Abstract
Background
Buffalo spermatozoa have a distinct membrane structure that makes them more vulnerable to cryopreservation, resulting in lower-quality post-thawed sperm. This decreases the success rate of artificial insemination in buffaloes. Understanding and addressing these specific vulnerabilities are essential for improving reproductive techniques in buffalo populations. The properties of cryopreserved buffalo bull semen were examined in this study regarding the impact of adding autologous platelet-rich plasma (PRP) to OptiXcell® or Tris egg yolk-based extenders. Ten buffalo bulls were used to collect semen. Each bull’s ejaculate was separated into two main equal amounts, each of which was then diluted with either OptiXcell® or Tris egg yolk-based extender, supplemented with various PRP concentrations (5%, 10%, and 15%), and the control (0%), before being cryopreserved according to established protocols. Following equilibration and thawing, the quality and functionality of the sperm were evaluated, along with the antioxidant enzyme activities (GSH and TAC), malondialdehyde (MDA) content, and in vivo fertilization rate of the thawed semen.
Results
All PRP concentrations in both extenders, particularly 10% PRP, improved the quality and functionality of the sperm in both equilibrated and frozen-thawed semen. Additionally, the antioxidant enzyme activities in both extenders were higher in the PRP-supplemented groups compared to the control group in thawed semen (P < 0.05). All post-thaw sperm quality, antioxidant enzyme activities, and functionality aside from DNA integrity were higher (P < 0.05) in the PRP-supplemented OptiXcell® than in the PRP-supplemented Tris egg yolk-based extender. The fertility of cryopreserved semen in the extenders supplemented with 10% and 15% PRP increased (P < 0.05) significantly more than that of the control extenders, with 10% PRP being the optimum concentration in OptiXcell® (80%) compared to that of Tris egg yolk-based extender (66.67%) and control of two extenders (53.33% and 46.67%, respectively).
Conclusions
Even though autologous PRP-supplemented extenders have a protective impact on equilibrated and cryopreserved semen, 10% PRP-supplemented OptiXcell® extenders are more effective at preserving post-thaw semen quality, functionality, and antioxidant capacity, which increases the in vivo fertility of buffalo bulls.
Keywords: OptiXcell® extender, Tris egg yolk-based extender, Platelet-rich plasma, Buffalo bull semen, In vivo fertilization
Background
In contrast to other species, buffalo spermatozoa have a different membrane structure that renders them more susceptible to cryopreservation and produces lower-quality post-thawed sperm [1], consequently decreasing the success rate of artificial insemination (AI) [2]. Cryo-damage in the form of sperm membrane rupture, nuclear DNA and mitochondrial impairment, and producing reactive oxygen species (ROS) caused by the freezing and thawing processes causes a 50% reduction in sperm motility [3]. Therefore, the biochemical characteristics and quantity of cryoprotectants in semen extenders protect spermatozoa against cryoinjuries [4]. Supplementing semen extenders with autologous platelet-rich plasma (PRP) provides spermatozoa with the requirements they require to recover from cryopreservation damage; this could be due to the effects of PRP ingredients [5]. The platelet-rich plasma contains around 800 protein molecules, chemoattractants, hormones, and cytokines [6]. Recently, PRP has been effectively employed in reproductive biology, and it can be added to both fresh [7] and cryopreserved semen, including bucks [8], rams [9], buffalo bulls [10], and humans [11], with good consequences and enhancements in semen functionality and quality. Moreover, PRP has been injected into the testes of many species, including bucks [12], leading to improved sperm functionality and quality. In men, PRP treats infertility related to non-obstructive azoospermia [13]. It also enhances frozen-thawed sperm properties and testicular function in rabbits [14], improves sperm morphology and quantity in rats, renews rat testicular tissue, and maintains sex hormone balance [15]. PRP improves antioxidant protection, decreases oxidative stress (OS), and defends rat testicular tissues from damage [15]. PRP is rich in cytokines, including platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), transforming growth factors (TGF), insulin-like growth factor I (IGF I), nerve growth factor (NGF), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF), and peptide hormones. PRP also contains ATP, calcium ions, zinc ions, histamine, serotonin, and superoxide dismutase (SOD). These components play a vital role in maintaining tissue and cell homeostasis. In the context of sperm quality and function, PRP’s factors have shown positive effects, such as enhancing sperm motility (TGF and VEGF), supporting sperm capacitation and acrosome reaction (zinc and calcium ions), protecting sperm from cryodamage, and improving post-cryopreservation quality (IGF-1, NGF, ATP, zinc ions, SOD, and platelet-activating factor) [11].
Ingredients of the extender seem to be one of the most crucial elements in the cryopreservation process because they preserve spermatozoa; similarly, egg yolk (20% v/v), which has been extensively employed as an impermeable cryoprotectant for the cryopreservation of spermatozoa in numerous species. Unfortunately, variations in egg yolk composition and the likelihood of microbial contamination affect sperm properties [16]. The preference is moving towards replacing the Tris egg yolk-based extender with one based on phospholipids or liposomes to lessen the harmful effects of egg yolk [17]. OptiXcell®, a commercial extender based on liposomes, has been shown to provide better cryoinjury protection than Tris egg yolk-based cryopreservation of bovine [18], equine [19], buffalo [20], and swine [21] semen.
As a result, we propose adding PRP to OptiXcell® and/or Tris egg yolk-based extenders to act as a cryodamage defense and improve the in vivo fertility of frozen-thawed buffalo bull semen. Furthermore, it is expected that the OptiXcell® extender will be more effective than the Tris egg yolk-based extender in the cryopreservation of buffalo bull semen. Consequently, this study aimed to compare the effects of OptiXcell® and Tris egg yolk-based extenders supplemented with various PRP concentrations on the quality and functionality of frozen-thawed sperm, as well as their antioxidant characteristics and in vivo fertility in buffalo-thawed spermatozoa.
Materials and methods
Ethical approval
The Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine at Kafrelsheikh University, Egypt allowed approval (IACUC#133/2021) for the present study involving the sampling.
Reagents and chemicals
Unless otherwise stated, all compounds utilized in the current study were bought from Sigma Aldrich Co., USA.
Experimental animals
The ethical committee of the Faculty of Veterinary Medicine, Kafrelsheikh University, Egypt, approved all the experimental techniques and procedures. Ten Egyptian buffalo (Bubalus bubalis) bulls (4–6 years old) were used; all were healthy, mature, fertile, and devoid of reproductive disorders and diseases. An artificial vagina obtained twenty-four ejaculates for every bull (two ejaculates per week). The animals (bulls and buffaloes) source as well as, the experiment was conducted at the Riwina Governmental Station, Animal Production Farm, Agricultural Research Center, Kafrelsheikh, Egypt, from September 2022 to November 2022.
Platelet-rich plasma preparation and activation
To prepare the PRP, two centrifugations of blood were employed [22]. Briefly, PRP was obtained from the blood of the same ten buffalo bulls under sterile conditions on the same day as semen collection. Blood samples were collected in sterilized sodium citrate tubes (10 mL, Becton Drive, BD Vacutainer®, Franklin, USA) and then centrifuged for 5 min at 300 g during the initial centrifugation. The recovered plasma was recently re-centrifuged for 17 min at 700 g to separate the PRP. According to Gutiérrez et al. [23], a calcium gluconate (9.3 mg/mL) solution is required for PRP activation.
Extender preparation
A Tris egg yolk-based extender was prepared using the following ingredients: 3.028 g Tris, 20 ml egg yolk, 1.678 g citric acid, 7.0 ml glycerol, 1.0 g glucose, 50,000 IU penicillin, and 25 mg gentamicin. These ingredients were liquefied in 73 mL of double-distilled water to make a final volume of 100 ml. The pH was adjusted to 6.8 [24] using a handheld pH meter (Twin pH, Horiba Ltd.) before adding the PRP. OptiXcell® was manufactured for use according to the manufacturer’s instructions and contains carbohydrates, antioxidants, buffers, phospholipids, glycerol, mineral salts, water, gentamicin, linomycin, specomycin, and tylosin (Ref. 026218, IMV Technologies).
Semen cryopreservation
Ejaculates from bulls with a minimum of 85% normal sperm morphology and 70% or more motility are only used (170 ejaculates). Each bull’s ejaculate was separated into two identical portions, each diluted to 80 × 106 (sperm/mL) using one of two semen extenders (OptiXcell® or Tris egg yolk-based). Each extender was added with several PRP concentrations (0, 5, 10, and 15%). The expanded semen samples were chilled (4 °C) for four hours for equilibration. After that, samples were put in 0.25 mL polyvinyl chloride straws (IMV, L’Aigle, France). The straws were placed in a biofreezer (Mini Digit-cool, ZH 400, IMV Technologies, L’Aigle, France) and frozen at -140 °C before being instantly submerged in liquid nitrogen for storage [25]. The frozen straws were thawed at 37 °C for 30 s for sperm evaluation.
Equilibrated and post-thawed semen evaluation
Sperm motility, viability, and abnormalities
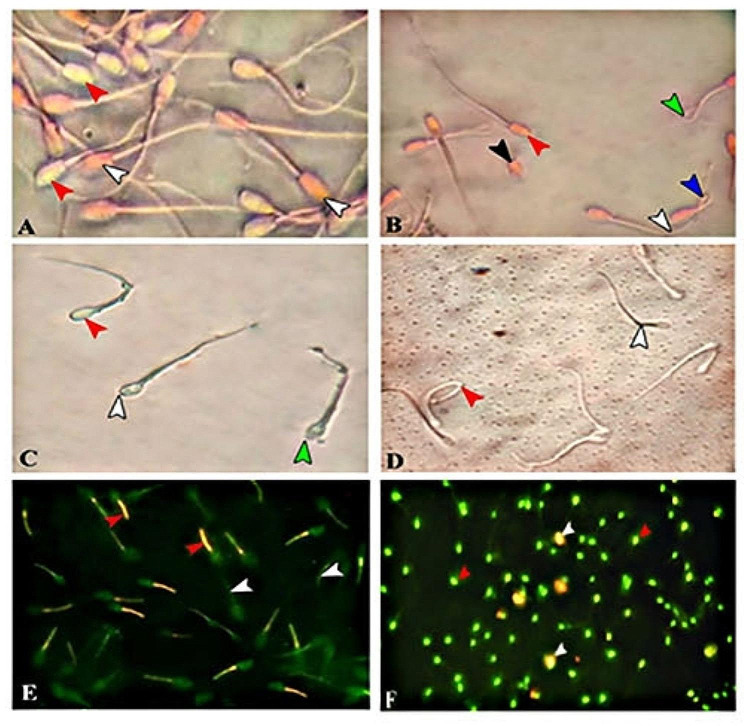
Using a computer-aided sperm motion analyzer system (CASA; Sperm Vision TM software; Minitube) for progressive motility (%) investigations. Five µL of semen were put into a clean, previously warmed-up Makler chamber (37 °C). Eight microscopic areas were chosen at random and examined for each evaluation [1]. Sperm viability and abnormality percentages were assessed by mixing 5 µl of nigrosine (Sigma-Aldrich) and semen and applying it to a glass slide before drying above a Bunsen flame. An Olympus Bx-53 light microscope (Tokyo, Japan) was used to classify sperm cells as live or dead (400x). The heads of live sperm were clear, but those of dead sperm were red (Fig. 1, panel A) [26]. The same slide measuring sperm viability percentage was used to examine sperm abnormalities (Fig. 1, panel B) through an oil immersion lens. At least 200 spermatozoa were counted in five microscopic areas to determine sperm abnormalities and viability.
Fig. 1.
Images illustrating the quality and functionality of sperm. Figure 1. Panel A: Sperm viability: The red arrow indicates live sperm, and the white arrow indicates dead sperm. Panel B: Sperm abnormality: The red arrow indicates normal sperm, the blue arrow indicates a lobed tail, the green arrow indicates a coiled tail, the black arrow indicates a detached head, and the white arrow indicates a bent tail. Panel C: Acrosome integrity: The red arrow indicates an intact acrosome, the white arrow indicates a vacuolated acrosome, and the green arrow indicates a ruptured acrosome. Panel D: Membrane integrity: The red arrow indicates HOST-positive and the white arrow indicates HOST-negative. Panel E: Mitochondrial activity: The red arrow indicates high mitochondrial membrane potential (MMP), and the white arrow indicates low MMP. Panel F: DNA fragmentation: The red arrow indicates intact DNA, and the white arrow indicates a damaged DNA
Acrosome integrity
To test the acrosome’s integrity (%), a formal citrate solution (2.92 g tri-sodium citrate dihydrate and 1 ml formaldehyde (37%) liquefied in 100 ml distilled water) was used [27]. In brief, 500 µL of the equilibrated or post-thawed semen specimen was combined with 50 µL of formal citrate solution. Five ml of the resulting mixture were put on a glass slide, coverslipped, and inspected under an oil immersion, 1000x bright-field microscope (Olympus Optical Co., Ltd., Japan). The proportion of acrosomal abnormalities (Fig. 1. Panel C; ruptured and vacuolated) in 200 spermatozoa was determined in 5 microscopic fields [28].
Membrane integrity
To evaluate the sperm plasma membrane’s functional integrity (%), the hypo-osmotic swelling test (HOST) was applied [29]. In detail, ten µL of semen suspended in 100 µL of 150 mOsm/kg hypo-osmotic solution (0.0735 g sodium citrate and 0.1351 g fructose dissolving in 10 mL Milli-Q water) were incubated in a water bath for 30 to 60 min at 37 °C. Post-incubation, sperm tail coiling or bending was evaluated by placing two µL of a thoroughly mixed sample onto a heated (38 °C) slide, covering it with an already-heated coverslip, and viewing the results using a phase-contrast microscope at 400 × magnifications. It was discovered that sperm cells with coiling and/or expanded tails had functioning plasma membranes (Fig. 1, panel D; HOST-positive).
Mitochondrial activity
The mitochondrial activity of sperm cells was measured using the lipophilic cation JC-10 (JC-10 Assay for Flow Cytometry, Sigma-Aldrich, St. Louis, MO, USA). JC-10 fluorescence reversibly transforms from green (a monomeric state) to orange (a multimeric state) as mitochondrial membrane potential improves. In summary, 1 × 106 sperm/mL of equilibrated or thawed semen were placed into polypropylene tubes. The pellet was immersed in 500 µL of JC-10 and maintained at 37 °C for 1 h. After being washed with 1 mL of PBS and centrifuged for 10 min at 800 g, the samples were centrifuged again and resuspended in 1 mL of PBS. The samples were then subjected to flow cytometry analysis. Frequency plots were used to quantify the orange (JC-10 aggregates) or green (JC-10 monomers) fluorescence of the two populations using emission filters of 595 nm and 535 nm. The green color represented lower mitochondrial membrane potential (MMP), while the orange represented higher MMP (Fig. 1, panel E) [30].
DNA fragmentation
Evaluation of DNA integrity by the acridine orange assay, according to Martins et al. [31]. In brief, on glass slides, semen smears were prepared and then fixed in Carnoy’s solution (glacial acetic acid and methanol in a 1:3 proportion) overnight. The slides were air-dried and then incubated in the tampon solution (15 mmol/L Na2HPO4, pH 2.5, and 80 mmol/L citric acid) for 5 min at 75 °C. Afterward, the slides were stained with a 0.2 mg/mL acridine orange stain. After washing the slides with water, they were covered and assessed with an epifluorescence microscope. From each semen sample, 100 cells were examined. Fluoresced green indicated normal DNA content, but those with aberrant DNA fluoresced various colors, ranging from yellow-green to red (Fig. 1, panel F) [32].
Biochemical parameter measurements of post-thawed semen
Glutathione reductase (GSH)
The GSH concentration of sperm was assessed using the technique developed by Beutler et al. [33]. Briefly, the semen samples were centrifuged at 1,000 g for 5 min at 22 °C after being precipitated with 50% trichloracetic acid (vol/vol). The reaction solution contained 0.25 mL of 5,5′-dithiobis-2-nitrobenzoic acid, 0.5 mL supernatant, and 2.0 mL of phosphate buffer. The solution was incubated at room temperature for 5 min. The GSH activity at 412 nm was measured with a spectrophotometer, and the levels of GSH were given in nmol/l [33].
Total antioxidant capacity (TAC)
According to Kumar et al. [34], the concentration of TAC was determined using a colorimetric assay kit (Catalogue No. K274, BioVision). This assay relied on Trolox as an antioxidant standard. The antioxidants converted Cu2+ ions to Cu+. The reduced Cu+ ions were then chelated with a colorimetric probe, resulting in a broad absorbance peak around 570 nm. This peak was proportional to the total antioxidant capacity and expressed as mmol/ml.
Malondialdehyde (MDA) concentrations
Malondialdehyde (MDA) concentrations in the semen samples were determined using the thiobarbituric acid reaction [35]. Briefly, a 500 µL aliquot of sperm was centrifuged for ten min at 800 g. The pellets were then collected, reconstituted in PBS, and re-centrifuged thrice. Finally, 1 mL of deionized water was added to the sperm, which was then snap-frozen and kept at -70 °C until analysis. The samples were thawed before the lipid peroxidation assay. Thiobarbituric acid-reactive substances were measured by comparing the absorption with the standard curve of MDA equivalents produced by the acid-catalyzed hydrolysis of 1, 1, 3, 3- tetramethoxypropane. MDA was represented as nmol/ml.
In vivo fertilization test
A total of 120 healthy cyclic (15 buffalo/PRP concentration/extender) buffalo were used, and each buffalo received two injections of 20 µg Busereline acetate (Receptal, GnRH agonist, Intervet International, Netherlands) at 9-day intervals, as well as a single injection of 750 pg cloprostenol sodium (Cloprostenol sodium, Estrumate, Coopers Animal Health L.T.D., Berkhamsted, England) on Day 7, and AI was performed on Day 10 for buffaloes [36]. 240 frozen-thawed straws (20 × 106 spermatozoa) from two extenders were used for the insemination process. Each estrous buffalo received two inseminations, one in the morning and one in the evening. Insemination was done in December 2022, and on Day 45, after insemination, the linear probe ultrasonography was used to diagnose pregnancy in all inseminated buffalo.
Statistical analysis
The data were statistically assessed via analysis of variance using SAS’s [37] General Linear Model Procedure. Differences between means were examined using Duncan’s multiple range test [38]. The Chi-square (χ2) test was used to investigate the pregnancy rate. All differences were applied at P < 0.05. OptiXcell® and Tris egg yolk-based extenders and conception rates were compared using a Student t-test.
Results
Effect of adding different concentrations of PRP-supplemented OptiXcell® and Tris egg yolk-based extenders on the quality and functionality of equilibrated semen
PRP-supplemented extenders positively affect sperm quality and functionality, especially when using 10% PRP-supplemented extenders during the equilibration period. There are no significant differences in semen quality and functionality between OptiXcell® and Tris egg yolk-based extenders, except for MMP (%) of 5%, 10%, and 15% PRP-supplemented OptiXcell® (Tables 1 and 2).
Table 1.
Effect of PRP concentrations and extender types on equilibrated sperm quality (mean ± SEM)
| Extender | PRP (%) | N | Progressive motility (%) | Viability (%) | Abnormality (%) |
|---|---|---|---|---|---|
| OptiXcell® A | 0% | 48 | 57.17 ± 1.20b | 79.67 ± 1.56b | 13.83 ± 0.95a |
| 5% | 48 | 60.33 ± 2.57ab | 82.50 ± 1.28ab | 11.83 ± 1.25a | |
| 10% | 48 | 64.33 ± 2.04a | 85.50 ± 1.23a | 7.50 ± 0.99b | |
| 15% | 48 | 62.83 ± 1.74ab | 84.33 ± 1.33a | 8.33 ± 0.96b | |
| Tris egg yolk-based B | 0% | 48 | 54.50 ± 0.76c | 78.17 ± 1.60b | 15.67 ± 1.33a |
| 5% | 48 | 56.67 ± 0.80bc | 79.83 ± 1.25ab | 13.17 ± 0.98ab | |
| 10% | 48 | 61.50 ± 1.88a | 82.83 ± 1.51a | 10.83 ± 1.14b | |
| 15% | 48 | 60.17 ± 1.45ab | 81.77 ± 0.98ab | 10.33 ± 0.56b |
N = 48 ejaculates. Within columns, values with different superscripts differ significantly for each parameter (P < 0.05)
Within the same column, means bearing a, b, and c differ among PRP concentrations (0%, 5%, 10% and 15%) within the same extender (OptiXcell® or Tris egg yolk-based). A and B differ between extender types (OptiXcell® or Tris egg yolk-based), at the same PRP concentrations (0%, 5%, 10% and 15%)
PRP, Platelet-rich plasma
Table 2.
Effect of PRP concentrations and extender types on equilibrated sperm functionality (mean ± SEM)
| Extender | PRP (%) | N | Acrosome integrity (%) | Plasma membrane integrity (%) |
MMP (%) | DNA integrity (%) |
|---|---|---|---|---|---|---|
| OptiXcell® | 0% | 48 | 71.17 ± 1.85b | 57.83 ± 1.30b | 52.33 ± 2.43b | 90.33 ± 1.59b |
| 5% | 48 | 75.00 ± 1.57ab | 61.17 ± 1.74ab | 56.17 ± 1.35abA | 93.33 ± 1.36ab | |
| 10% | 48 | 79.17 ± 2.12a | 67.17 ± 2.18a | 59.50 ± 2.43aA | 95.00 ± 1.21a | |
| 15% | 48 | 77.50 ± 1.52a | 65.5 ± 2.51a | 58.33 ± 1.87abA | 94.67 ± 1.09a | |
| Tris egg yolk-based | 0% | 48 | 68.33 ± 1.38b | 54.17 ± 1.76c | 48.50 ± 1.59b | 89.83 ± 1.42b |
| 5% | 48 | 72.33 ± 1.52a | 56.83 ± 1.45bc | 50.17 ± 0.91abB | 92.17 ± 0.60ab | |
| 10% | 48 | 76.17 ± 1.14a | 62.17 ± 1.89a | 53.00 ± 1.07aB | 94.33 ± 0.62a | |
| 15% | 48 | 74.83 ± 1.17a | 61.00 ± 1.51ab | 51.83 ± 0.79abB | 93.50 ± 0.43a |
N = 48 ejaculates. Within columns, values with different superscripts differ significantly for each parameter (P < 0.05)
Within the same column, means bearing a, b, and c differ among PRP concentrations (0%, 5%, 10% and 15%) within the same extender (OptiXcell® or Tris egg yolk-based). A and B differ between extender types (OptiXcell® or Tris egg yolk-based), at the same PRP concentrations (0%, 5%, 10% and 15%)
PRP, Platelet-rich plasma; MMP, Mitochondrial membrane potential
Post-thawed semen properties of control OptiXcell® and Tris egg yolk-based extenders
As shown in Tables 3, 4 and 5, and 6, except for DNA integrity (%), the control of OptiXcell® extender has better sperm quality, functionality, antioxidant enzyme activity, and lower malondialdehyde levels (P < 0.05) than that of Tris egg yolk-based extender.
Table 3.
Effect of PRP concentrations and extender types on post-thaw sperm quality (mean ± SEM)
| Extender | PRP (%) | N | Progressive motility (%) | Viability (%) | Abnormality (%) |
|---|---|---|---|---|---|
| OptiXcell® A | 0% | 48 | 46.50 ± 0.73c | 75.50 ± 0.62c | 18.67 ± 0.29a |
| 5% | 48 | 49.17 ± 0.60b | 78.67 ± 0.29b | 15.17 ± 0.48b | |
| 10% | 48 | 52.34 ± 0.96a | 80.83 ± 0.75a | 9.67 ± 0.49c | |
| 15% | 48 | 51.17 ± 0.30a | 80.17 ± 0.62ab | 10.83 ± 0.38c | |
| Tris egg yolk-based B | 0% | 48 | 43.67 ± 0.53c | 73.17 ± 0.40b | 21.17 ± 0.48a |
| 5% | 48 | 45.16 ± 0.58bc | 74.50 ± 0.55b | 18.93 ± 0.70b | |
| 10% | 48 | 47.25 ± 0.84a | 76.67 ± 0.88a | 14.17 ± 0.36c | |
| 15% | 48 | 45.67 ± 0.52ab | 76.00 ± 0.41a | 15.66 ± 0.22c |
N = 48 ejaculates. Within columns, values with different superscripts differ significantly for each parameter (P < 0.05)
Within the same column, means bearing a, b, and c differ among PRP concentrations (0%, 5%, 10% and 15%) within the same extender (OptiXcell® or Tris egg yolk-based). A and B differ between extender types (OptiXcell® or Tris egg yolk-based), at the same PRP concentrations (0%, 5%, 10% and 15%). PRP, Platelet-rich plasma
Table 4.
Effect of PRP concentrations and extender types on post-thaw sperm functionality (mean ± SEM)
| Extender | PRP (%) | N | Acrosome integrity (%) | Plasma membrane integrity (%) |
MMP (%) | DNA integrity (%) |
|---|---|---|---|---|---|---|
| OptiXcell® | 0% | 48 | 66.73 ± 0.36cA | 46.50 ± 0.56cA | 47.17 ± 0.31dA | 87.73 ± 0.56c |
| 5% | 48 | 70.67 ± 0.57bA | 48.67 ± 0.42bA | 51.83 ± 0.48cA | 91.68 ± 0.28b | |
| 10% | 48 | 74.33 ± 0.49aA | 52.30 ± 0.88aA | 54.50 ± 0.23aA | 93.50 ± 0.56a | |
| 15% | 48 | 72.83 ± 0.25aA | 50.53 ± 0.33bA | 53.17 ± 0.31bA | 92.83 ± 0.21a | |
| Tris egg yolk-based | 0% | 48 | 63.50 ± 0.76cB | 43.82 ± 0.42cB | 43.12 ± 0.70cB | 86.67 ± 0.89c |
| 5% | 48 | 66.44 ± 0.18bB | 45.37 ± 0.72bB | 46.59 ± 0.44bB | 90.67 ± 0.94b | |
| 10% | 48 | 69.17 ± 0.70aB | 47.87 ± 0.33aB | 48.83 ± 0.60aB | 92.67 ± 0.60a | |
| 15% | 48 | 67.77 ± 0.49abB | 46.74 ± 0.62abB | 47.33 ± 0.36abB | 92.17 ± 0.37a |
N = 48 ejaculates. Within columns, values with different superscripts differ significantly for each parameter (P < 0.05)
Within the same column, means bearing a, b, c, and d differ among PRP concentrations (0%, 5%, 10% and 15%) within the same extender (OptiXcell® or Tris egg yolk-based). A and B differ between extender types (OptiXcell® or Tris egg yolk-based), at the same PRP concentrations (0%, 5%, 10% and 15%). PRP, Platelet-rich plasma; MMP, Mitochondrial membrane potential
Table 5.
Effect of PRP concentrations and extender types on post-thaw sperm antioxidant activity and malondialdehyde levels (mean ± SEM)
| Extender | PRP (%) | N | GSH (nmol/l) | TAC (mmol/ml) | MDA (nmol/ml) |
|---|---|---|---|---|---|
| OptiXcell® A | 0% | 48 | 0.71 ± 0.01d | 19.67 ± 0.67d | 23.91 ± 0.48a |
| 5% | 48 | 0.95 ± 0.01c | 21.33 ± 0.42c | 18.23 ± 0.40b | |
| 10% | 48 | 1.49 ± 0.03a | 25.17 ± 0.65a | 11.77 ± 0.29c | |
| 15% | 48 | 1.42 ± 0.02b | 23.33 ± 0.42b | 12.75 ± 0.31c | |
| Tris egg yolk-based B | 0% | 48 | 0.63 ± 0.01d | 17.61 ± 0.49c | 26.28 ± 0.92a |
| 5% | 48 | 0.75 ± 0.03c | 19.77 ± 0.40b | 23.78 ± 0.79b | |
| 10% | 48 | 1.31 ± 0.02a | 22.83 ± 0.54a | 17.50 ± 0.62c | |
| 15% | 48 | 1.16 ± 0.02b | 20.60 ± 0.60b | 19.18 ± 0.40c |
N = 48 ejaculates. Within columns, values with different superscripts differ significantly for each parameter (P < 0.05)
Within the same column, means bearing a, b, c, and d differ among PRP concentrations (0%, 5%, 10% and 15%) within the same extender (OptiXcell® or Tris egg yolk-based). A and B differ between extender types (OptiXcell® or Tris egg yolk-based), at the same PRP concentrations (0%, 5%, 10% and 15%). PRP, Platelet-rich plasma; GSH, Glutathione reductase enzyme; TAC, Total antioxidant capacity; MDA, Malondialdehyde
Table 6.
Effect of PRP concentrations and extender types on the conception rate of buffalo
| Extender | PRP (%) | Conception rate (%) |
|---|---|---|
| OptiXcell® | 0% | 8/15 (53.33%)c |
| 5% | 8/15 (53.33%)c | |
| 10% | 12/15 (80%)aA | |
| 15% | 10/15 (66.67%)b | |
| Tris egg yolk-based | 0% | 7/15 (46.67%)b |
| 5% | 7/15 (46.67%)b | |
| 10% | 10/15 (66.67%)aB | |
| 15% | 9/15 (60%)a |
Number of inseminated buffalo = 15 buffalo per extender per PRP (%). Within a column, values with different superscripts differ significantly for each parameter (P < 0.05). Within a column, means bearing a, b, and c differ among PRP concentrations (0%, 5%, 10%, and 15%) within the same extender (OptiXcell® or Tris egg yolk-based). A and B differ between extender types (OptiXcell® or Tris egg yolk-based) at the same PRP concentrations (0%, 5%, 10% and 15%)
PRP, Platelet-rich plasma
Post-thawed sperm quality
All concentrations of PRP-supplemented OptiXcell® extenders, as well as the 10% and 15% of PRP-supplemented Tris egg yolk-based extenders, significantly improved (P < 0.05) sperm motility, vitality, and reduction of sperm abnormality when compared to their control groups. PRP-treated semen at all concentrations supplemented with OptiXcell® demonstrated significantly (P < 0.05) better motility, vitality, and reduced abnormality compared to Tris egg yolk-based extenders (Table 3).
Sperm functionality
In comparison to the control groups, semen extended with OptiXcell® and Tris egg yolk-based extenders supplemented with various PRP (5, 10, and 15%) concentrations showed significantly (P < 0.05) improved acrosome integrity (%), sperm plasma membrane integrity (%), DNA integrity (%), and mitochondrial membrane potential (%), as shown in Table 4. Except for DNA integrity (%), all concentrations of PRP-supplemented OptiXcell® significantly (P < 0.05) improved sperm functionality compared to the Tris egg yolk-based (Table 4).
Antioxidant enzyme activity and malondialdehyde level
The antioxidant capacity was significantly (P < 0.05) increased, and MDA levels were lowered in PRP-supplemented OptiXcell® as compared to PRP-supplemented Tris egg yolk-based extender. Supplementation of OptiXcell® and Tris egg yolk-based extenders with various concentrations of PRP (5, 10, and 15%) significantly (P < 0.05) increased TAC and GSH and lowered MDA levels compared to controls (Table 5).
In vivo fertilization rate (%)
The conception rate (%) was significantly (P < 0.05) higher in buffaloes inseminated with semen from 10% PRP-supplemented OptiXcell® than that from PRP-supplemented Tris egg yolk-based extenders. The conception rate (%) was significantly (P < 0.05) improved in 10% and 15% PRP-supplemented OptiXcell® and Tris egg yolk-based extenders compared to 5% PRP and control groups of the two extenders, with 10% PRP being the optimal concentration with cryopreserved semen in the OptiXcell® extender (80%) compared to those of the Tris egg yolk-based extender (66.67%) and the control of two extenders (53.33% and 46.67%, respectively) as shown in Table 6.
Discussion
Our study indicated that there were no significant differences in semen quality and functionality between equilibrated controls of two extenders, as well as between equilibrated PRP-supplemented OptiXcell® or Tris egg yolk-based extenders, except for MMP%. This finding is consistent with the results of Chaudhari et al. [39], who also found no significant difference between Tris egg yolk extender and OptiXcell® regarding equilibrated semen quality and functionality, especially during the winter season.
Cryoprotectants in extenders are essential for semen cryopreservation; modifying semen extenders to include biological additions like PRP for sperm preservation may improve sperm qualities and functionality, boost antioxidant activity, and reduce the lipid peroxidation (MDA) profile, ultimately allowing for higher conception rates [10]. This study discovered that spermatozoa cryopreserved in PRP-supplemented OptiXcell® performed significantly better than PRP-supplemented Tris egg yolk-based extenders concerning post-thaw sperm viability, progressive motility, and decrease in abnormalities; this improvement was related to the composition of OptiXcell® and the action of PRP-supplementation, whereas the reversible binding and fusing of OptiXcell® liposomes to spermatozoa during cryopreservation have reduced cryoinjuries to the mitochondria and axonemal machinery [40]. Furthermore, a multitude of bioactive components in PRP, such as FGF, NGF, and TGF, may be connected to this improvement. FGF boosts protein kinase B signaling pathways, extracellular signal-regulated kinase, and the phosphorylation of FGF receptors on sperm flagella, resulting in increased sperm motility proportions [41]. TGF also has anti-inflammatory properties, increases sperm motility, and significantly affects reproduction success [42]. Furthermore, NGF greatly improved sperm viability and motility and reduced necrosis; its reduction resulted in human seminal abnormalities [43]. In contrast, it is hypothesized that decreased sperm viability, motility, and increased sperm abnormality in Tris egg yolk-based extenders compared to OptiXcell® may be caused by a cellular energy production mechanism that is delayed as a result of the presence of high-density lipoproteins, corticosteroid hormones, and other granular substances in egg yolk [44]. Regarding post-thaw motility, the findings of this study are comparable to those of further investigations carried out on buffalo [45] and bovine subjects [46]. The freezing process causes spermatozoa to undergo cryocapacitation because of protein (tyrosine) phosphorylation and calcium ion saturation, eventually destabilizing the sperm plasma membrane [47]. Our findings suggested that the more significant proportion of sperm with an intact acrosomal membrane in PRP-supplemented OptiXcell® compared to PRP-supplemented Tris egg yolk-based extenders may be attributable to the fact that OptiXcell® lessened the effects of cryocapacitation in cryopreserved semen [20]. These findings concur with Stewart et al. [48]and Ansari et al. [40].
Additionally, insulin-like growth factor 1’s actions in PRP, which lessen cryopreservation damage by preserving acrosomal membrane-associated proteins, may be responsible for a higher percentage of sperm with a normal acrosomal membrane [49]. Our results demonstrate that the PRP-supplemented OptiXcell® extender considerably increased the proportion of spermatozoa with an intact plasma membrane following cryopreservation, consistent with Naz et al. [20]. Moreover, as PRP’s protein composition mechanically preserves sperm membranes by decreasing the possibility of ice crystal formation or thawing at different phases of the cryopreservation process, it also adds to the buffering action of PRP, which minimizes osmotic stress [50].
This study found that the post-thaw mitochondrial membrane potential of PRP-supplemented OptiXcell® was much greater than that of PRP-supplemented Tris egg yolk-based extenders. This improvement may be attributable to OptiXcell® having lower sperm abnormalities and cryodamage than Tris egg yolk-based extenders, which are the main factors affecting mitochondrial membrane potential [51]. Additionally, this might be explained by the presence of vascular endothelial growth factor in PRP, which affects mitochondrial potential maintenance and OS protection [52]. This study found that PRP-free OptiXcell® and the PRP-free Tris egg yolk-based extender produced spermatozoa with identical DNA integrity [40]. But in contrast to the control group, PRP supplementation with extenders reduced OS and ROS generation and subsequently improved sperm DNA integrity [53].
In this study, we found that PRP-free Tris egg yolk-based extender had lower antioxidant activity than that of PRP-free OptiXcell® extender; this could be because Tris egg yolks contain fewer antioxidants or because microbial contamination of the egg yolks produced ROS, which could have led to spermatozoa’s lipid peroxidation [54]. PRP affects antioxidant enzymes in mammalian cells to protect cells from damage [55]. Our studies show that the addition of PRP to semen extenders before cryopreservation dramatically raises TAC and GSH levels in buffalo bull semen while reducing MDA levels, proving PRP’s potent antioxidant action by Bader et al. [56], who discovered that 2% PRP could repair the adverse consequences of H2O2-induced OS on human spermatozoa, and Yan et al. [11], who also found that human sperm that had undergone PRP treatment had lower levels of ROS. The semen quality, functionality, and antioxidant parameters of different concentrations of PRP-supplemented extenders (Tris egg yolk and/or OptiXcell®) increased gradually from 0% PRP (control) to 5% PRP to reach the highest at 10% PRP, then stabilized to decrease at 15%. This may be due to the elevated levels of extender amino acids in PRP components. This leads to increased osmotic pressure and extender hypertonicity, as Ali et al. [57] and Khlifaoui et al. [58] reported. These adverse effects can significantly decrease sperm antioxidant enzyme content, sperm quality, and in vivo fertility.
This study’s in vivo fertilization rate was significantly higher when cryopreserved semen was supplemented with 10% and 15% PRP in the OptiXcell® and Tris egg yolk-based extenders. These results suggest that the supplementation of PRP improves sperm DNA integrity, which in turn can enhance bull sperm fertility. Studies have shown that sperm DNA degradation can hurt fertility [59]. Additionally, the improved fertilization rate may be attributed to the fact that PRP enhances the immune response and has antimicrobial and anti-inflammatory properties, reducing the risk of uterine infection after breeding buffaloes. Uterine infection is a significant cause of poor embryonic survival, and by lowering this risk, PRP can lead to better embryonic recovery and higher pregnancy rates [60].
Conclusion
Compared to Tris egg yolk-based extender, OptiXcell® was more successful in cryopreserving bull semen. Additionally, extenders supplemented with PRP have properties that defend against cryodamage and improve in vivo fertility. The optimal concentration for cryopreserving buffalo bull semen is a 10% PRP-containing OptiXcell® extender, which enhances post-thaw sperm quality, functionality, antioxidant enzyme activity, and spermatozoa fertility.
Acknowledgements
The authors appreciate the assistance of the International Livestock Management Training Center and the whole Riwina Animal Production Farm team. This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2023/R/1445), Al-Kharj, Saudi Arabia. This research also funded partially by Zarqa University-Jordan. Moreover, this paper is published from the project 6PFE of the University of Life Sciences “King Mihai I” from Timisoara and the Research Institute for Biosecurity and Bioengineering from Timisoara. We also appreciated the AlMaarefa University, Saudi Arabia and Mutah University-Jordan.
Author contributions
MSS., MAA., EST., conceptualization, methodology, investigation, and data curation. FR., IMI., ME., drafted the manuscript and performing the statistical analysis being supervised by AMI., OM., RO., Writing, review and editing of the manuscript, supervision, formal analysis, and visualization was performed by NAM., AA., MS. All authors read and approved the final manuscript.
Data availability
Upon request from the corresponding authors.
Declarations
Ethics approval and consent to participate
The ethical principles outlined by the journal have been upheld, and the study has obtained the necessary ethical approval. Additionally, the research complies with the EU standards regarding the protection and utilization of animals for scientific purposes and/or feed legislation. The Institutional Animal Care and Use Committee of the Faculty of Veterinary Medicine at Kafrelsheikh University, Egypt allowed approval (IACUC#133/2021) for the present study involving the sampling.
Consent to publish
Not applicable.
Competing interests
The authors disclose no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ostan Mihaela, Email: mihaela_ostan@usvt.ro.
Mustafa Shukry, Email: mostafa.ataa@vet.kfs.edu.eg.
References
- 1.El-Khawagah AR, Kandiel MM, Samir H. Effect of quercetin supplementation in extender on sperm kinematics, extracellular enzymes release, and oxidative stress of Egyptian buffalo bulls frozen–thawed semen. Front Veterinary Sci. 2020;7:604460. doi: 10.3389/fvets.2020.604460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahzad Q, Mehmood MU, Khan H, ul Husna A, Qadeer S, Azam A, Naseer Z, Ahmad E, Safdar M, Ahmad M. Royal jelly supplementation in semen extender enhances post-thaw quality and fertility of Nili-Ravi buffalo bull sperm. Anim Reprod Sci. 2016;167:83–8. doi: 10.1016/j.anireprosci.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Layek S, Mohanty T, Kumaresan A, Parks J. Cryopreservation of bull semen: evolution from egg yolk based to soybean based extenders. Anim Reprod Sci. 2016;172:1–9. doi: 10.1016/j.anireprosci.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Andrabi S. Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod Domest Anim. 2009;44(3):552–69. doi: 10.1111/j.1439-0531.2008.01240.x. [DOI] [PubMed] [Google Scholar]
- 5.Irmak G, Demirtaş TT, Gümüşderelioğlu M. Sustained release of growth factors from photoactivated platelet rich plasma (PRP) Eur J Pharm Biopharm. 2020;148:67–76. doi: 10.1016/j.ejpb.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Pantos K, Nitsos N, Kokkali G, Vaxevanoglou T, Markomichali C, Pantou A, Grammatis M, Lazaros L, Sfakianoudis K. Ovarian rejuvenation and folliculogenesis reactivation in peri-menopausal women after autologous platelet-rich plasma treatment. Hum Reprod. 2016;31(Suppl 1):i301. [Google Scholar]
- 7.Hernández-Corredor L, León-Restrepo S, Bustamante-Cano J, Báez-Sandoval G, Jaramillo X. Effect of the incorporation of plasma rich of platelets on the spermatozoa physiology of ram semen. J Dairy Vet Anim Res. 2020;9(1):34–8. doi: 10.15406/jdvar.2020.09.00275. [DOI] [Google Scholar]
- 8.Salama MS, Shehabeldin AM, Ashour MA, Al-Ghadi MQ, Marghani BH, El-kon I, Shukry M. Effect of the addition of platelet-rich plasma to Boer Buck semen on sperm quality and antioxidant activity before and after cryopreservation and in vivo fertility. Small Ruminant Res. 2024;230:107167. doi: 10.1016/j.smallrumres.2023.107167. [DOI] [Google Scholar]
- 9.Alcay S, Aktar A, Koca D, Kilic M, Akkasoglu M, Yilmaz M, SAĞIRKAYA H. Autologous platelet rich plasma have positive effect on ram spermatozoa during cryopreservation in non-breeding season. Kafkas Universitesi Veteriner Fakultesi Dergisi 2022, 28.
- 10.El-Sherbiny HR, Abdelnaby EA, Samir H, Fathi M. Addition of autologous platelet rich plasma to semen extender enhances cryotolerance and fertilizing capacity of buffalo bull spermatozoa. Theriogenology. 2022;194:104–9. doi: 10.1016/j.theriogenology.2022.09.029. [DOI] [PubMed] [Google Scholar]
- 11.Yan B, Zhang Y, Tian S, Hu R, Wu B. Effect of autologous platelet-rich plasma on human sperm quality during cryopreservation. Cryobiology. 2021;98:12–6. doi: 10.1016/j.cryobiol.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Kashkool MH, AL-Delemi DHJ. Testicular injection of autologous platelet Rich plasma (PRP) to improve of semen parameters of goats. J Surv Fisheries Sci. 2023;10(3S):4026–37. [Google Scholar]
- 13.Al-Nasser R, Khrait Z, Jamali S. The effectiveness of autologous platelet-rich plasma (prp) in the therapy of infertile men with non-abstractive azoospermia. J Reprod Med Gynecol Obstet. 2018;3(11):D0I. [Google Scholar]
- 14.Abdulla AK, Rebai T, Al-Delemi DHJ. Protective effects of Autologous platelet-rich plasma (PRP) on the outcome of cryopreservation in rabbit sperm. Cell Mol Biol. 2022;68(4):113–21. doi: 10.14715/cmb/2022.68.4.14. [DOI] [PubMed] [Google Scholar]
- 15.Gazia MA. Histological study on the possible ameliorating effect of platelet rich plasma on ischemia/reperfusion injury in testicular torsion model in adult albino rat. Egypt J Histol. 2020;43(2):614–29. doi: 10.21608/ejh.2019.9860.1091. [DOI] [Google Scholar]
- 16.Vishwanath R, Shannon P. Storage of bovine semen in liquid and frozen state. Anim Reprod Sci. 2000;62(1–3):23–53. doi: 10.1016/S0378-4320(00)00153-6. [DOI] [PubMed] [Google Scholar]
- 17.Röpke T, Oldenhof H, Leiding C, Sieme H, Bollwein H, Wolkers W. Liposomes for cryopreservation of bovine sperm. Theriogenology. 2011;76(8):1465–72. doi: 10.1016/j.theriogenology.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Miguel-Jiménez S, Mogas T, Peña A, Tamargo C, Hidalgo C, Muiño R, Rodríguez-Gil J, Morató R. Post-thaw changes in sperm membrane and ROS following cryopreservation of dairy bull semen using four different commercial extenders. In: Physiology of Reproduction in Male and Semen Technology (Abstracts A191E to A205E): 32nd Meeting of the European Embryo Transfer Association (AETE): 2016; 2016: 9–10.
- 19.Wilhelm K, Graham J, Squires E. Effects of phosphatidylserine and cholesterol liposomes on the viability, motility, and acrosomal integrity of stallion spermatozoa prior to and after cryopreservation. Cryobiology. 1996;33(3):320–9. doi: 10.1006/cryo.1996.0032. [DOI] [PubMed] [Google Scholar]
- 20.Naz S, Umair M, Iqbal S. Comparison of tris egg yolk-based, Triladyl® and Optixell® extender on post‐thaw quality, kinematics and in vivo fertility of Nili Ravi Buffalo (Bubalus bubalis) bull spermatozoa. Andrologia. 2018;50(8):e13063. doi: 10.1111/and.13063. [DOI] [PubMed] [Google Scholar]
- 21.He L, Bailey J, Buhr M. Incorporating lipids into boar sperm decreases chilling sensitivity but not capacitation potential. Biol Reprod. 2001;64(1):69–79. doi: 10.1095/biolreprod64.1.69. [DOI] [PubMed] [Google Scholar]
- 22.Nazari L, Salehpour S, Hoseini S, Zadehmodarres S, Ajori L. Effects of autologous platelet-rich plasma on implantation and pregnancy in repeated implantation failure: a pilot study. Int J Reproductive Biomed. 2016;14(10):625. doi: 10.29252/ijrm.14.10.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gutiérrez CM, López C, Giraldo CE, Carmona JU. Study of a two-step centrifugation protocol for concentrating cells and growth factors in bovine platelet-rich plasma. Veterinary Medicine International 2017, 2017. [DOI] [PMC free article] [PubMed]
- 24.Badr M, Rawash Z, Azab A, Dohreg R, Ghattas T, Fathi M. Spirulina platensis extract addition to semen extender enhances cryotolerance and fertilizing potentials of buffalo bull spermatozoa. Anim Reprod. 2021;18:e20200520. doi: 10.1590/1984-3143-ar2020-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalal J, Kumar A, Kumar P, Honparkhe M, Malik VS, Singhal S, Kaur S, Brar PS. Improvement in cryosurvival of buffalo bull (Bubalus bubalis) sperm by altering freezing rate within critical temperature range. Asian Pac J Reprod. 2018;7(2):72–8. doi: 10.4103/2305-0500.228016. [DOI] [Google Scholar]
- 26.Susilowati S, Triana IN, Sardjito T, Suprayogi TW, Wurlina W, Mustofa I. Effect of simmental bull seminal plasma protein in egg yolk-citrate extender on Kacang Buck semen fertility. Cryobiology. 2020;97:20–7. doi: 10.1016/j.cryobiol.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Iqbal S, Andrabi SMH, Riaz A, Durrani AZ, Ahmad N. Trehalose improves semen antioxidant enzymes activity, post-thaw quality, and fertility in Nili Ravi buffaloes (Bubalus bubalis) Theriogenology. 2016;85(5):954–9. doi: 10.1016/j.theriogenology.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Khan M, Ijaz A. Assessing undiluted, diluted and frozen-thawed Nili-Ravi buffalo bull sperm by using standard semen assays. Italian J Anim Sci. 2007;6(sup2):784–7. doi: 10.4081/ijas.2007.s2.784. [DOI] [Google Scholar]
- 29.Kumar P, Kumar D, Sikka P, Singh P. Sericin supplementation improves semen freezability of buffalo bulls by minimizing oxidative stress during cryopreservation. Anim Reprod Sci. 2015;152:26–31. doi: 10.1016/j.anireprosci.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Alkhawagah AR, Ricci A, Banchi P, Martino NA, Poletto ML, Donato GG, Nervo T, Vincenti L. Effect of epidermal growth factor (EGF) on Cryopreserved Piedmontese Bull Semen characteristics. Animals. 2022;12(22):3179. doi: 10.3390/ani12223179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martins C, Dode M, Báo S, Rumpf R. The use of the acridine orange test and the TUNEL assay to assess the integrity of freeze-dried bovine spermatozoa DNA. 2007. [PubMed]
- 32.Ansari M, Rakha B, Andrabi S, Ullah N, Iqbal R, Holt W, Akhter S. Thioglycol in extender improves the post-thaw quality of buffalo (Bubalus bubalis) bull spermatozoa. 2014. [DOI] [PubMed]
- 33.Beutler E, Duron O, Kelly BM. Improved method for determination of blood glutathione. 1963. [PubMed]
- 34.Kumar P, Saini M, Kumar D, Jan M, Swami DS, Sharma R. Quantification of leptin in seminal plasma of buffalo bulls and its correlation with antioxidant status, conventional and computer-assisted sperm analysis (CASA) semen variables. Anim Reprod Sci. 2016;166:122–7. doi: 10.1016/j.anireprosci.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Placer ZA, Cushman LL, Johnson BC. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966;16(2):359–64. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 36.Hoque MN, Talukder AK, Kamal MM, Jha AK, Bari FY, Shamsuddin M. Ovulation synchronization in water buffaloes guided by milk progesterone Elisa. J Embryo Transf. 2011;26(2):105–9. [Google Scholar]
- 37.Pituch KA, Stevens JP. Applied multivariate statistics for the social sciences: analyses with SAS and. IBM’s SPSS: Routledge; 2015. [Google Scholar]
- 38.Duncan DB. Multiple range and multiple F tests. biometrics 1955, 11(1):1–42.
- 39.Chaudhari D, Patel J, Hadiya K, Dhami A. Influence of seasons and extenders on quality and freezability of gir bull semen under middle Gujarat climate. Indian J Veterinary Sci Biotechnol. 2017;13(3):95–100. [Google Scholar]
- 40.Ansari M, Rakha B, Akhter S, Ashiq M. OPTIXcell improves the postthaw quality and fertility of buffalo bull sperm. Theriogenology. 2016;85(3):528–32. doi: 10.1016/j.theriogenology.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 41.Saucedo L, Buffa GN, Rosso M, Guillardoy T, Gongora AD, Munuce MJ, Vazquez MH, Marin Briggiler CI. Fibroblast Growth Factor Receptors (FGFRs) in human male germ cells: evidence for their expression, functionality and involvement in sperm motility regulation. 2015. [DOI] [PMC free article] [PubMed]
- 42.Sharkey DJ, Tremellen KP, Briggs NE, Dekker GA, Robertson SA. Seminal plasma transforming growth factor-β, activin A and follistatin fluctuate within men over time. Hum Reprod. 2016;31(10):2183–91. doi: 10.1093/humrep/dew185. [DOI] [PubMed] [Google Scholar]
- 43.Castellini C, Mattioli S, Dal Bosco A, Cotozzolo E, Mancinelli AC, Rende M, Stabile AM, Pistilli A. Nerve growth factor receptor role on rabbit sperm storage. Theriogenology. 2020;153:54–61. doi: 10.1016/j.theriogenology.2020.04.042. [DOI] [PubMed] [Google Scholar]
- 44.Amirat L, Anton M, Tainturier D, Chatagnon G, Battut I, Courtens JL. Modifications of bull spermatozoa induced by three extenders: Biociphos, low density lipoprotein and Triladyl, before, during and after freezing and thawing. Reproduction. 2005;129(4):535–43. doi: 10.1530/rep.1.00011. [DOI] [PubMed] [Google Scholar]
- 45.Singh A, Kumar A, Honparkhe M, Kaur S, Kaur H, Ghuman S, Brar P. Comparison of in vitro and in vivo fertilizing potential of buffalo bull semen frozen in egg yolk-, soya bean lecithin‐and liposome‐based extenders. Reprod Domest Anim. 2018;53(1):195–202. doi: 10.1111/rda.13092. [DOI] [PubMed] [Google Scholar]
- 46.Ansari MS, Rakha BA, Akhter S. Cryopreservation of bull semen in OptiXcell® and conventional extenders: comparison of semen quality and fertility. Anim Sci Papers Rep 2017, 35(3).
- 47.Reddy NSS, Mohanarao GJ, Atreja S. Effects of adding taurine and trehalose to a tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm quality following cryopreservation. Anim Reprod Sci. 2010;119(3–4):183–90. doi: 10.1016/j.anireprosci.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Stewart JL, Shipley CF, Katich AS, Po E, Ellerbrock RE, Lima FS, Canisso IF. Cryopreservation of white-tailed deer (Odocoileus virginianus) semen using soybean-, liposome-, and egg yolk-based extenders. Anim Reprod Sci. 2016;171:7–16. doi: 10.1016/j.anireprosci.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Selvaraju S, Krishnan BB, Archana SS, Ravindra JP. IGF1 stabilizes sperm membrane proteins to reduce cryoinjury and maintain post-thaw sperm motility in buffalo (Bubalus bubalis) spermatozoa. Cryobiology. 2016;73(1):55–62. doi: 10.1016/j.cryobiol.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 50.Taher-Mofrad SMJ, Topraggaleh TR, Ziarati N, Bucak MN, Nouri M, Seifi S, Esmaeili V, Rahimizadeh P, Shahverdi A. Knockout serum replacement is an efficient serum substitute for cryopreservation of human spermatozoa. Cryobiology. 2020;92:208–14. doi: 10.1016/j.cryobiol.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Alcay S, Toker MB, Onder NT, Gokce E. Royal jelly supplemented soybean lecithin-based extenders improve post-thaw quality and incubation resilience of goat spermatozoa. Cryobiology. 2017;74:81–5. doi: 10.1016/j.cryobiol.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Tohidnezhad M, Wruck C-J, Slowik A, Kweider N, Beckmann R, Bayer A, Houben A, Brandenburg L-O, Varoga D, Sönmez T-T. Role of platelet-released growth factors in detoxification of reactive oxygen species in osteoblasts. Bone. 2014;65:9–17. doi: 10.1016/j.bone.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 53.Zeron Y, Tomczak M, Crowe J, Arav A. The effect of liposomes on thermotropic membrane phase transitions of bovine spermatozoa and oocytes: implications for reducing chilling sensitivity. Cryobiology. 2002;45(2):143–52. doi: 10.1016/S0011-2240(02)00123-2. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi T, Miyazaki T, Natori M, Nozawa S. Protective role of superoxide dismutase in human sperm motifity: superoxide dismutase activity and lipid peroxide in human seminal plasma and spermatozoa. Hum Reprod. 1991;6(7):987–91. doi: 10.1093/oxfordjournals.humrep.a137474. [DOI] [PubMed] [Google Scholar]
- 55.Lai L, Mohamed MNA, Ali MRM, Teng-Keen K, Yusof A. Effect of platelet-rich plasma treatment on antioxidant enzymes’ activity following hamstring injury among Malaysian athletes. Sains Malaysiana. 2016;45(5):769–75. [Google Scholar]
- 56.Bader R, Ibrahim J, Moussa M, Mourad A, Azoury J, Azoury J, Alaaeddine N. In vitro effect of autologous platelet-rich plasma on H2O2‐induced oxidative stress in human spermatozoa. Andrology. 2020;8(1):191–200. doi: 10.1111/andr.12648. [DOI] [PubMed] [Google Scholar]
- 57.Ali Al Ahmad M, Chatagnon G, Amirat-Briand L, Moussa M, Tainturier D, Anton M, Fieni F. Use of glutamine and low density lipoproteins isolated from egg yolk to improve buck semen freezing. Reprod Domest Anim. 2008;43(4):429–36. doi: 10.1111/j.1439-0531.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- 58.Khlifaoui M, Battut I, Bruyas JF, Chatagnon G, Trimeche A, Tainturier D. Effects of glutamine on post-thaw motility of stallion spermatozoa: an approach of the mechanism of action at spermatozoa level. Theriogenology. 2005;63(1):138–49. doi: 10.1016/j.theriogenology.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Serafini R, Love CC, Coletta A, Mari G, Mislei B, Caso C, Di Palo R. Sperm DNA integrity in frozen-thawed semen from Italian Mediterranean Buffalo bulls and its relationship to in vivo fertility. Anim Reprod Sci. 2016;172:26–31. doi: 10.1016/j.anireprosci.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Segabinazzi LG, Canisso IF, Podico G, Cunha LL, Novello G, Rosser MF, Loux SC, Lima FS, Alvarenga MA. Intrauterine blood plasma platelet-therapy mitigates persistent breeding-induced endometritis, reduces uterine infections, and improves embryo recovery in mares. Antibiotics. 2021;10(5):490. doi: 10.3390/antibiotics10050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request from the corresponding authors.