Abstract
Interleukin (IL)‐6 plays pleiotropic roles in human hematopoiesis and immune responses by acting on not only the IL‐6 receptor‐α subunit (IL‐6Rα)+ but also IL‐6Rα− hematopoietic progenitors via soluble IL‐6R. The Notch ligand Delta‐1 has been identified as an important modulator of the differentiation and proliferation of human hematopoietic progenitors. Here, it was investigated whether these actions of IL‐6 are influenced by Delta‐1. When CD34+CD38− hematopoietic progenitors were cultured with stem cell factor, flt3 ligand, thrombopoietin and IL‐3, Delta‐1, in combination with the IL‐6R/IL‐6 fusion protein FP6, increased the generation of glycophorin A+ erythroid cells but counteracted the effects of IL‐6 and FP6 on the generation of CD14+ monocytic and CD15+ granulocytic cells. Although freshly isolated CD34+CD38− cells expressed no or only low levels of IL‐6Rα, its expression was increased in myeloid progenitors after culture but remained negative in erythroid progenitors. It was found that Delta‐1 acted in synergy with FP6 to enhance the generation of erythroid cells from the IL‐6Rα− erythroid progenitors. In contrast, Delta‐1 antagonized the effects of IL‐6 and FP6 on the development of monocytic and granulocytic cells, as well as CD14−CD1a+ dendritic cells, from the IL‐6Rα+ myeloid progenitors. These results indicate that Delta‐1 interacts differentially with gp130 activation in IL‐6Rα− erythroid and IL‐6Rα+ myeloid progenitors. The present data suggest a divergent interaction between Delta‐1 and gp130 activation in human hematopoiesis. (Cancer Sci 2007; 98: 1597–1603)
Interleukin (IL)‐6 is a multifunctional cytokine that regulates hematopoiesis and various immune responses.( 1 ) Although gp130, a common signal transducer for the IL‐6 family of cytokines, is ubiquitously expressed in hematopoietic progenitor cells, the expression of the IL‐6 receptor‐α subunit (IL‐6Rα), a binding subunit of IL‐6R, is limited to a subset of human hematopoietic progenitors. However, after binding to soluble IL‐6R (sIL‐6R), IL‐6 associates with gp130 and consequently stimulates the growth of IL‐6Rα− human hematopoietic progenitors in the presence of stem cell factor (SCF) or flt3 ligand (Flt3L).( 2 , 3 , 4 , 5 ) Nevertheless, little is known about how the actions of IL‐6 on human hematopoietic progenitors are regulated.
Notch signaling, an evolutionarily conserved pathway involved in the cell fate decisions of various types of progenitor cells in numerous developmental systems,( 6 ) has been shown to play crucial roles in hematopoiesis.( 7 , 8 , 9 ) In humans, subsets of hematopoietic cells and stromal cells express Notch receptors, including Notch‐1, Notch‐2 and Notch‐4, and Notch ligands, including Delta‐1, Delta‐4 and Jagged‐1.( 9 , 10 , 11 , 12 , 13 , 14 ) It has been reported that co‐culture with these Notch ligands or activation of Notch‐1 or Notch‐4 modifies the differentiation and proliferation of human CD34+CD38− or CD34+ hematopoietic progenitors.( 9 , 11 , 12 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ) Previous studies have shown that Delta‐1 inhibits the differentiation of CD34+CD38− and CD34+ hematopoietic progenitors toward myeloid lineages and promotes their differentiation along particular lymphoid lineages, such as T, natural killer (NK) and plasmacytoid dendritic cells, at the expense of their B cell fate.( 15 , 16 , 17 , 18 , 19 ) Delta‐1 also modulates the differentiation of monocytes.( 9 , 10 , 23 , 24 )
The present study was conducted to determine whether Delta‐1 affects the IL‐6Rα‐ and sIL‐6R‐mediated actions of IL‐6 on human hematopoietic progenitors. Instead of using the sIL‐6R/IL‐6 complex, the sIL‐6R‐mediated effects of IL‐6 were assessed using a recombinant IL‐6R/IL‐6 fusion protein, FP6, which originally was engineered to efficiently stimulate gp130.( 25 ) Here, it is shown that Delta‐1 acts synergistically with FP6 to augment the generation of erythroid cells from IL‐6Rα− erythroid progenitors, but counteracts the effects of IL‐6 and FP6 on the generation of granulocytic, monocytic and dendritic cells from IL‐6Rα+ myeloid progenitors. Thus, Delta‐1 interacts differentially with gp130 activation in IL‐6Rα− erythroid and IL‐6Rα+ myeloid progenitors. The present data disclose that Delta‐1 displays a divergent interaction with gp130 activation in human hematopoiesis.
Materials and Methods
Cell preparation. Human cord blood samples were taken from newborns of healthy mothers at Mie University Hospital according to institutional guidelines after informed consent was obtained from the mothers. This study was approved by the Institutional Review Board of Mie University Hospital. CD34+CD38− cells were isolated as described previously.( 16 ) Briefly, CD34+ cells were enriched from mononuclear cells using CD34 immunomagnetic beads (MACS; Miltenyi Biotec, Auburn, CA, USA), and then stained with fluorescein isothiocyanate (FITC)‐conjugated anti‐CD38 and phycoerythrin (PE)‐conjugated anti‐CD34 antibodies (Becton Dickinson, San Jose, CA, USA). CD34+CD38− cells were sorted using a FACSVantage or FACSAria (Becton Dickinson Immunocytometry Systems, Mountain View, CA, USA).
Recombinant growth factors and reagents. Recombinant human IL‐3, IL‐6, SCF, thrombopoietin (TPO), granulocyte–macrophage colony‐stimulating factor (GM‐CSF), granulocyte colony‐stimulating factor (G‐CSF) and erythropoietin (EPO) were prepared as previously described.( 24 , 25 ) Recombinant FP6, which was engineered to link sIL‐6R with IL‐6, was produced as described previously.( 25 ) Recombinant Flt3L was purchased from R&D Systems (Minneapolis, MN, USA). The cytokines were added at the following concentrations: IL‐3, 10 ng/mL; IL‐6, 10 ng/mL; SCF, 50 ng/mL; TPO, 20 ng/mL; GM‐CSF, 10 ng/mL; G‐CSF, 10 ng/mL; EPO, 2 U/mL; FP6, 100 ng/mL; Flt3L, 50 ng/mL. A recombinant Delta‐1 construct consisting of an extracellular domain of Delta‐1 and six myc tags as well as anti‐myc antibody 9E10 F(ab′)2 fragments were kind gifts from Dr I.D. Bernstein (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) or prepared as previously described.( 10 ) Delta‐1 was used at a concentration of 1 µg/mL. A recombinant human gp130/Fc chimeric protein was purchased from R&D Systems. Purified human IgG (Sigma‐Aldrich, St Louis, MO, USA) was used as a control for the gp130/Fc chimeric protein.
Culture. Liquid cultures were performed in serum‐free medium (StemSpan; Stem Cell Technologies, Vancouver, BC, Canada) supplemented with 50 U/mL penicillin, 50 µg/mL streptomycin and the indicated cytokines in the presence or absence of Delta‐1. For immobilization of Delta‐1, non‐tissue culture‐treated plates (Falcon; BD Labware, Franklin Lakes, NJ, USA) were pre‐coated with 5 µg/mL of anti‐myc antibody 9E10 F(ab′)2 fragments and 2.5 µg/mL of recombinant human fibronectin fragment CH‐296 (Takara Bio Inc., Otsu, Shiga Japan).( 16 ) Viable cell numbers were counted using the Trypan blue dye exclusion method. Semisolid colony assays were carried out in 35‐mm culture dishes containing 1 mL of α‐modified Eagle's medium (α‐MEM; ICN Biomedicals, Aurora, OH, USA), 30% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT, USA), 1% deionized fraction V bovine serum albumin (BSA; Sigma‐Aldrich), 1.3% methylcellulose (Shinetsu Kagaku, Tokyo, Japan), 2 mM L‐glutamine (Gibco‐BRL, Gaithersburg, MD, USA), 50 U/mL penicillin, 50 µg/mL streptomycin, 5 × 10−5 M 2‐mercaptoethanol (Sigma‐Aldrich), SCF, Flt3L, TPO, GM‐CSF, G‐CSF and EPO. After 12–14 days, colony types were determined by in situ observation of the plates under an inverted microscope. Mixed colonies, erythroid bursts and colonies consisting of granulocytes and/or macrophages were scored as CFU‐Mix, BFU‐E and CFU‐GM, respectively.
Flow cytometric analysis and cell sorting. For flow cytometric analysis, the following murine monoclonal antibodies (mAb) were used: anti‐HLA‐DR‐FITC, anti‐CD14‐FITC, anti‐CD11c‐PE, anti‐CD14‐PE, anti‐glycophorin A (GPA)‐PE and anti‐CD14‐allophyocyanin (APC) (all from Becton Dickinson); anti‐CD15‐FITC, anti‐CD36‐FITC, anti‐CD40‐FITC, anti‐CD71‐FITC, anti‐CD80‐FITC, anti‐CD86‐FITC, anti‐CD38‐APC, anti‐GPA‐APC and biotinylated anti‐IL‐6Rα (all from BD PharMingen, San Diego, CA, USA); anti‐CD1a‐FITC and anti‐CD1a‐PE (both from Coulter, Miami, FL, USA). The following isotype controls were used: mouse IgG1‐FITC, IgG2a‐FITC and IgG1‐PE (all from Becton Dickinson); mouse IgM‐FITC, IgG2b‐FITC, IgG2b‐PE and IgG2b‐APC (all from BD PharMingen). The IL‐6Rα expression level was examined by incubation with a biotinylated anti‐IL‐6Rα mAb and the indicated mAbs, followed by a streptavidin‐PE conjugate (BD PharMingen). Flow cytometric analysis was carried out using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems). Dead cells were excluded using propidium iodide or 7‐amino‐actinomycin‐D (BD PharMingen) staining. In some experiments, cell populations were sorted using a FACSVantage or FACSAria (Becton Dickinson Immunocytometry Systems). The purity of the populations routinely exceeded 95%.
Quantitative real‐time reverse transcription–polymerase chain reaction (RT‐PCR). Quantitative real‐time RT‐PCR for hairy enhancer of split (HES)‐1 was performed as described previously.( 16 , 24 ) Briefly, total RNA was prepared from cultured cells using an RNeasy Blood Mini Kit (Qiagen, Tokyo, Japan), and cDNA were synthesized using Omniscript reverse transcriptase (Qiagen) and oligo dT primers according to the manufacturer's instructions. All amplifications were carried out using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and an ABI PRISM 7700 Sequence Detector (Applied Biosystems). The primers and probes were as follows: HES‐1 forward primer, 5′‐TGGAAATGACAGTGAAGCACC‐3′; HES‐1 reverse primer, 5′‐GTTCATGCACTCGCTGAAGC‐3′; HES‐1 probe, 5′‐(FAM)‐CGCAGATGACGGCTGCGCTG‐(TAMRA)‐3′; glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) forward primer, 5′‐GAAGGTGAAGGTCGGAGT‐3′; GAPDH reverse primer, 5′‐GAAGATGGTGATGGGATTTC‐3′; GAPDH probe, 5′‐(FAM)‐TTGCCATCAATGACCCCTTCA TTGAC‐(TAMRA)‐3′. The reaction conditions were: incubation at 50°C for 2 min and then 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. All samples were run in duplicate. HES‐1 mRNA expression was normalized to the GAPDH gene expression. Quantitative real‐time RT‐PCR for GATA‐2 was conducted using Power SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer's instructions. The primers were as follows: GATA‐2 forward primer, 5′‐CACAAGATGAATGGGCAGAA; GATA‐2 reverse primer, 5′‐TCATGGTCAGTGGCCTGTTA; eukaryotic translation elongation factor 1‐β‐2 (EEF1B2) forward primer, 5′‐CATGCCCTACGTTGGTATAATCAC‐3′; EEF1B2 reverse primer, 5′‐ACATCGGCAGGACCATATTTG‐3′.( 26 ) The reaction conditions were: incubation at 50°C for 2 min and then 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 45 s. Quantitative real‐time RT‐PCR for GATA‐1 was performed as previously described.( 27 ) All samples were run in duplicate. GATA‐2 and GATA‐1 mRNA expressions were normalized using the endogenous EEF1B2 expression as an internal standard.( 27 )
Data analysis. Statistical comparisons were made using Student's t‐test. Values of P < 0.05 were considered to indicate statistical significance.
Results
Delta‐1 differentially influences the effects of gp130 activation on the generation of erythroid and myeloid cells from CD34+CD38− cells. First, whether Delta‐1 in conjunction with IL‐6 or the sIL‐6R/IL‐6 fusion protein FP6 affects the production of primitive, erythroid and myeloid progenitors from human CD34+CD38− primitive hematopoietic progenitors was examined. CD34+CD38− cells were cultured for 6–7 days with SCF, flt3L, TPO and IL‐3 (4GF), 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1, and then replated into semisolid cultures. FP6 increased the generation of mixed and erythroid colonies from CD34+CD38− cells. Delta‐1 in combination with FP6 further increased the production of mixed and erythroid colonies from CD34+CD38− cells (Fig. 1). However, no significant effects were observed for the production of myeloid colonies (data not shown). Because the production of hematopoietic progenitors from CD34+CD38− cells was modified by Delta‐1 in combination with FP6, we next studied whether Delta‐1 influences the effects of IL‐6 and FP6 on the generation of erythroid and myeloid cells from human CD34+CD38− primitive hematopoietic progenitors. CD34+CD38− cells were cultured for 18–20 days with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1, and then analyzed for the numbers of GPA+ erythroid, CD14+ monocytic and CD15+ granulocytic cells. As shown in Fig. 2a, cultures containing FP6 supported the generation of considerable numbers of GPA+ erythroid cells. In addition, the expression levels of GPA and transferrin R (CD71) on the erythroid cells that developed in FP6‐containing cultures were higher than those in cultures that contained 4GF or 4GF + IL‐6 (data not shown), suggesting that FP6 supported the differentiation of hematopoietic progenitors into relatively mature erythroid cells, as observed for the complex of sIL‐6R and IL‐6.( 5 , 25 ) Although Delta‐1 alone did not affect the generation of erythroid cells, Delta‐1 in combination with FP6 increased the number of erythroid cells (Fig. 2a). The expression levels of GPA and transferrin R on the erythroid cells in cultures containing 4GF + FP6 + Delta‐1 were similar to those in cultures containing 4GF + FP6 (data not shown). These findings suggest that Delta‐1 acts in synergy with FP6 to enhance the production of erythroid cells from CD34+CD38− cells. In contrast, IL‐6 and FP6 stimulated the development of CD14+ monocytic cells (Fig. 2b). Delta‐1 suppressed the generation of monocytic cells with 4GF and also counteracted the stimulatory effects of IL‐6 and FP6 on the generation of monocytic cells from CD34+CD38− cells (Fig. 2b). Similar effects were observed for CD15+ granulocytic cells (Fig. 2c). Thus, the effects of IL‐6 or FP6 on the generation of erythroid and myeloid cells from CD34+CD38− progenitor cells were differentially modulated by Delta‐1.
Figure 1.
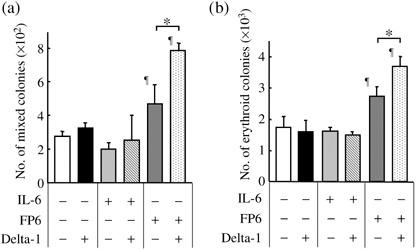
Delta‐1 in combination with FP6, increases the generation of mixed and erythroid progenitors from CD34+CD38− cells. CD34+CD38− cells (500 cells/well) were incubated with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1. After 6–7 days of culture, aliquots of the cultured cells were replated into semisolid cultures and the numbers of CFU‐mix, BFU‐E and CFU‐GM colonies derived from 500 CD34+CD38− cells were calculated. The numbers of CFU‐mix and BFU‐E colonies are shown as the mean ± SD of triplicate cultures. *P < 0.05 compared to the respective control culture. Data are representative of three independent experiments. ¶ P < 0.05 compared to cells cultured with 4GF.
Figure 2.
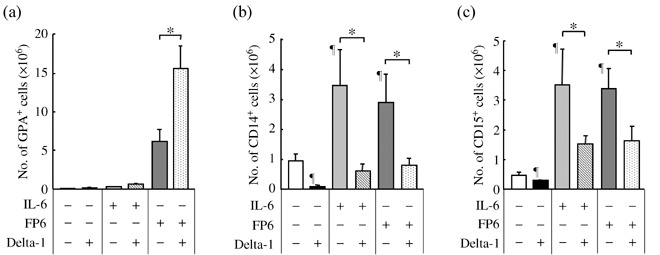
Delta‐1 differentially influences the effects of gp130 activation on the generation of erythroid and myeloid cells from CD34+CD38− cells. CD34+CD38− cells (500 cells/well) were incubated with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1. After 18–20 days of culture, the numbers of (a) GPA+ erythroid, (b) CD14+ monocytic and (c) CD15+ granulocytic cells were counted. Data are shown as the mean ± SD of triplicate cultures. ¶ P < 0.05 compared to cells cultured with 4GF. *P < 0.05 compared to the respective control culture. Representative data of three experiments are shown.
Delta‐1 acts in synergy with the IL‐6Ra/IL‐6 fusion protein FP6 on IL‐6Rα− erythroid progenitors. CD34+CD38− hematopoietic progenitors contained CFU‐Mix in addition to BFU‐E and CFU‐GM. Therefore, we sought to clarify whether Delta‐1 influences the effects of IL‐6 and FP6 on erythroid and myeloid lineage‐committed progenitors. To this end, CD34+CD38− cells were cultured for 6–7 days with 4GF, and a relatively immature cell population was selected based on negative expressions of the mature lineage markers (Lin: CD14, CD15, CD1a and GPA). The IL‐6Rα expression levels on the Lin− cell fraction were then analyzed. Although freshly isolated CD34+CD38− cells expressed no or only low levels of IL‐6Rα, a subset of the cells expressed higher levels of IL‐6Rα after culture (Fig. 3a). The IL‐6Rα−Lin− (R1 region) and IL‐6Rα+Lin− (R2 region) cell fractions were sorted and tested for colony formation. The IL‐6Rα− cell population mainly formed BFU‐E colonies, while the IL‐6Rα+ subpopulation largely generated CFU‐GM colonies (Fig. 3b). Virtually no mixed colonies were detected (data not shown). These data demonstrate that the CD34+CD38− cell‐derived IL‐6Rα−Lin− and IL‐6Rα−Lin− cell populations predominantly consist of erythroid and myeloid progenitors, respectively.
Figure 3.
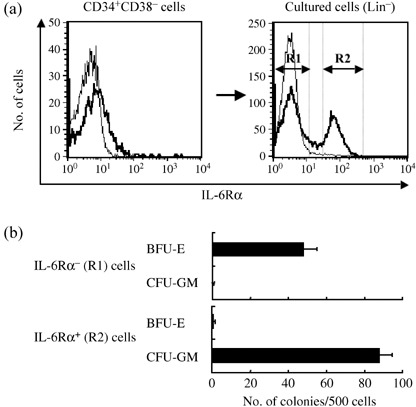
(a) IL‐6Rα expression levels in freshly isolated CD34+CD38− cells and Lin− cells derived from CD34+CD38− cells cultured with 4GF for 6–7 days. Bold lines represent the expression of IL‐6Rα and thin lines represent the staining of the isotype control. IL‐6Rα−Lin− (R1 region) and IL‐6Rα+Lin− (R2 region) cells are shown. (b) IL‐6Rα−Lin− and IL‐6Rα+Lin− cells were isolated and subjected to semisolid cultures (500 cells/well) with 4GF, GM‐CSF, G‐CSF and EPO. Colonies were scored on day 14 of culture.
When the IL‐6Rα−Lin− cells (Fig. 3a, R1 region) were incubated for 11–14 days with 4GF, 4GF + IL‐6 or 4GF + FP6, only the cultures containing FP6 supported the generation of significant numbers of GPA+ erythroid cells. To verify that FP6 actually bound to and activated gp130 on the IL‐6Rα− erythroid progenitors, whether a soluble human gp130/Fc chimeric protein could inhibit the effects of FP6 by blocking the binding of FP6 to gp130 on the cell surface was tested.( 2 , 28 ) As expected, FP6‐mediated generation of GPA+ erythroid cells was almost completely inhibited by the addition of a high concentration (1 µg/mL) of the soluble human gp130/Fc chimeric protein. No effects were seen with purified IgG (Fig. 4a). Next, it was investigated whether Delta‐1 affects the FP6‐mediated development of erythroid cells from the IL‐6Rα− erythroid progenitors by incubating the cells for 11–14 days with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1. Although Delta‐1 alone or in combination with IL‐6 did not support the generation of GPA+ cells, Delta‐1 in conjunction with FP6 significantly increased the generation of GPA+ cells from the IL‐6Rα−Lin− cells (Fig. 4b). These results indicate that Delta‐1 acts synergistically with FP6 on IL‐6Rα− erythroid progenitors to enhance the generation of relatively mature erythroid cells. To confirm whether Delta‐1 actually activates Notch signaling in the IL‐6Rα− erythroid progenitors, the IL‐6Rα−Lin− cells were incubated for 24 h with 4GF + FP6 in the presence or absence of Delta‐1, and then the expression of HES‐1, a target gene of Notch signaling,( 6 ) was analyzed using quantitative real‐time RT‐PCR. The HES‐1 expression levels were higher in cells cultured with Delta‐1 than in cells cultured without FP6 (Fig. 4c, left panel). Elevated expression of the transcription factor GATA‐2 has been shown to increase the production of erythroid cells from primitive hematopoietic progenitors.( 29 , 30 , 31 , 32 ) Therefore, the expression levels of GATA‐2 in IL‐6Rα−Lin− cells was assessed before and after culture with or without Delta‐1 for 24 h. The expression of GATA‐2 was significantly increased after incubation of IL‐6Rα−Lin− cells with Delta‐1 (Fig. 4c, middle panel). The expression of GATA‐1, which plays an important role in the regulation of erythropoiesis, was also analyzed.( 31 , 32 ) It was observed that Delta‐1 also increased GATA‐1 expression in the presence of 4GF and FP6 (Fig. 4c, right panel).
Figure 4.
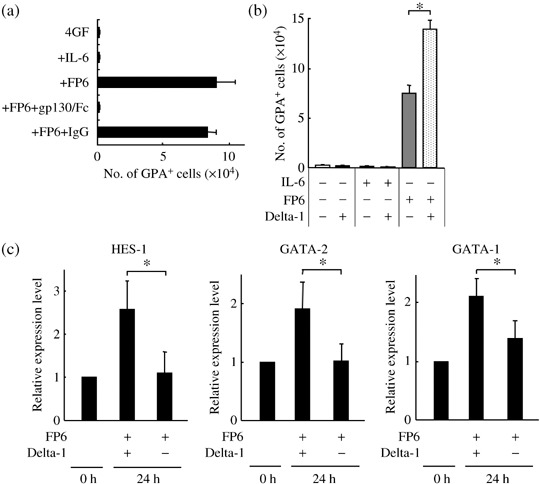
Delta‐1 acts synergistically with FP6 on IL‐6Rα− early erythroid progenitors. (a) IL‐6Rα−Lin− cells (2500 cells/well) were cultured with 4GF, 4GF + IL‐6, 4GF + FP6, 4GF + FP6 + sgp130‐Fc (1 µg/mL) or 4GF + FP6 + IgG. The numbers of CD36+GPA+ cells were counted after 11–14 days of culture. Data are shown as the mean ± SD of triplicate cultures. (b) IL‐6Rα−Lin− cells (2500 cells/well) were cultured in liquid cultures with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1. The numbers of GPA+ cells were counted after 11–14 days of culture. Data are shown as the mean ± SD of triplicate cultures. *P < 0.05 compared to cells cultured with 4GF + FP6. (c) Quantitative real‐time RT‐PCR analysis of HES‐1, GATA‐2 and GATA‐1 gene expression levels in IL‐6Rα−Lin− cells and cells cultured for 24 h with 4GF + FP6 in the presence or absence of Delta‐1. Each gene expression level in cultured cells is expressed relative to the corresponding value in untreated cells. Data are shown as the mean ± SD of three independent experiments. *P < 0.05 compared to the gene expression level in cells cultured with 4GF + FP6.
Delta‐1 counteracts the effects of IL‐6 and FP6 on IL‐6Rα+ myeloid progenitors. Next, whether Delta‐1 influences the effects of IL‐6 and FP6 on myeloid progenitors was investigated. Because the CD34+CD38− cell‐derived myeloid progenitors expressed relatively higher levels of IL‐6Rα, IL‐6Rα+Lin− cells (Fig. 3a, R2 region) were incubated in suspension cultures with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1, and the numbers of CD14+ monocytic and CD15+ granulocytic cells were counted after 11–14 days of culture. The addition of IL‐6 and FP6 to the cultures increased the numbers of CD14+ cells. Delta‐1 alone had only a slight inhibitory effect on the generation of CD14+ cells. However, Delta‐1 substantially antagonized the stimulatory effects of IL‐6 and FP6 on the generation of CD14+ monocytic cells from the IL‐6Rα+ myeloid progenitors (Fig. 5a). Similar results were observed for the generation of CD15+ granulocytic cells (Fig. 5b). The presence of CD14−CD1a+ cells was also detected in the cultures. These cells expressed other dendritic‐cell‐related markers such as CD11c, CD80, CD86 and HLA‐DR( 33 , 34 ) (data not shown). Therefore, the effects of Delta‐1, IL‐6, FP6 or their combinations on the generation of CD14−CD1a+ dendritic cells after 7–11 days in culture were examined. In contrast to CD14+CD1a− monocytic cells, the generation of CD14−CD1a+ dendritic cells was suppressed by IL‐6 and FP6, but stimulated by Delta‐1 (Fig. 5c,d). Nevertheless, Delta‐1 counteracted the effects of IL‐6 and FP6 on the generation of CD14−CD1a+ dendritic cells (Fig. 5c,d). To verify whether Delta‐1 indeed activated Notch signaling in IL‐6Rα+ myeloid progenitors, the IL‐6Rα+Lin− cells were incubated for 24 h with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1, and then the expression of HES‐1 was examined using quantitative real‐time RT‐PCR. Delta‐1 substantially increased the expression of HES‐1 compared with the level in cells cultured without Delta‐1 (Fig. 5e). GATA‐2 expression has been reported to be associated with the growth of myeloid as well as erythroid progenitors.( 31 ) However, GATA‐2 expression was not significantly affected by incubation with Delta‐1 (data not shown). Taken together, the present data suggest that Delta‐1‐induced Notch signaling counteracts the effects of IL‐6 and FP6 on the generation of granulocytic, monocytic and dendritic cells by antagonizing the effects of gp130 activation on IL‐6Rα+ myeloid progenitors.
Figure 5.
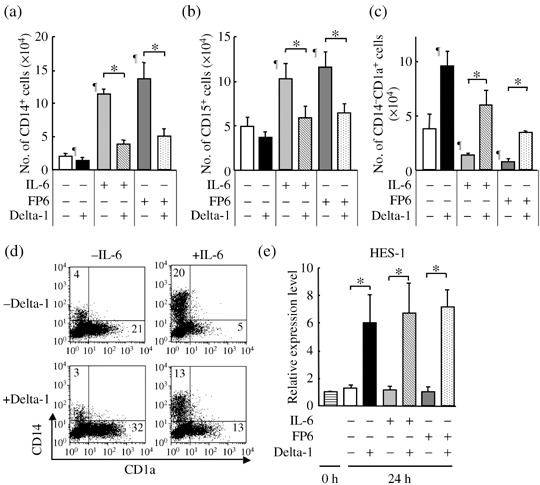
Delta‐1 antagonizes the effects of IL‐6 and FP6 on IL‐6Rα+ myeloid progenitors. IL‐6Rα+Lin− cells (2500 cells/well) were cultured with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1. The numbers of (a) CD14+, (b) CD15+ and (c) CD14−CD1a+ cells were counted after 11–14 days of culture. Data are shown as the mean ± SD of triplicate cultures. ¶P < 0.05 compared to cells cultured with 4GF. *P < 0.05 compared to the respective control culture. (d) Expression levels of CD1a and CD14 on cells after 7–11 days in culture. Representative data are shown. (e) Quantitative real‐time RT‐PCR analysis of HES‐1 gene expression in IL‐6Rα+Lin− cells and cells cultured for 24 h with 4GF, 4GF + IL‐6 or 4GF + FP6 in the presence or absence of Delta‐1. HES‐1 expression in cultured cells is expressed relative to the value in untreated cells. Data are shown as the mean ± SD of three independent experiments. *P < 0.05 compared to the gene expression in cells in the linked cultures.
Discussion
In the current study, it was first found that the effects of gp130 activation on the generation of erythroid and myeloid cells from CD34+CD38− primitive hematopoietic progenitors were differentially modulated by Delta‐1. It was further found that Delta‐1 acted in synergy with FP6 to augment the generation of GPA+ erythroid cells from IL‐6Rα− early erythroid progenitors, but antagonized the effects of IL‐6 and FP6 on the generation of CD14+ monocytic, CD15+ granulocytic and CD14−CD1a+ dendritic cells from IL‐6Rα+ myeloid progenitors. These results demonstrate that Delta‐1 interacts differentially with gp130 activation in IL‐6Rα− erythroid and IL‐6Rα+ myeloid progenitors.
Consistent with a previous report, CD34+CD38− cell‐derived erythroid progenitors did not express IL‐6Rα.( 3 ) However, the sIL‐6/IL‐6 fusion protein FP6 supported their erythroid differentiation, as observed for another type of engineered sIL‐6R/IL‐6 hybrid protein, H‐IL‐6.( 35 ) The authors have previously reported the presence of low levels of Notch‐1 on GPA1ow/– immature erythroid cells, but not on GPAhigh relatively mature erythroid cells.( 10 ) By incubating IL‐6Rα− erythroid progenitors with Delta‐1 in serum‐free cultures, it has been shown here that Delta‐1 activates Notch signaling in erythroid progenitors and, in conjunction with FP6, potentiates the generation of relatively mature erythroid cells from the erythroid progenitors. It was further observed that Delta‐1 increased the expression levels of GATA‐2 and GATA‐1 in erythroid progenitors. As seen in Delta‐1, other studies have reported that Delta‐4 has a positive influence on the production of erythroid progenitors from human primitive hematopoietic progenitors.( 36 , 37 ) Interestingly, the expression of Delta‐4 by bone marrow stromal cells is enhanced by gp130 activation.( 38 ) Because significant levels of the sIL‐6R/IL‐6 complex are detectable in plasma,( 39 ) these findings indicate that Delta‐1‐ or Delta‐4‐induced Notch signaling stimulates the growth of erythroid progenitors through a direct or indirect interaction with the sIL‐6R/IL‐6 complex. On the other hand, Delta‐1 did not significantly affect erythroid differentiation in the context of gp130 signaling. Notch‐1 activation has been shown to inhibit the erythroid differentiation of erythroid cell lines.( 40 , 41 ) Delta‐1 was recently reported to inhibit the EPO‐dependent differentiation of erythroid progenitors derived from adult peripheral blood.( 42 ) Nevertheless, the authors have previously observed that Notch‐1 expression decreases as erythroid progenitors differentiate.( 10 ) These findings imply that Notch signaling stimulates the growth of erythroid progenitors but may negatively influence their erythroid differentiation, depending on the cytokine milieu.
Freshly isolated CD34+CD38− cells expressed no or only low levels of IL‐6Rα. However, higher levels of IL‐6Rα were detected in myeloid progenitors generated after culture of the cells. These observations suggest that human primitive hematopoietic progenitors express higher levels of IL‐6Rα as they differentiate along the myeloid lineage. Previous studies, including the authors’, have shown that IL‐6 inhibits the differentiation of hematopoietic progenitors into dendritic cells and promotes their differentiation into macrophages.( 34 , 43 , 44 , 45 , 46 ) The authors have also reported that Delta‐1 directs dendritic over monocytic cell fate of CD34+ cell‐derived macrophage/dendritic cell progenitors.( 23 ) In the present study, we have extended these studies and demonstrated that, although Delta‐1 does not significantly affect the production of myeloid progenitors from CD34+CD38− cells, it opposes the IL‐6Rα‐ and sIL‐6R‐mediated effects of IL‐6 on the generation of various lineages of myeloid cells, such as monocytic, granulocytic and dendritic cells, by antagonizing the effects of gp130 activation in myeloid progenitors. Kamakura et al. reported that Notch‐induced Hes‐1 affects STAT3 activity, a downstream gene of gp130 activation in neural cells.( 47 ) On the other hand, GATA‐2 has been shown to be involved in the modulation of myeloid differentiation by Notch activation.( 48 , 49 ) Although Delta‐1 increased the expression levels of GATA‐2 in erythroid progenitors, its expression was not changed in myeloid progenitors. Therefore, further studies are needed to elucidate how Notch and gp130 signaling interact in hematopoiesis and how Notch signaling differentially modulates gp130 signaling in IL‐6Rα− erythroid and IL‐6Rα+ myeloid progenitors.
The IL‐6R/IL‐6 fusion protein FP6, but not IL‐6, induced the generation of relatively mature erythroid cells from IL‐6Rα− erythroid progenitors. This finding supports the notion that sIL‐6R is the principal element controlling the actions of IL‐6 on IL‐6Rα− hematopoietic cells.( 2 , 39 ) Although significant levels of the sIL‐6R/IL‐6 complex are detectable in plasma,( 39 ) the production of sIL‐6R is elevated in various diseases, such as chronic inflammatory diseases and cancers.( 2 , 39 ) The present results have revealed that the sIL‐6R‐ as well as IL‐6Rα‐mediated actions of IL‐6 are modulated by Delta‐1‐induced Notch signaling. It has been suggested that there is an interaction between Notch and gp130 signaling in neural stem cells.( 50 ) Very recently, an interaction between Delta‐1 and gp130 activation was reported to induce the expansion of human hematopoietic stem cells.( 51 ) The authors have also observed that Delta‐1 in combination with FP6 increased the production of primitive hematopoietic progenitors from CD34+CD38− cells. These data, together with the data obtained in the present study, suggest that there is an extensive relationship between Delta‐1 and gp130 in the human hematopoietic system.
References
- 1. Naka T, Nishimoto N, Kishimoto T. The paradigm of IL‐6: from basic science to medicine. Arthritis Res 2002; 4: 233–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rose‐John S, Scheller J, Elson G, Jones SA. Interleukin‐6 biology is coordinated by membrane‐bound and soluble receptors: role in inflammation and cancer. J Leukoc Biol 2006; 80: 227–36. [DOI] [PubMed] [Google Scholar]
- 3. Tajima S, Tsuji K, Ebihara Y et al . Analysis of interleukin 6 receptor and gp130 expressions and proliferative capability of human CD34+ cells. J Exp Med 1996; 184: 1357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ebihara Y, Tsuji K, Lyman SD et al . Synergistic action of flt3 and gp130 signaling in human hematopoiesis. Blood 1997; 90: 4363–8. [PubMed] [Google Scholar]
- 5. Sui X, Tsuji K, Tajima S et al . Erythropoietin‐independent erythrocyte production: signals through gp130 and c‐kit dramatically promote erythropoiesis from human CD34+ cells. J Exp Med 1996; 183: 837–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lai EC. Notch signaling: control of cell communication and cell fate. Development 2004; 131: 965–73. [DOI] [PubMed] [Google Scholar]
- 7. Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Ann Rev Immunol 2005; 23: 945–74. [DOI] [PubMed] [Google Scholar]
- 8. Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol 2004; 5: 247–53. [DOI] [PubMed] [Google Scholar]
- 9. Ohishi K, Katayama N, Shiku H, Varnum‐Finney B, Bernstein ID. Notch signalling in hematopoiesis. Semin Cell Dev Biol 2003; 14: 143–50. [DOI] [PubMed] [Google Scholar]
- 10. Ohishi K, Varnum‐Finney B, Flowers D, Anasetti C, Myerson D, Bernstein ID. Monocytes express high amounts of Notch and undergo cytokine specific apoptosis following interaction with the Notch ligand, Delta‐1. Blood 2000; 95: 2847–54. [PubMed] [Google Scholar]
- 11. Vercauteren SM, Sutherland HJ. Constitutively active Notch4 promotes early human hematopoietic progenitor cell maintenance while inhibiting differentiation and causes lymphoid abnormalities in vivo. Blood 2004; 104: 2315–22. [DOI] [PubMed] [Google Scholar]
- 12. Karanu FN, Murdoch B, Miyabayashi T et al . Human homologues of Delta‐1 and Delta‐4 function as mitogenic regulators of primitive human hematopoietic cells. Blood 2001; 97: 1960–7. [DOI] [PubMed] [Google Scholar]
- 13. Li L, Milner LA, Deng Y et al . The human homolog of rat Jagged1 expressed by marrow stroma inhibits differentiation of 32D cells through interaction with Notch1. Immunity 1998; 8: 43–55. [DOI] [PubMed] [Google Scholar]
- 14. Karanu FN, Murdoch B, Gallacher L et al . The Notch ligand Jagged‐1 represents a novel growth factor of human hematopoietic stem cells. J Exp Med 2000; 192: 1365–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jaleco AC, Neves H, Hooijberg E et al . Differential effects of Notch ligands Delta‐1 and Jagged‐1 in human lymphoid differentiation. J Exp Med 2001; 194: 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ohishi K, Varnum‐Finney B, Bernstein ID. Delta‐1 enhances marrow and thymus repopulating ability of human CD34+CD38− cord blood cells. J Clin Invest 2002; 110: 1165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neves H, Weerkamp F, Gomes AC et al . Effects of Delta1 and Jagged1 on early human hematopoiesis: correlation with expression of notch signaling‐related genes in CD34+ cells. Stem Cells 2006; 24: 1328–37. [DOI] [PubMed] [Google Scholar]
- 18. La Motte‐Mohs RN, Herer E, Zuniga‐Pflucker JC. Induction of T‐cell development from human cord blood hematopoietic stem cells by Delta‐like 1 in vitro . Blood 2005; 105: 1431–9. [DOI] [PubMed] [Google Scholar]
- 19. Olivier A, Lauret E, Gonin P, Galy A. The Notch ligand delta‐1 is a hematopoietic development co‐factor for plasmacytoid dendritic cells. Blood 2006; 107: 2694–701. [DOI] [PubMed] [Google Scholar]
- 20. Lauret E, Catelain C, Titeux M et al . Membrane‐bound delta‐4 notch ligand reduces the proliferative activity of primitive human hematopoietic CD34+CD38low cells while maintaining their LTC‐IC potential. Leukemia 2004; 18: 788–97. [DOI] [PubMed] [Google Scholar]
- 21. Carlesso N, Aster JC, Sklar J, Scadden DT. Notch1‐induced delay of human hematopoietic progenitor cell differentiation is associated with altered cell cycle kinetics. Blood 1999; 93: 838–48. [PubMed] [Google Scholar]
- 22. De Smedt M, Reynvoet K, Kerre T et al. Active form of Notch imposes T cell fate in human progenitor cells. J Immunol 2002; 169: 3021–9. [DOI] [PubMed] [Google Scholar]
- 23. Ohishi K, Varnum‐Finney B, Serda RE, Anasetti C, Bernstein ID. The Notch ligand, Delta‐1, inhibits the differentiation of monocytes into macrophages but permits their differentiation into dendritic cells. Blood 2001; 98: 1402–7. [DOI] [PubMed] [Google Scholar]
- 24. Hoshino N, Katayama N, Shibasaki T et al . A novel role for Notch ligand Delta‐1 as a regulator of human Langerhans cell development from blood monocytes. J Leukoc Biol 2005; 78: 921–9. [DOI] [PubMed] [Google Scholar]
- 25. Kimura T, Wang J, Minamiguchi H et al . Signal through gp130 activated by soluble interleukin (IL)‐6 receptor (R) and IL‐6 or IL‐6R/IL‐6 fusion protein enhances ex vivo expansion of human peripheral blood‐derived hematopoietic progenitors. Stem Cells 2000; 18: 444–52. [DOI] [PubMed] [Google Scholar]
- 26. Hallahan AR, Pritchard JI, Hansen S et al . The SmoA1 mouse model reveals that notch signaling is critical for the growth and survival of sonic hedgehog‐induced medulloblastomas. Cancer Res 2004; 64: 7794–800. [DOI] [PubMed] [Google Scholar]
- 27. Yamamura K, Ohishi K, Katayama N et al . Pleiotropic role of histone deacetylases in the regulation of human adult erythropoiesis. Br J Haematol 2006; 135: 242–53. [DOI] [PubMed] [Google Scholar]
- 28. Jostock T, Müllberg J, Özbek S et al . Soluble gp130 is the natural inhibitor of soluble interleukin‐6 receptor transsignaling responses. Eur J Biochem 2001; 268: 160–7. [DOI] [PubMed] [Google Scholar]
- 29. Kitajima K, Masuhara M, Era T, Enver T, Nakano T. GATA‐2 and GATA‐2/ER display opposing activities in the development and differentiation of blood progenitors. EMBO J 2002; 21: 3060–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Briegel K, Lim KC, Plank C, Beug H, Engel JD, Zenke M. Ectopic expression of a conditional GATA‐2/estrogen receptor chimera arrests erythroid differentiation in a hormone‐dependent manner. Genes Dev 1993; 7: 1097–109. [DOI] [PubMed] [Google Scholar]
- 31. Labbaye C, Valtieri M, Barberi T et al . Differential expression and functional role of GATA‐2, NF‐E2, and GATA‐1 in normal adult hematopoiesis. J Clin Invest 1995; 95: 2346–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohneda K, Yamamoto M. Roles of hematopoietic transcription factors GATA‐1 and GATA‐2 in the development of red blood cell lineage. Acta Haematol 2002; 108: 237–45. [DOI] [PubMed] [Google Scholar]
- 33. Shortman Naik SH. Steady‐state and inflammatory dendritic‐cell development. Nat Rev Immunol 2007; 7: 19–30. [DOI] [PubMed] [Google Scholar]
- 34. Encabo A, Solves P, Mateu E, Sepulveda P, Caebonell‐Uberos F, Minana MD. Selective generation of different dendritic cell precursors from CD34+ cells by interleukin‐6 and interleukin‐3. Stem Cells 2004; 22: 725–40. [DOI] [PubMed] [Google Scholar]
- 35. Baiocchi M, Marcucci I, Rose‐John S, Serlupi‐Crescenzi O, Biffoni M. An IL‐6/IL‐6 soluble receptor (IL‐6R) hybrid protein (H‐IL‐6) induces EPO‐independent erythroid differentiation in human CD34(+) cells. Cytokine 2000; 12: 1395–9. [DOI] [PubMed] [Google Scholar]
- 36. Dando JS, Tavian M, Catelain C et al . Notch/Delta4 interaction in human embryonic liver CD34+CD38− cells: positive influence on BFU‐E production and LTC‐IC potential maintenance. Stem Cells 2005; 23: 550–60. [DOI] [PubMed] [Google Scholar]
- 37. Sugimoto A, Yamamoto M, Suzuki M et al . Delta‐4 Notch ligand promotes erythroid differentiation of human umbilical cord blood CD34+ cells. Exp Hematol 2006; 34: 424–32. [DOI] [PubMed] [Google Scholar]
- 38. Suzuki M, Yamamoto M, Sugimoto A, Nakamura S, Motoda R, Orita K. Delta‐4 expression on a stromal cell line is augmented by interleukin‐6 via STAT3 activation. Exp Hematol 2006; 34: 1143–50. [DOI] [PubMed] [Google Scholar]
- 39. Jones SA, Rose‐John S. The role of soluble receptors in cytokine biology: the agonistic properties of the sIL‐6R/IL‐6 complex. Biochim Biophys Acta 2002; 1592: 251–63. [DOI] [PubMed] [Google Scholar]
- 40. Lam LT, Ronchini C, Norton J, Capobianco AJ, Bresnick EH. Suppression of erythroid but not megakaryocytic differentiation of human K562 erythroleukemic cells by notch‐1. J Biol Chem 2000; 275: 19 676–84. [DOI] [PubMed] [Google Scholar]
- 41. Ishiko E, Matsumura I, Ezoe S et al . Notch signals inhibit the development of erythroid/megakaryocytic cells by suppressing GATA‐1 activity through the induction of HES1. J Biol Chem 2005; 280: 4929–39. [DOI] [PubMed] [Google Scholar]
- 42. Tachikawa Y, Matsushima T, Abe Y et al . Pivotal role of Notch signaling in regulation of erythroid maturation and proliferation. Eur J Haematol 2006; 77: 273–81. [DOI] [PubMed] [Google Scholar]
- 43. Mitani H, Katayama N, Araki H et al . Activity of interleukin 6 in the differentiation of monocytes to macrophages and dendritic cells. Br J Haematol 2000; 109: 288–95. [DOI] [PubMed] [Google Scholar]
- 44. Chomarat P, Banchereau J, Davoust J, Palucka AK. IL‐6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol 2000; 1: 510–4. [DOI] [PubMed] [Google Scholar]
- 45. Menetrier‐Caux C, Montmain G, Dieu MC et al . Inhibition of the differentiation of dendritic cells from CD34+ progenitors by tumor cells: role of interleukin‐6 and macrophage colony‐stimulating factor. Blood 1998; 92: 4778–91. [PubMed] [Google Scholar]
- 46. Ratta M, Fagnoni F, Curti A et al . Dendritic cells are functionally defective in multiple myeloma: the role of interleukin‐6. Blood 2002; 100: 230–7. [DOI] [PubMed] [Google Scholar]
- 47. Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK‐STAT signalling. Nat Cell Biol 2004; 6: 547–54. [DOI] [PubMed] [Google Scholar]
- 48. De Pooter RF, Schmitt TM, De La Pompa JL et al . Notch signaling requires GATA‐2 to inhibit myelopoiesis from embryonic stem cells and primary hemopoietic progenitors. J Immunol 2006; 176: 5267–75. [DOI] [PubMed] [Google Scholar]
- 49. Kumano K, Chiba S, Shimizu K et al . Notch1 inhibits differentiation of hematopoietic cells by sustaining GATA‐2 expression. Blood 2001; 98: 3283–9. [DOI] [PubMed] [Google Scholar]
- 50. Chojnaki A, Shimiazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates Notch 1 expression and activation in the self‐renewal of mammalian forebrain neural stem cells. J Neurosci 2003; 23: 1730–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suzuki T, Yokoyama Y, Kumano K et al . Highly efficient ex vivo expansion of human hematopoietic stem cells using Delta1‐Fc chimeric protein. Stem Cells 2006; 24: 2456–65. [DOI] [PubMed] [Google Scholar]


