacomitinib) and third-generation (3G) (osimertinib) generation EGFR-TKIs (2-4). Based on the results of a phase 3 study comparing osimertinib with gefitinib (4), osimertinib is recommended as the standard of care in the first-line setting for NSCLC patients with EGFR mutations. However, most patients who receive osimertinib experience acquired resistance, such as on-target EGFR mutations (C797S and G724S), mesenchymal-epithelial transition (MET) amplification, or bypass mechanisms, and EGFR-TKIs are not effective after the recurrence by acquired resistance (5-7). Currently, the mechanism of resistance to EGFR-TKIs remains incompletely elucidated. Overcoming primary and acquired resistance to EGFR-TKIs is warranted.
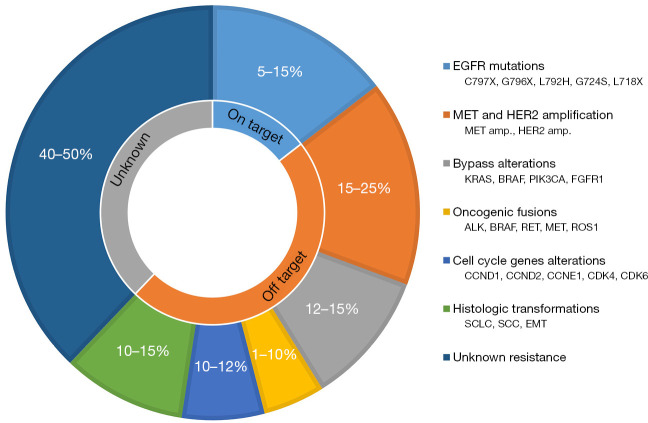
Acquired resistance to osimertinib remains a critical unresolved issue; thus, elucidating the underlying mechanisms of resistance is crucial. Acquired resistance can be divided into two categories: target-dependent (on-target) and target-independent (off-target) (8). Figure 1 illustrates the mechanisms underlying the resistance to first-line osimertinib treatment. Target-dependent resistance to osimertinib in the initial treatment might be less common than that in later-line treatments, and EGFRC797X was identified in only 7% of the patients in the analysis of resistance mechanisms within the FLAURA study (4). In later-line osimertinib, EGFRC797S was identified in approximately 10–20% of patients with disease progression, and rare EGFR mutations, such as G796X, L792X, G724S, and L718X, were identified (8).
Figure 1.
Mechanisms of resistance to first-line osimertinib treatment. Prevalence of individual resistance mechanisms categorized by target, off-target, and unknown reasons. EGFR, epidermal growth factor receptor; MET, mesenchymal-epithelial transition; HER2, human epidermal growth factor receptor 2; amp, amplification; KRAS, Kirsten rat sarcoma viral oncogene homolog; BRAF, v-raf murine sarcoma viral oncogene homologue B; PIK3CA, PI3K P110α catalytic subunit; FGFR1, fibroblast growth factor receptor 1; ALK, anaplastic lymphoma kinase; RET, rearranged during transfection; ROS1, proto-oncogene 1; CCND, cyclin D; CCNE1; cyclin E1; CDK, cyclin-dependent kinase; SCLC, small cell lung cancer; SCC, squamous cell carcinoma; EMT, epithelial-to-mesenchymal transition.
The resistance mechanism resulting from the activation of bypass signaling pathways requires the dual inhibition of both driver alterations to overcome resistance. Table 1 displays the prevalence of resistance mechanisms that activate the bypass signaling pathway after first-line osimertinib treatment in patients with EGFR-mutant NSCLC (9,10). MET amplification as major resistance mechanisms involving bypass signaling after first-line osimertinib administration was identified in 7–15% (9,10). Other resistance mechanisms include 3.7% of cases rearrangement of the rearranged during transfection (RET) rearrangements, 3.0% of anaplastic lymphoma kinase (ALK) rearrangements, 3.7% of v-raf murine sarcoma viral oncogene homologue B (BRAF) rearrangements, 2.0% of human epidermal growth factor receptor 2 (HER2) amplifications, 2–7% of Kirsten rat sarcoma viral oncogene homolog (KRAS) mutations, and 4.0% of PI3K P110α catalytic subunit (PIK3CA) mutations (9,10). Further resistance mechanisms encompass histological transformation from NSCLC to small cell lung cancer (SCLC), and squamous cell carcinoma identified in 2–15% of patients with disease progression after osimertinib treatment (11). Inactivation of RB1 and TP53 is associated with an increased risk of SCLC (11). Moreover, alterations in cell cycle genes, such as cyclin D1, D2, and E1, are observed in approximately 10% of patients who experience disease progression after osimertinib treatment, and are associated with shorter survival (11). Epithelial-to-mesenchymal transition (EMT) has also been detected in patients with EGFR-mutant NSCLC, implicating it in therapeutic resistance to osimertinib (12). However, the mechanism of resistance is unknown in approximately 40–50% of patients with first-line osimertinib resistance (11).
Table 1. Bypass resistance pathways after first-line osimertinib in EGFR-mutant NSCLC.
| Bypass mechanism | Prevalence (%) | References |
|---|---|---|
| MET amplifications | 7–15 | (9,10) |
| RET rearrangements | 3.7 | (9) |
| ALK rearrangements | 3.0 | (9) |
| BRAF rearrangements | 3.7 | (9) |
| HER2 amplifications | 2.0 | (10) |
| KRAS mutations | 2–7 | (9,10) |
| PIK3CA mutations | 4.0 | (9) |
EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; MET, mesenchymal-epithelial transition; RET, rearranged during transfection; ALK, anaplastic lymphoma kinase; BRAF, v-raf murine sarcoma viral oncogene homologue B; HER2, human epidermal growth factor receptor 2; KRAS, Kirsten rat sarcoma viral oncogene homolog; PIK3CA, PI3K P110α catalytic subunit.
2G EGFR-TKIs, such as dacomitinib and afatinib, are pan-human EGFR (HER) inhibitors with superior efficacy compared with 1G EGFR-TKIs for the initial treatment of EGFR-mutant NSCLC (2,3). Recently, Elkrief et al. reported a phase 1/2 study of osimertinib and dacomitinib to mitigate primary and acquired resistance in EGFR-mutant lung adenocarcinoma, and this trial included the patients with EGFR-mutant NSCLC progressing on osimertinib due to acquired secondary EGFR mutations (7). Although the overall response rate (ORR) to this combination was 73%, the ORR for the acquired resistance setting was 14%, and cutaneous toxicities occurred in 93% of patients with greater toxicities (7). Therefore, dacomitinib, with or without osimertinib, could not successfully overcome acquired resistance after first-line osimertinib treatment. Another study showed that 1G or 2G EGFR-TKIs following the development of osimertinib resistance yielded limited efficacy with an ORR of 6.9% (6).
Tumors resistant to TKIs are biologically complex with multiple mechanisms of resistances. An understanding of the mechanisms of resistance to osimertinib requires both pre-clinical and clinical studies; however, no established treatment for disease progression after first-line osimertinib treatment has been approved. Physicians select targeted therapy, platinum-based chemotherapy, and immune checkpoint inhibitors (ICIs) based on known resistance mechanisms. Investigated targeted therapies after osimertinib include 1G or 2G EGFR-TKI, 1G plus 3G EGFR-TKI, brigatinib plus cetuximab (8), and next-generation EGFR-TKIs to overcome on-target resistance mechanisms. Expected therapies focusing on off-target mechanisms include osimertinib plus MET inhibitors and osimertinib combined with other TKIs or inhibitors of oncogenic fusions (8). When chemotherapy is deemed appropriate, platinum-based chemotherapy with or without bevacizumab and osimertinib may be preferred. Similar to other regimens, ICIs, anti-vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor 2 (VEGFR2) antibodies, anti-EGFR antibodies, and antibody-drug conjugates (ADCs) were administered after failure of osimertinib (8,11). Recently, a phase III FLAURA 2 study comparing the efficacy of first-line osimertinib with or without platinum-pemetrexed chemotherapy reported that osimertinib chemotherapy resulted in significantly longer progression-free survival (PFS) than osimertinib monotherapy in 557 patients with EGFR-mutated advanced NSCLC (13). Moreover, the RELAY phase III trial demonstrated that PFS significantly improved with erlotinib plus ramucirumab as an anti-VEGFR2 antibody compared with erlotinib plus placebo (median, 19.4 vs. 12.4 months), regardless of the EGFR mutation status (14). However, the combination with osimertinib and bevacizumab yielded a controversial result in recent studies (15,16). A phase 1/2 study by Yu et al. met the primary endpoint with a 12-month PFS rate of 76% (15), whereas the randomized phase 2 WJOG9717L trial failed to demonstrate improved PFS with osimertinib plus bevacizumab over osimertinib alone (median, 22.1 vs. 20.1 months) (16). To overcome acquired resistance to osimertinib, a combination of chemotherapies may be effective, but not by combining osimertinib with a VEGF inhibitor.
Sequential therapeutic agents for treating osimertinib resistance have been developed in clinical studies. Recently, promising novel agents include a HER3-directed ADC [patritumab deruxtecan (HER3-DXd)] and an EGFR-MET bispecific antibody (amivantamab) in patients with osimertinib-resistance (17). ADCs bind a cytotoxic payload through a linker to a specific monoclonal antibody against a tumor antigen. ADCs targeting HER2, HER3, Trophoblast antigen 2 (TROP-2) and MET have potential applications in lung cancer treatment (18).
HER3-DXd is a promising ADC comprising a fully human monoclonal antibody to HER3 conjugated to a topoisomerase I inhibitor. The phase II HERTHENA-Lung01 trial for HER3-DXd was recently conducted in patients with EGFR-mutated NSCLC following the failure of EGFR-TKIs, including osimertinib (19). The ORR, PFS, and overall survival (OS) for the HER3-DXd group were 39.8%, 5.5 and 11.9 months, respectively (19). Currently, a phase III study comparing HER3-DXd vs. platinum-based chemotherapy is ongoing in patients with EGFR-mutated NSCLC after the failure of EGFR-TKI treatment. Furthermore, trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan (T-DXd) as HER2-directed ADCs were investigated for the patients with EGFR-mutated NSCLC (20,21). The phase II TRAEMOS trial examined the clinical efficacy of T-DM1 plus osimertinib in patients with EGFR-mutated NSCLC after progression on EGFR-TKIs, including osimertinib; however, the ORR and median PFS were 13% and 2.8 months, respectively, showing disappointing results (20). Conversely, T-DXd was reported as a promising treatment for patients with previously treated HER2 positive (overexpression or mutations) NSCLC (without patients with EGFR-mutation), exhibiting ORR, median PFS, and OS of 55%, 8.2 and 17.8 months, respectively (21). Ongoing research is examining the use of T-DXd in early studies for HER2-positive patients, including those with EGFR-mutated NSCLC, following the unsuccessful treatment with EGFR-TKIs. Although initial data on ADCs in EGFR-mutated NSCLC after osimertinib resistance has been reported, exploring the potential of HER3-DXd may hold promise.
MET amplification is a significant mechanism causing resistance to osimertinib. A therapeutic strategy targeting MET gene alterations has been developed in clinical studies to overcome osimertinib resistance. Amivantamab, a fully humanized bispecific antibody, is designed to target both EGFR and MET mutations/amplification. Inhibiting ligand binding for both receptors. Recently, the phase III MARIPOSA-2 study compared amivantamab plus lazertinib with chemotherapy to amivantamab plus chemotherapy in 657 patients with EGFR-mutated advanced NSCLC after disease progression with osimertinib (22). This study demonstrated significant improvements in PFS, ORR, duration of response, and intracranial PFS with amivantamab plus chemotherapy and amivantamab plus lazertinib with chemotherapy compared with chemotherapy alone (median PFS, 6.3 and 8.3 vs. 4.2 months, respectively; P<0.001) (22). Although amivantamab plus chemotherapy could be a potential second-line therapy after osimertinib disease progression, its survival benefit appears limited, while the toxicity of this combination was a critical issue with more than 70% grade 3 adverse events in the MARIPOSA-2 study. The prospect of long-term survival remains unclear, highlighting the need for an established biomarker to predict responses to amivantamab-containing regimens for the most promising approaches after osimertinib disease progression.
Currently, many physicians resort to platinum-based chemotherapy as a second-line option for disease progression after osimertinib. However, the therapeutic efficacy of platinum-based chemotherapy is limited, leading to recurrence in most patients with EGFR-mutated NSCLC within 6 months of systemic chemotherapy.
However, the use of immunotherapy as a potential treatment for patients with EGFR-mutated NSCLC remains controversial. Therefore, researchers explored combination therapies involving ICIs with chemotherapy and anti-VEGF antibodies, specifically analyzing data from IMPower 150. The combination of atezolizumab, bevacizumab, carboplatin, and paclitaxel (ABCP) yielded a higher ORR (71% vs. 42%), duration of response (11.1 vs. 4.7 months), and median OS (not reached vs. 17.5 months) than the combination without atezolizumab [bevacizumab, carboplatin, and paclitaxel (BCP)] in patients with EGFR-mutated NSCLC who had experienced failure with at least one prior EGFR-TKI (23). As opposed to BCP, the ABCP combination improved OS in patients with sensitizing EGFR mutations that have progressed on TKI treatment (24). However, the question of whether the combination of ICI plus chemotherapy and antiangiogenic agents can overcome acquired resistance to osimertinib remains unanswered.
Looking towards novel therapies, the development of ADCs and bispecific antibodies is underway, holding promise as effective regimens for osimertinib-resistant EGFR-mutated patients. The discovery of activating EGFR mutations marked a paradigm shift in the therapeutic landscape for advanced or metastatic NSCLC. Despite this breakthrough, the optimal regimens for managing disease progression following the failure of first-line osimertinib therapy remain uncertain. To address acquired resistance to osimertinib, a combination of chemotherapy with osimertinib, HER3-DXd, amivantamab plus chemotherapy, or new-generation EGFR-TKIs is highly anticipated. Moreover, the identification of predictive markers for these novel regimens is vital for enhancing outcomes. A more profound comprehension of the mechanisms underlying resistance to osimertinib will significantly influence the advancement of clinical studies, the creation of innovative therapeutic agents, and the optimization of therapeutic sequencing.
Supplementary
The article’s supplementary files as
Acknowledgments
The authors thank Editage (www.editage.jp) for English language editing.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-193/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-193/coif). The authors have no conflicts of interest to declare.
References
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. 10.1056/NEJMoa040938 [DOI] [PubMed] [Google Scholar]
- 2.Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. 10.1016/S1470-2045(17)30608-3 [DOI] [PubMed] [Google Scholar]
- 3.Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. 10.1016/S1470-2045(16)30033-X [DOI] [PubMed] [Google Scholar]
- 4.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. 10.1056/NEJMoa1913662 [DOI] [PubMed] [Google Scholar]
- 5.Yu HA, Tian SK, Drilon AE, et al. Acquired Resistance of EGFR-Mutant Lung Cancer to a T790M-Specific EGFR Inhibitor: Emergence of a Third Mutation (C797S) in the EGFR Tyrosine Kinase Domain. JAMA Oncol 2015;1:982-4. 10.1001/jamaoncol.2015.1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morimoto K, Yamada T, Takeda T, et al. Clinical Efficacy and Safety of First- or Second-Generation EGFR-TKIs after Osimertinib Resistance for EGFR Mutated Lung Cancer: A Prospective Exploratory Study. Target Oncol 2023;18:657-65. 10.1007/s11523-023-00991-5 [DOI] [PubMed] [Google Scholar]
- 7.Elkrief A, Makhnin A, Moses KA, et al. Brief Report: Combination of Osimertinib and Dacomitinib to Mitigate Primary and Acquired Resistance in EGFR-Mutant Lung Adenocarcinomas. Clin Cancer Res 2023;29:1423-8. 10.1158/1078-0432.CCR-22-3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper AJ, Sequist LV, Lin JJ. Third-generation EGFR and ALK inhibitors: mechanisms of resistance and management. Nat Rev Clin Oncol 2022;19:499-514. 10.1038/s41571-022-00639-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoenfeld AJ, Chan JM, Kubota D, et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin Cancer Res 2020;26:2654-63. 10.1158/1078-0432.CCR-19-3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol 2018;29:viii740. 10.1093/annonc/mdy424.063 [DOI] [Google Scholar]
- 11.Fu K, Xie F, Wang F, et al. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J Hematol Oncol 2022;15:173. 10.1186/s13045-022-01391-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng CH, Chen LY, Lin YC, et al. Epithelial-mesenchymal transition (EMT) beyond EGFR mutations per se is a common mechanism for acquired resistance to EGFR TKI. Oncogene 2019;38:455-68. 10.1038/s41388-018-0454-2 [DOI] [PubMed] [Google Scholar]
- 13.Planchard D, Jänne PA, Cheng Y, et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med 2023;389:1935-48. 10.1056/NEJMoa2306434 [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:1655-69. 10.1016/S1470-2045(19)30634-5 [DOI] [PubMed] [Google Scholar]
- 15.Yu HA, Schoenfeld AJ, Makhnin A, et al. Effect of Osimertinib and Bevacizumab on Progression-Free Survival for Patients With Metastatic EGFR-Mutant Lung Cancers: A Phase 1/2 Single-Group Open-Label Trial. JAMA Oncol 2020;6:1048-54. 10.1001/jamaoncol.2020.1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenmotsu H, Wakuda K, Mori K, et al. Randomized Phase 2 Study of Osimertinib Plus Bevacizumab Versus Osimertinib for Untreated Patients With Nonsquamous NSCLC Harboring EGFR Mutations: WJOG9717L Study. J Thorac Oncol 2022;17:1098-108. 10.1016/j.jtho.2022.05.006 [DOI] [PubMed] [Google Scholar]
- 17.Girard N. New Strategies and Novel Combinations in EGFR TKI-Resistant Non-small Cell Lung Cancer. Curr Treat Options Oncol 2022;23:1626-44. 10.1007/s11864-022-01022-7 [DOI] [PubMed] [Google Scholar]
- 18.Araki T, Kanda S, Horinouchi H, et al. Current treatment strategies for EGFR-mutated non-small cell lung cancer: from first line to beyond osimertinib resistance. Jpn J Clin Oncol 2023;53:547-61. 10.1093/jjco/hyad052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu HA, Goto Y, Hayashi H, et al. HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor-Mutated Non-Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy. J Clin Oncol 2023;41:5363-75. 10.1200/JCO.23.01476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotta K, Aoe K, Kozuki T, et al. A Phase II Study of Trastuzumab Emtansine in HER2-Positive Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:273-9. 10.1016/j.jtho.2017.10.032 [DOI] [PubMed] [Google Scholar]
- 21.Li BT, Smit EF, Goto Y, et al. Trastuzumab Deruxtecan in HER2-Mutant Non-Small-Cell Lung Cancer. N Engl J Med 2022;386:241-51. 10.1056/NEJMoa2112431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Passaro A, Wang J, Wang Y, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol 2024;35:77-90. 10.1016/j.annonc.2023.10.117 [DOI] [PubMed] [Google Scholar]
- 23.Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019;7:387-401. 10.1016/S2213-2600(19)30084-0 [DOI] [PubMed] [Google Scholar]
- 24.Nogami N, Barlesi F, Socinski MA, et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups With EGFR Mutations or Metastases in the Liver or Brain. J Thorac Oncol 2022;17:309-23. 10.1016/j.jtho.2021.09.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as