Abstract
We have previously shown that overexpression of thymidylate synthetase (TS) resulted in pemetrexed (MTA) resistance. To investigate another mechanism of MTA resistance, we investigated the expression of ATP‐binding cassette (ABC)‐transporters in MTA‐resistant lung cancer cell lines and found that the gene and protein expression of ABCC11/MRP8 (ABCC11) was higher in MTA‐resistant cells than in the parental cells. The MTA resistant cells showed cross‐resistance to methotrexate (MTX), which is a substrate for ABCC11, and intracellular MTX accumulation in MTA‐resistant cells was lower than in the parental cells. We then tested the effect of decreasing the expression of ABCC11 by siRNA and found that decreased expression of ABCC11 enhanced MTA cytotoxicity and increased intracellular MTX accumulation in MTA‐resistant cells. These findings suggested that ABCC11 directly confers resistance to MTA by enhancing efflux of the intracellular anti‐cancer drug. Next, we analyzed the relationship between ABCC11 gene expression and MTA sensitivity of 13 adenocarcinoma cells, but there was no correlation. The ABCC11 gene has been shown to have a functional single‐nucleotide polymorphism (SNP), 538G>A. We then classified 13 lung adenocarcinoma cell lines into three groups based on the genotype of this ABCC11 SNP: G/G, G/A and A/A. The A/A group showed a significant reduction in the IC50 of MTA compared with the combined G/G and G/A groups, indicating that the SNP (538G>A) in the ABCC11 gene is an important determinant of MTA sensitivity. These results showed that ABCC11 may be one of the biomarkers for MTA treatment in adenocarcinomas. (Cancer Sci 2010; 101: 2404–2410)
Currently, several folate‐based antimetabolites are used for cancer treatment and have been studied intensively in clinical trials. Nearly 60 years ago, methotrexate (MTX) was the first antifolate used in oncology clinics that was shown to be active for the chemotherapeutic treatment of childhood acute lymphoblastic leukemia.( 1 ) Methotrexate and its polyglutamate forms are potent inhibitors of the dihydrofolate reductase (DHFR) enzyme that plays a key role in intracellular metabolism and is essential for DNA synthesis and cell growth.( 2 ) Recently, a new generation antifolate, pemetrexed (MTA) was approved for the treatment of malignant pleural mesothelioma and non‐small‐cell lung cancer (NSCLC).( 3 , 4 ) Pemetrexed is transported into the cell, predominantly via the reduced folate carrier (RFC), and is metabolized to polyglutamated forms. Pemetrexed was found to be one of the best substrates for mammalian folylpoly‐γ‐glutamate synthetase (FPGS), and it is believed that MTA polyglutamation and its polyglutamated metabolites play important roles in determining both the selectivity and antitumor activity of this agent. The polyglutamated metabolites of MTA are most active against thymidylate synthase (TS), followed by DHFR, glycinamide ribonucleotide formyltransferase (GARFT) and aminoimidazole carboxamide formyl transferase (AICARFT), and natural folate competes with this inhibition in all cases.( 5 , 6 ) The above characteristics distinguish MTA from the older generation antifolates. Furthermore, it was recently reported that treatment with MTA in combination with cisplatin provides a similar efficacy, with better tolerability and more convenient administration than gemcitabine with cisplatin, which has been approved as one of the first lines of treatment for advanced‐stage (stage IIIB or IV) NSCLC.( 4 ) Thus, MTA shows promise as a new treatment for lung cancer.
Recently, we showed that TS gene expression may have an important role in the acquired resistance and sensitivity to MTA in lung cancer.( 7 ) However, there are several other mechanisms of resistance to antifolate drugs, and the important factors for antifolate resistance including MTA remain to be fully elucidated. Decreased intracellular concentration of anti‐cancer agents is often indicative of drug resistance, and some ATP‐binding cassette (ABC) transporters including MRP1/ABCC1 (ABCC1), MRP2/ABCC2 (ABCC2), MRP3/ABCC3 (ABCC3), MRP4/ABCC4 (ABCC4), MRP5/ABCC5 (ABCC5), BCRP/ABCG2 (ABCG2) and MRP8/ABCC11 (ABCC11) play a role in the cellular efflux of drugs and may confer resistance to MTX.( 8 , 9 , 10 ) Consistent with this efflux capability and the substantially decreased cellular accumulation of MTX, some of these ABC transporters may also confer resistance to MTA. Therefore, we developed an in vitro model of acquired resistance to MTA and examined the association between ABC transporters and MTA resistance to examine the mechanism underlying its MTA resistance.
Materials and Methods
Cell lines and chemicals. The following 13 human lung adenocarcinoma cell lines were used in the present study: NCI‐H23; SK‐LC‐10; VMRC‐LCF; SK‐LC‐1; VMRC‐LCD; ACC‐LC‐314; ACC‐LC‐MS; ACC‐LC‐176; NCU‐LC‐201; ACC‐LC‐94; PC‐14; RERF‐LC‐MT; and PC‐9. Cells from a human small‐cell lung cancer (SCLC) cell line, PC‐6, were kindly provided by Daiichi Pharmaceuticals (Tokyo, Japan). Cells from the MTA‐resistant PC‐6 cells were established in our laboratory by continuous exposure to stepwise‐increasing concentrations of MTA as described previously.( 7 ) Finally, two sublines that grew in a medium containing 1.6 μM MTA were named PC‐6/MTA‐1.6 and PC‐6/MTA‐1.6‐2. A subline growing in a medium containing 4.0 μM MTA was also named PC‐6/MTA‐4.0. These cells were cultured in RPMI 1640 supplemented with 10% heat‐inactivated FBS and 1% (v/w) penicillin/streptomycin in a humidified chamber (37°C, 5% CO2). Before each experiment, cells were cultured in MTA‐free medium for 7 days. The MTA was obtained from Eli Lilly and Company (Indianapolis, IN, USA). The MTX was obtained from Wako Chemicals (Osaka, Japan). Etoposide and cisplatin were obtained from Bristol Myers (Tokyo, Japan). Docetaxel was obtained from Sanofi Aventis (Tokyo, Japan). Vinorelbine was obtained from Kyowa Hakko Kogyo (Tokyo, Japan).
Total RNA extraction and quantitative real‐time RT‐PCR. Total RNA was extracted using an RNeasy Mini kit (Qiagen, Alameda, CA, USA) according to the manufacturer’s instructions. Real‐time PCR was performed using the LightCycler FastStart DNA SYBR Green kit (Roche Diagnostics, Indianapolis, IN, USA). Melting curve analysis was used to control for specificity of the amplification products. The number of transcripts was calculated from a standard curve obtained by plotting the input of four different known transcript concentrations versus the PCR cycle number at which the detected fluorescence intensity reached a fixed value. The PCR program consisted of 45 cycles of 94°C for 15 s and 60°C for 1 min. For each sample, the data were normalized to those of the housekeeping gene glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH).
Intracellular MTX concentration. PC‐6 and PC‐6/MTA‐1.6 cells (1 × 107) were treated with 80 μM MTX for 1, 2 or 4 h. After washing five times with cold PBS, the cells were resuspended in RPMI (1.5 mL) and homogenized. After centrifugation, the supernatant was stored at −80°C until analysis. The intracellular MTX concentration was determined using a fluorescence polarization immunoassay (FPIA).( 11 )
Protein extraction and western blotting. Equal amounts of total cell lysates were solubilized in the sample buffer (50 mM Tris‐HCl [pH 6.8], 2% SDS, 1 mM EDTA, 10% glycerol), which contained Complete, Mini, Protease Inhibitor Cocktail Tablets and the PhosSTOP Phosphatase Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN, USA). Subsequently, these lysates were electrophoresed on a 10% Ready Gel Tris‐HCl Gel (Bio‐Rad Laboratories, Harculies, CA, USA) and transferred to Immobilon‐P filters (Millipore, Billerica, MA, USA). The filters were first incubated with primary antibodies for 3 h (ABCC11) or 1 h (α‐tubulin) at room temperature and then with horseradish peroxidase‐conjugated secondary antibodies (GE Healthcare Bioscience, Amersham Place, UK). The anti‐ABCC11 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and the anti‐α‐tubulin antibody was obtained from Sigma‐Aldrich (St Louis, MO, USA).
Concentration of MTA resulting in 50% cell survival (IC50). Cells were cultured at 5000 cells per well in 96‐well tissue culture plates. To assess cell viability, stepwise 10‐fold dilutions of the anticancer drugs were added 2 h after plating, and the cultures were incubated at 37°C for 96 h. At the end of the culture period, 20 μL 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium, inner salt solution (MTS) (CellTiter 96 Aqueous One Solution Cell Proliferation Assay; Promega, Madison, WI, USA) was added, and the cells were incubated for a further 4 h. Absorbance was then measured at 490 nm using an ELISA plate reader. Cellular chemosensitivity is expressed as the relationship between MTA concentration and its effect by using GraphPad Prism (version 4, GraphPad Software, La Jolla, CA, USA).
Transfection and small interfering RNA experiments. Cells were transfected with siRNA oligonucleotides using the siPORT NeoFX Transfection Agent (Ambion, Austin, TX, USA) for 24 h to produce a final RNA concentration of 50 nM in serum‐free Opti‐MEM (Invitrogen, Curlsbad, CA, USA). After transfection, total RNA was extracted, or the cells were cultured at 5000 per well in 96‐well tissue culture plates for 2 h and incubated for 48 h after adding stepwise dilutions of MTA. Finally, the absorbance was measured by MTS solution as described above. We also investigated the intracellular MTX concentration by FPIA in PC‐6/MTA‐1.6 cells (1 × 107) transfected with siRNA for 24 h after exposure to 80 μM MTX for 2 h as described above. The siRNA oligonucleotides for ABCC11 (predesigned siRNA, ID s39907 [namely siABCC11]) and the negative control siRNA (Silencer Negative Control 1 siRNA [namely siNEG]) were purchased from Ambion.
Genomic DNA extraction and detection of the 538(G>A) SNP in the ABCC11 gene. Genomic DNA was extracted from PC‐6, PC‐6/MTA‐1.6 and 13 adenocarcinoma cell lines using the QIAamp DNA Mini kit (Qiagen) according to the manufacturer’s instructions. The single‐nucleotide polymorphism (SNP) (538G>A) in the ABCC11 gene was detected using StepOnePlus Real‐Time PCR Systems (Applied Biosystems; Foster City, CA, USA) and Taqman Drug Metabolism Genotyping Assays (Assay ID; C_25999969, JSNP ID; rs17822931, Applied Biosystems) according to the manufacturer’s instructions.
Statistical analysis. Differences in gene expression level, intracellular MTX accumulation and the IC50 values for MTA between samples were evaluated using the Student’s unpaired t test. Correlation between expression of the ABCC11 gene and IC50 values for MTA was evaluated using Spearman’s test. The level of significance was set at 5% using two‐sided analysis.
Results
Characteristics of the MTA‐resistant cell lines. The sensitivities of PC‐6, PC‐6/MTA‐1.6, PC‐6/MTA‐1.6‐2 and PC‐6/MTA‐4.0 to a panel of chemotherapeutic drugs are summarized in Table 1. The IC50 for MTA was 32.5 times, 33.4 times and 436 times higher in the PC‐6/MTA‐1.6, PC‐6/MTA‐1.6‐2 and PC‐6/MTA‐4.0 cells, respectively, than in the parent PC‐6 cells. The MTA‐resistant cells showed cross‐resistance to MTX but not to the other four drugs tested: etoposide, docetaxel, vinorelbine and cisplatin.
Table 1.
The IC50 drug concentrations for a panel of chemotherapeutic drugs in MTA‐resistant and parental cells
| Cell lines | PC‐6 | PC‐6/MTA‐1.6 | PC‐6/MTA‐1.6‐2 | PC‐6/MTA‐4.0 | |||
|---|---|---|---|---|---|---|---|
| Drug | IC50(95% CI) | IC50(95% CI) | RR | IC50(95% CI) | RR | IC50(95% CI) | RR |
| Pemetrexed (μM) | 0.2253 (0.1689–0.3004) | 7.327 (4.156–12.92) | 32.52 | 7.513 (3.599–15.69) | 33.4 | 98.24 (31.11–310.2) | 436 |
| Methotrexate (nM) | 2.207 (1.244–3.303) | 5868 (1331–25880) | 2895 | 6542 (3591–11920) | 3227 | 21700 (8241–57150) | 9832 |
| Etoposide (nM) | 833.6 (542.1–1282) | 756.8 (537.8–1065) | 0.908 | 853.8 (581.4–1254) | 1.02 | 652.0 (502.9–845.3) | 0.782 |
| Docetaxel (nM) | 1.146 (0.4742–2.767) | 1.516 (0.5373–4.279) | 1.323 | 1.216 (0.3262–4.535) | 1.06 | 1.352 (0.7734–2.364) | 1.18 |
| Vinorelbine (pM) | 29.11 (18.96–44.69) | 19.08 (11.72–31.07) | 0.655 | 23.44 (14.13–38.87) | 0.81 | 36.00 (25.20–51.43) | 1.237 |
| Cisplatin (μM) | 30.19 (2.034–395.6) | 26.85 (9.783–73.68) | 0.889 | 46.82 (8.081–271.3) | 1.55 | 46.35 (14.33–150.0) | 1.535 |
MTA, pemetrexed; RR, resistance ratio: (IC50 in resistant subline)/(IC50 in the parental cells).
Expression levels of ABC transporters in MTA‐resistant cells. We examined the gene expression levels of drug efflux transporters of the ABC family by real‐time PCR. This analysis indicated that the gene expression of ABCC11 in PC‐6/MTA‐1.6 and PC‐6/MTA‐1.6‐2 cells was increased compared with that in the PC‐6 cells (Fig. 1; 3.6‐fold increase, P < 0.0001 and 3.1‐fold increase, P < 0.0001, respectively), whereas the expression of ABCG2, ABCC1, ABCC2, ABCC3 and ABCC5 was not different between these cells. The gene expression levels of the ABC family in PC‐6/MTA‐4.0 were not increased compared with that in the PC‐6 cells. Then, we further examined the expression level of the ABCC11 protein in PC‐6, PC‐6/MTA‐1.6 and PC‐6/MTA‐1.6‐2 by western blotting. Compared with the PC‐6 cells, the level of ABCC11 protein was up‐regulated in PC‐6/MTA‐1.6 and PC‐6/MTA‐1.6‐2 cells (Fig. 2). Because the level of ABCC11 protein was higher in the PC‐6/MTA‐1.6 cells than in the PC‐6/MTA‐1.6‐2 cells, we mainly used PC‐6/MTA‐1.6 in the following experiments.
Figure 1.
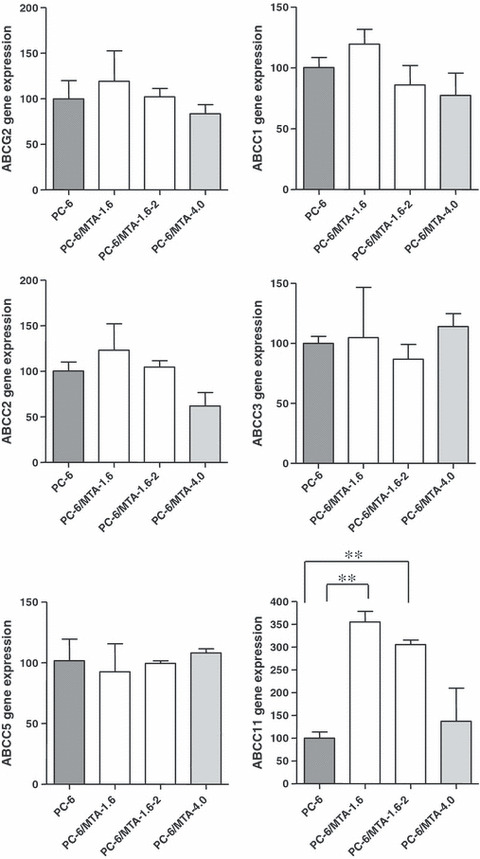
Gene expression levels of drug efflux transporters of the ABC family by real‐time PCR in PC‐6 cells and pemetrexed (MTA)‐resistant cell lines, PC‐6/MTA‐1.6, PC‐6/MTA‐1.6‐2 and PC‐6/MTA‐4.0 cells. Comparison of ABCG2, ABCC1, ABCC2, ABCC3, ABCC5 and ABCC11 gene expression levels. The expression level of each gene was determined by real‐time PCR. **P < 0.0001.
Figure 2.
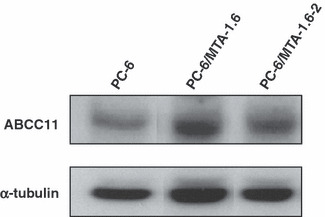
Comparison of ABCC11 protein levels between PC‐6, PC‐6/MTA‐1.6 and PC‐6/MTA‐1.6‐2 cells using western blotting. α‐Tubulin was assayed as a loading control.
Intracellular MTX concentration. We tried to determine whether a higher expression of ABCC11 transporter in the drug‐resistant cells resulted in a greater efflux of drugs and thereby a lower intracellular MTA concentration. We therefore assayed the intracellular accumulation of MTX, which is known to be a substrate for ABCC11( 12 ) similar to MTA in the PC‐6 and PC‐6/MTA‐1.6 cells using an FPIA. After treatment with 80 μM of MTX over a period of 4 h, intracellular MTX accumulation was lower in the PC‐6/MTA‐1.6 cells than in the parental PC‐6 cells at each time‐point assayed (Fig. 3).
Figure 3.

Comparison of the intracellular methotrexate (MTX) concentration between PC‐6 and PC‐6/MTA‐1.6 cell lines using the FPIA method. The intracellular MTX concentration was measured in PC‐6 and PC‐6/MTA‐1.6 cells after exposure to MTX for 1, 2 and 4 h, respectively. Open circle (○), PC‐6; open triangle (△), PC‐6/MTA‐1.6.
Inhibition of MTA cytotoxicity by ABCC11 siRNA. To further investigate the role of ABCC11 in the drug resistance of these cell lines, we assayed the effect of knockdown of ABCC11, using ABCC11 siRNA, on the cytotoxicity of MTA towards these cells. The PC‐6/MTA‐1.6 cells were transfected with siRNA directed against ABCC11, and control siRNA‐transfected or non‐transfected cells were used as controls. Cells were harvested at 24 h after transfection, and extracts were prepared and analyzed by quantitative real‐time RT‐PCR. The expression level of the ABCC11 gene was decreased at 24 h after transfection with siRNA against ABCC11 (Fig. 4a). We then tested if ABCC11 siRNA would enhance the cytotoxicity of MTA towards the PC‐6/MTA‐1.6 cells. The PC‐6/MTA‐1.6 cells were transfected with ABCC11 or negative control siRNA, or were not transfected. After 24 h, the cells were treated with MTA for 48 h and cell viability was assayed using a MTS assay. The MTA was more cytotoxic towards cells transfected with ABCC11 siRNA than towards cells transfected with control siRNA or toward non‐transfected cells (Fig. 4b). Since negative control siRNA does not affect gene expression, these results indicate that the decreased expression of ABCC11 induced by the siRNA altered the cytotoxicity of MTA towards these cells. The intracellular MTX concentration after treatment of 80 μM MTX for 2 h was also increased in the PC‐6/MTA‐1.6 cells transfected with ABCC11 siRNA compared with the cells transfected with negative control siRNA (Fig. 4c).
Figure 4.

Modification of pemetrexed (MTA) cytotoxicity by ABCC11 siRNA. (a) Gene expression levels of ABCC11 in PC‐6 and PC‐6/MTA‐1.6 cells transfected with siRNA directed against ABCC11 (siABCC11), control siRNA‐transfected (siNEG) or non‐transfected PC‐6/MTA‐1.6 cells. **P < 0.0001. NS, not significantly different. (b) Cytotoxicity to MTA was measured in PC‐6 and PC‐6/MTA‐1.6 cells transfected with siRNA (siABCC11 or siNEG) or non‐transfected. Closed circle (•), PC‐6; closed triangle (), PC‐6/MTA‐1.6; open triangle (△), PC‐6/MTA‐1.6 transfected with ABCC11 siRNA; open circle (○), PC‐6/MTA‐1.6 transfected with negative‐control siRNA. (c) The intracellular methotrexate (MTX) concentration was measured in PC‐6/MTA‐1.6 cells transfected with siRNA (siABCC11 or siNEG) after exposure to MTX for 2 h. *P < 0.05.
Relationship between ABCC11 and lung adenocarcinoma. Because MTA shows efficacy to NSCLC, especially lung adenocarcinoma clinically,( 4 ) we further studied the relationship among ABCC11 expression levels in 13 lung adenocarcinoma cell lines. These cell lines were analyzed for ABCC11 expression using real‐time PCR and the level of ABCC11 expression was compared with the IC50 values for MTA in each cell line. At first, we examined the relationship between the ABCC11 gene and MTA cytotoxicity, but correlation was not observed in this study (Fig. 5). It was recently reported that the ABCC11 gene has a nonsynonymous SNP 538G>A (rs17822931; Gly180Arg), which is important for determination of the type of human earwax that is expressed.( 13 ) Because cells with allele G show higher excretory activity for ABCC11 substrates cells with allele A, the G/G and G/A genotypes correspond to wet earwax and the A/A genotype corresponds to dry earwax.( 14 ) PC‐6 and PC‐6/MTA‐1.6 have been shown to have the G/A genotype that exhibits higher excretory activity. To determine if the ABCC11 SNP 538G>A might play a role in the drug sensitivity of cancer cells, we classified 13 adenocarcinoma cell lines into three groups (G/G, G/A and A/A) based on the genotype of ABCC11 SNP 538 that was present in each cell line. We then compared the distribution of the IC50 values for MTA within each group Table 2. Four of the cell lines had a G/G genotype, two cell lines had a G/A genotype and seven cell lines had an A/A genotype. The IC50 for MTA showed no significant difference between the three groups (Fig. 6a). However, a comparison of the combined G/G and G/A groups with the A/A group did show a significant difference (Fig. 6b).
Figure 5.
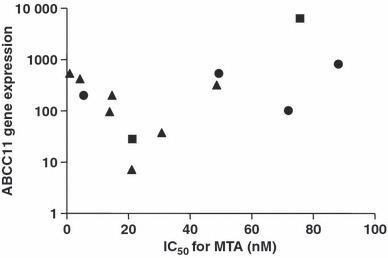
Relationship between IC50 values for pemetrexed (MTA) and ABCC11 gene expression in 13 lung adenocarcinoma cell lines. Each symbol refers to the genotype of ABCC11. Closed circle (•), 538G/G; closed square (), 538G/A; closed triangle (), 538A/A. No significant correlation was observed between the ABCC11 gene expression levels and MTA sensitivity (n = 13, r = 0.2418, P = 0.4262).
Table 2.
The status of ABCC11 SNP538 and IC50 for MTA in 13 adenocarcinoma cell lines
| SNP538 | IC50 for MTA (nM) | 95% CI | |
|---|---|---|---|
| NCI‐H23 | G/G | 72.16 | 45.08–115.5 |
| SK‐LC‐10 | G/G | 5.648 | 4.175–7.639 |
| VMRC‐LCF | G/G | 88.38 | 36.94–211.4 |
| SK‐LC‐1 | G/G | 49.48 | 36.51–67.06 |
| VMRC‐LCD | G/A | 21.39 | 14.78–30.96 |
| ACC‐LC‐314 | G/A | 75.88 | 49.16–117.1 |
| ACC‐LC‐MS | A/A | 31.01 | 19.39–49.60 |
| ACC‐LC‐176 | A/A | 1.102 | 0.8365–1.453 |
| NCU‐LC‐201 | A/A | 4.394 | 3.552–5.436 |
| ACC‐LC‐94 | A/A | 21.19 | 16.87–26.63 |
| PC‐14 | A/A | 14.74 | 11.14–19.51 |
| RERF‐LC‐MT | A/A | 48.94 | 33.91–70.63 |
| PC‐9 | A/A | 14.01 | 12.51–15.69 |
MTA, pemetrexed.
Figure 6.
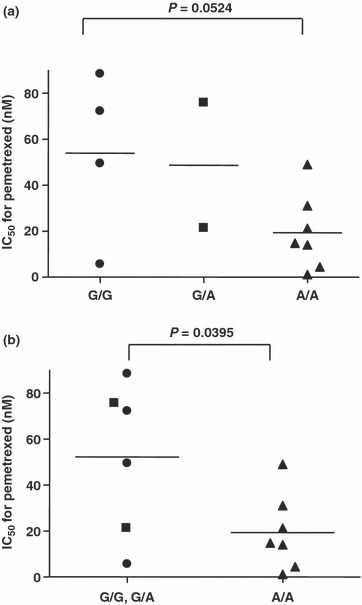
Distribution of IC50 values for pemetrexed (MTA) in 13 lung adenocarcinoma cell lines classified as ABCC11 538(G>A) SNP types. Closed circle (•), 538G/G; closed square (), 538G/A; closed triangle (), 538A/A. (a) Comparison of three genotype groups: 538G/G, 538G/A and 538A/A. (b) Comparison of the combined 538G/G and 538G/A groups and 538A/A group.
Discussion
In the present study, we found that increased gene expression of ABCC11 directly confers resistance to MTA in MTA‐resistant cells. Furthermore, we found that the SNP (538G>A) in ABCC11 plays a role in MTA sensitivity in lung adenocarcinoma cell lines. This is the first report that ABCC11 and the ABCC11 SNP determines MTA efficacy in lung cancer cells.
The ABC transporters play a biologically important role as membrane transporters or ion channel modulators.( 15 ) ABCC1 through to ABCC12 belong to the ABCC family, which is one subfamily of the ABC transporters.( 16 , 17 ) These transporters confer drug resistance by effluxing anticancer agents or their metabolites from cells.( 17 ) ABCC11 was recently identified and we previously showed that ABCC11 confers 5‐fluorouracil (5‐FU) resistance on a lung cancer cell line by enhancing the efflux for the active metabolite fluorodeoxyuridine monophosphate (FdUMP).( 9 , 18 , 19 , 20 , 21 ) However, little is known regarding the ability of ABCC11 to mediate the transport of other anti‐cancer agents. In this study, we compared the expression levels of the ABC transporter gene in MTA‐resistant cells with that in PC‐6 cells to determine which transporter contributes resistance to MTA. Compared with parental cells, only ABCC11 gene expression was significantly increased in PC‐6/MTA‐1.6 and PC‐6/MTA‐1.2 cells, and we therefore focused on the ABCC11 gene. We found that MTX accumulation was reduced in PC‐6/MTA‐1.6 cells, concomitant with the increased expression of ABCC11. In addition, modification of ABCC11 gene expression by ABCC11 siRNA in PC‐6/MTA‐1.6 cells altered the cytotoxicity of MTA and intracellular MTX concentration. These data suggest that MTA or its metabolites might be a substrate of the ABCC11 transporter and also that ABCC11 overexpression might be one of the determinants for acquired resistance to MTA. Interestingly, we found that ABCC11 expression was not increased in higher resistant PC‐6/MTA‐4.0 cells. Previously, we have shown the overexpression of TS in PC‐6/MTA‐4.0 cells, but not in PC‐6/MTA‐1.6 cells.( 7 ) It has also been reported that TS gene expression is higher in squamous cell carcinoma compared with adenocarcinoma( 22 ) and cisplatin/pemetrexed therapy is not effective in patients with squamous cell carcinoma histology compared with adenocarcinoma.( 3 ) These results indicate that TS is the most powerful molecule for resistance to MTA, while ABCC11 is associated with MTA resistance when TS expression is at low levels.
Once taken up into cells via the RFC, antifolates including MTA undergo polyglutamylation catalyzed by FPGS. This polyglutamate conjugation results in cellular retention of antifolates and inhibits transporter‐mediated antifolate efflux. ABCC1 through to ABCC5, as well as ABCG2, have the facility to transport antifolates including MTX.( 23 ) ABCC1 through to ABCC4 only extrude the monoglutamate form of MTX, and di‐ or longer chain polyglutamates are not transported at all. However, ABCC5 can extrude the di‐glutamate form and even the mono‐glutamate form( 24 ) and, moreover, ABCG2 can extrude the mono‐, di‐ and tri‐glutamate forms.( 10 , 25 ) Based on these data, it has been considered that ABCC5 and ABCG2 contribute mainly to the efflux of antifolate and its polyglutamates. However, in this study, in contrast to ABCC11, the gene expression of ABCC5 and ABCG2 was not increased in MTA‐resistant cells compared with the parental cells. It has been reported that during a 1–4 h antifolate pulse exposure, no substantial antifolate polyglutamylation occurs.( 26 ) In this respect, we found that the intracellular MTX concentration in the MTA‐resistant cells was dramatically lower than that in the parental cells after 4 h exposure to an antifolate (Fig. 3). This result suggests that ABCC11 does have a potent ability to extrude non‐glutamated or mono‐glutamated MTA in the early phase of drug treatment. However, it is unknown whether ABCC11 also has the ability to extrude polyglutamate forms, as do ABCC5 and ABCG2.
Given the importance of ABCC11 expression for MTA‐acquired resistance in cancer cells, we then analyzed the relationship between the levels of ABCC11 gene expression and natural resistance to MTA in lung cancer cells. However, there was no correlation between the IC50 value of adenocarcinoma cell lines for MTA and the levels of ABCC11 expression (Fig. 5). The SNP (538G>A) in the ABCC11 gene is known to determine the characteristics of human earwax and it has been reported that earwax type may be related to colostrum secretion, axillary osmidrosis and breast cancer risk.( 27 , 28 , 29 , 30 ) Earwax type, and this SNP in ABCC11, also show geographical gradient distributions.( 13 ) For example, the dry type is seen frequently (80–95%) among East Asians but is uncommon (0–3%) among Europeans and people of African origin, and is observed at intermediate (30–50%) frequencies in native North Americans. Our results indicate that the IC50 value for MTA was significantly different for the combined G/G and G/A groups and the A/A group in NSCLC cell lines because G/G or G/A SNP‐type ABCC11 transporters can extrude MTA, but the A/A type cannot.( 14 ) These data suggest that the SNP (538G>A) in ABCC11 might play an important role in cellular MTA sensitivity. It was recently reported that, in a subgroup analysis of MTA‐cisplatin as a first‐line treatment for advanced NSCLC, this therapy was more efficacious in East Asian patients than in all other cases.( 31 ) Since most East Asians have dry earwax, which is dependent on the A/A SNP‐type of ABCC11, this finding also supports our hypothesis of the importance of the ABCC11 SNP‐genotype in the regulation of MTA sensitivity. However, whether ABCC11 expression and/or the ABCC11 genotype is important still remains to be elucidated. Additional investigation will be needed to confirm our hypothesis. Furthermore, ABCC11 is highly expressed in the liver( 19 ) and may have a role in drug metabolisms, so the relationship among pharmacogenetics, pharmacokinetics and pharmacodynamics should be examined clinically as SNP of ABCG2 highly expressed in the liver is associated with diarrhea in gefitinib treatment.( 32 )
The number of lung cancer patients has increased in recent years and the use of MTA as a multi‐targeting drug is very important for therapy of these tumors. In future, the relationship between the SNP (538G>A) in the ABCC11 genotype and the efficacy rate of MTA chemotherapy for patients with lung cancer should be investigated in clinical studies. It will also be important to confirm if ABCC11 might be useful as a biomarker of MTA sensitivity in lung cancer.
Acknowledgment
This work was supported by a grant from the program for developing the supporting system for upgrading education and research from the Ministry of Education, Culture, Sports, Science and Technology‐Japan. We thank Shino Nakamura for her skillful technical assistance.
References
- 1. Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4‐aminopteroyl‐glutamic acid. N Engl J Med 1948; 238: 787–93. [DOI] [PubMed] [Google Scholar]
- 2. Chen MJ, Shimada T, Moulton AD et al. The functional human dihydrofolate reductase gene. J Biol Chem 1984; 259: 3933–43. [PubMed] [Google Scholar]
- 3. Vogelzang NJ, Rusthoven JJ, Symanowski J et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003; 21: 2636–44. [DOI] [PubMed] [Google Scholar]
- 4. Scagliotti GV, Parikh P, Von Pawel J et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy‐naive patients with advanced‐stage non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 3543–51. [DOI] [PubMed] [Google Scholar]
- 5. Shih C, Chen VJ, Gossett LS et al. LY231514, a pyrrolo[2,3‐d]pyrimidine‐based antifolate that inhibits multiple folate‐requiring enzymes. Cancer Res 1997; 57: 1116–23. [PubMed] [Google Scholar]
- 6. Shih C, Habeck LL, Mendelsohn LG, Chen VJ, Schultz RM. Multiple folate enzyme inhibition: mechanism of a novel pyrrolopyrimidine‐based antifolate LY231514 (MTA). Adv Enzyme Regul 1998; 38: 135–52. [DOI] [PubMed] [Google Scholar]
- 7. Ozasa H, Oguri T, Uemura T et al. Significance of thymidylate synthase for resistance to pemetrexed in lung cancer. Cancer Sci 2009; 101: 161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hooijberg JH, De Vries NA, Kaspers GJ, Pieters R, Jansen G, Peters GJ. Multidrug resistance proteins and folate supplementation: therapeutic implications for antifolates and other classes of drugs in cancer treatment. Cancer Chemother Pharmacol 2006; 58: 1–12. [DOI] [PubMed] [Google Scholar]
- 9. Guo Y, Kotova E, Chen ZS et al. MRP8, ATP‐binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines 2′,3′‐dideoxycytidine and 9′‐(2′‐phosphonyl‐methoxyethyl)adenine. J Biol Chem 2003; 278: 29509–14. [DOI] [PubMed] [Google Scholar]
- 10. Chen ZS, Robey RW, Belinsky MG et al. Transport of methotrexate, methotrexate polyglutamates, and 17beta‐estradiol 17‐(beta‐D‐glucuronide) by ABCG2: effects of acquired mutations at R482 on methotrexate transport. Cancer Res 2003; 63: 4048–54. [PubMed] [Google Scholar]
- 11. Pesce MA, Bodourian SH. Evaluation of a fluorescence polarization immunoassay procedure for quantitation of methotrexate. Ther Drug Monit 1986; 8: 115–21. [DOI] [PubMed] [Google Scholar]
- 12. Chen ZS, Guo Y, Belinsky MG, Kotova E, Kruh GD. Transport of bile acids, sulfated steroids, estradiol 17‐beta‐D‐glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11). Mol Pharmacol 2005; 67: 545–57. [DOI] [PubMed] [Google Scholar]
- 13. Yoshiura K, Kinoshita A, Ishida T et al. A SNP in the ABCC11 gene is the determinant of human earwax type. Nat Genet 2006; 38: 324–30. [DOI] [PubMed] [Google Scholar]
- 14. Toyoda Y, Sakurai A, Mitani Y et al. Earwax, osmidrosis, and breast cancer: why does one SNP (538G>A) in the human ABC transporter ABCC11 gene determine earwax type? FASEB J 2009; 23: 2001–13. [DOI] [PubMed] [Google Scholar]
- 15. Klein I, Sarkadi B, Váradi A. An inventory of the human ABC proteins. Biochim Biophys Acta 1999; 1461: 237–62. [DOI] [PubMed] [Google Scholar]
- 16. Bryan J, Aguilar‐Bryan L. The ABCs of ATP‐sensitive potassium channels: more pieces of the puzzle. Curr Opin Cell Biol 1997; 9: 553–9. [DOI] [PubMed] [Google Scholar]
- 17. Haimeur A, Conseil G, Deeley RG, Cole SP. The MRP‐related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab 2004; 5: 21–53. [DOI] [PubMed] [Google Scholar]
- 18. Yabuuchi H, Shimizu H, Takayanagi S, Ishikawa T. Multiple splicing variants of two new human ATP‐binding cassette transporters, ABCC11 and ABCC12. Biochem Biophys Res Commun 2001; 288: 933–9. [DOI] [PubMed] [Google Scholar]
- 19. Bera TK, Lee S, Salvatore G, Lee B, Pastan I. MRP8, a new member of ABC transporter superfamily, identified by EST database mining and gene prediction program, is highly expressed in breast cancer. Mol Med 2001; 7: 509–16. [PMC free article] [PubMed] [Google Scholar]
- 20. Tammur J, Prades C, Arnould I et al. Two new genes from the human ATP‐binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene 2001; 273: 89–96. [DOI] [PubMed] [Google Scholar]
- 21. Oguri T, Bessho Y, Achiwa H et al. MRP8/ABCC11 directly confers resistance to 5‐fluorouracil. Mol Cancer Ther 2007; 6: 122–7. [DOI] [PubMed] [Google Scholar]
- 22. Ceppi P, Volante M, Saviozzi S et al. Squamous cell carcinoma of the lung compared with other histotypes shows higher messenger RNA and protein levels for thymidylate synthase. Cancer 2006; 107: 1589–96. [DOI] [PubMed] [Google Scholar]
- 23. Assaraf YG. The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat 2006; 9: 227–46. [DOI] [PubMed] [Google Scholar]
- 24. Wielinga P, Hooijberg JH, Gunnarsdottir S et al. The human multidrug resistance protein MRP5 transports folates and can mediate cellular resistance against antifolates. Cancer Res 2005; 65: 4425–30. [DOI] [PubMed] [Google Scholar]
- 25. Volk EL, Schneider E. Wild‐type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Res 2003; 63: 5538–43. [PubMed] [Google Scholar]
- 26. Spinella MJ, Brigle KE, Freemantle SJ, Sierra EE, Goldman ID. Comparison of methotrexate polyglutamylation in L1210 leukemia cells when influx is mediated by the reduced folate carrier or the folate receptor Lack of evidence for influx route‐specific effects. Biochem Pharmacol 1996; 52: 703–12. [DOI] [PubMed] [Google Scholar]
- 27. Miura K, Yoshiura K, Miura S et al. A strong association between human earwax‐type and apocrine colostrum secretion from the mammary gland. Hum Genet 2007; 121: 631–3. [DOI] [PubMed] [Google Scholar]
- 28. Yoo WM, Pae NS, Lee SJ, Roh TS, Chung S, Tark KC. Endoscopy‐assisted ultrasonic surgical aspiration of axillary osmidrosis: a retrospective review of 896 consecutive patients from 1998 to 2004. J Plast Reconstr Aethet Surg 2006; 59: 978–82. [DOI] [PubMed] [Google Scholar]
- 29. Petrakis NL. Cerumen phenotype and epithelial dysplasia in nipple aspirates of breast fluid. Am J Phys Anthropol 1983; 62: 115–8. [DOI] [PubMed] [Google Scholar]
- 30. Petrakis NL. Cerumen genetics and human breast cancer. Science 1971; 173: 347–9. [DOI] [PubMed] [Google Scholar]
- 31. Orlando M, Lee JS, Yang C et al. Efficacy of pemetrexed‐cisplatin (PC) in East Asian patients: subgroup analysis of a phase III study comparing PC vs gemcitabine‐cisplatin (GC) in first‐line treatment of advanced non‐small cell lung cancer (NSCLC). J Clin Oncol 2009; 27(15 Suppl): A8045. [Google Scholar]
- 32. Cusatis G, Gregorc V, Li J et al. Pharmacogenetics of ABCG2 and adverse reactions to gefitinib. J Natl Cancer Inst 2006; 98: 1739–42. [DOI] [PubMed] [Google Scholar]


