Abstract
Dkk‐3 is proposed to be a new specific marker for tumor endothelial cells. Here we analyzed the clinical relevance of Dkk‐3 expression in pancreas adenocarcinomas and determined its role on endothelial cell growth in vitro. Microvessel density in tumor samples was immunohistochemically determined using Dkk‐3 and CD31 as endothelial cell markers, respectively. Based on the median microvessel density as a cut‐off point, patients were categorized into high and low microvessel density groups and a correlation with survival and clinical parameters was assessed. Moreover, the role of Dkk‐3 expression on chemosensitivity of endothelial cells was analyzed. In contrast to CD31 staining, Dkk‐3‐positive vessels were found only in tumor tissue and Dkk‐3 microvessel density significantly correlated negative with tumor grading. In survival analysis the median survival time was 7 months for patients with Dkk‐3 low, and 15 months for Dkk‐3 high microvessel density (P = 0.0013). Subset analysis of patients receiving gemcitabine therapy showed that overall survival was significantly decreased in Dkk‐3 low tumors than in high tumors (P = 0.009). In Cox regression Dkk‐3 emerged as a significant independent parameter (P = 0.024). Dkk‐3 overexpression in endothelial cells resulted in significantly enhanced growth inhibition after 5‐fluorouracil or gemcitabine treatment compared to control endothelial cells and cancer cell lines. Dkk‐3 low microvessel density was associated with tumor progression and worse clinical outcome. Overexpression of Dkk‐3 enhanced endothelial cell growth inhibition to chemotherapeutic drugs. Therefore, we suggest that Dkk‐3 high microvessel density may help to select patients who may benefit from chemotherapy. (Cancer Sci 2009)
In spite of significant advances which have been made in improving the safety of its resection, pancreatic cancer still has an extremely poor prognosis with an overall 1‐ and 5‐year survival of 12% and 1%, respectively, whereas the median survival remains to be 6 months.( 1 , 2 ) For the majority of patients, curative resections are not possible due to the lack of an effective early screening test.( 3 ) In addition, cell resistance to cytotoxic agents and radiation are two other factors accounting for the poor prognosis of pancreatic cancer.( 4 , 5 ) Therefore, new potential biomarkers predicting therapy response as well as for therapeutic target structures in pancreatic cancer are urgently needed.
After tumors reach a size of approximately 1–2 mm3, growth, spread, and metastasis are strictly dependent on angiogenesis.( 6 ) Angiogenesis is therefore of key importance for the progression of most solid tumors and may be used as a potential prognostic marker. In the tumor microenvironment, proangiogenic factors prevail over antiangiogenic activities generating a proangiogenic response, which results in an increased blood vessel growth.( 7 , 8 ) However, during tumor‐induced angiogenesis endothelial cells develop morphological abnormalities as well as altered expression of proteins which can be used as specific marker for diagnostic purposes. In a study comparing gene expression patterns of endothelial cells derived from blood vessels of normal and malignant colorectal tissue, Dkk‐3 was shown to be one of the genes expressed at substantially higher levels in tumor endothelium than in normal endothelium.( 9 )
Dkk‐3 is a member of the dickkopf family of secreted modulators of the Wnt signaling pathway which consists of four different genes in vertebrates (Dkk‐1/2/3/4).( 10 ) Dkk‐3 has been proposed to act as a tumor suppressor, as it is down‐regulated in a number of different tumors and its overexpression suppresses tumor growth in vitro.( 10 ) However, Dkk‐3 knock‐out mice showed no enhanced tumor formation.( 11 )
We have previously shown that Dkk‐3 expression is highly up‐regulated in tumor vessels of various tumors but not or low expressed in matched normal tissue, suggesting the importance of Dkk‐3 in tumor vessel biology.( 12 ) Here we address whether Dkk‐3 is also expressed in tumor endothelial cells in pancreatic cancer and whether the expression is correlated with tumor angiogenesis and tumor progression analyzed in a retrospective study on 154 tumor samples. Furthermore, we analyzed the importance of Dkk‐3 expression on endothelial cell growth after chemotherapeutic treatment in vitro.
Materials and Methods
Cell culture and determination of dose–response curve. Human umbilical vein endothelial cells (HUVEC) were obtained from Clonetics and cultivated according to the supplier's instructions (Cambrex Bio Science, Walkersville, IA, US). Human pancratic cancer cell lines Capan‐1 and Panc‐1 were obtained from ATCC‐LGC (Wesel, Germany) and cultivated according to the supplier's instructions. Generation of recombinant hDkk‐3 adenovirus was described previously.( 12 ) For determination of the dose–response curve, HUVEC were transfected with hDkk‐3 and control adenovirus, respectively. After 24 h cells were seeded in 96‐well plates at a density of 4000 cells/well and allowed to adhere overnight before the medium was changed and 5‐fluorouracil or gemcitabine were added. After 48‐h cell growth was determined using the cell counting kit‐8 (CCK8; Dojindo, Gaithersburg, MD, US). Pancreatic cancer cell lines were plated in triplicate in 96‐well pates at a density of 4000 cells/well and exposed to 5‐fluorouracil or gemcitabine at different concentrations for 72 h. Percent growth relative to untreated controls was calculated based on the CCK8 read‐out and IC50 values were defined as the concentration of drug that produced 50% reduction in control absorbance. Calculations of dose–response curves were done using Prim‐5 (GraphPad, La Jolla, CA, US).
Western blot analysis. Cells were harvested and lyzed as described previously.( 13 ) Total protein (20 µg) was denaturated, separated on 10% polyarcylamid gel, and transferred on an Immuno‐BlotTM polyvinylidene difluoride (PVDF) membrane (Bio‐Rad, Hercules, CA, USA). After blocking with 3% skim milk powder dissolved in PBS, the membrane was probed with polyclonal antihuman Dkk‐3 antiserum (0.1 µg/mL; R&D systems, Minneapolis, MN, USA) or antitubulin antibody (1:1000 dilution; Sigma, Taufkirchen, Germany) for 1–2 h, respectively. After additional washing steps, the membrane was incubated with HRP‐conjugated rabbit‐antigoat IgG (1:2500 dilutions) for 1 h. Signals were detected by chemiluminescence (Pierce, Rockford, IL, USA) using a CL Hyperfilm (Amersham, Vienna, Austria).
Patient and tissue samples. The study was conducted according to the regulations of the local ethics committee and Austrian law. A total number of 154 patients with pancreatic ductal adenocarcinoma diagnosed between 1987 and 2003 at the Department of Pathology, Medical University of Innsbruck, were included in this retrospective study (Table 1). All tumors were retrospectively reclassified on hematoxylin–eosin stained slides, and histological type and tumor grade were reassessed by a pathologist using standard diagnostic criteria. Clinical data were obtained by reviewing the charts and, if available, contacting the treating physicians. Tumors were histologically classified according to the World Health Organization classification and staged according to the International Union Against Cancer tumor‐node‐metastasis classification (UICC).( 14 ) A subset of 66 patients received standard gemcitabine‐based chemotherapy according to their clinical stage and performance status. Overall survival time was defined by the period from the date of initial diagnosis to the date of death or last contact. Normal pancreatic tissues were analyzed using a commercially available tissue microarray of normal pancreatic tissues obtained from US Biomax (Rockville, MD, USA).
Table 1.
Patient characteristics
| Variable | Pancreatic cancern = 154 (%) |
|---|---|
| Sex | |
| Male | 84 (54) |
| Female | 70 (46) |
| Age | |
| Mean | 66 |
| Median | 67 |
| Range | 37–86 |
| Stage (UICC) | |
| Ia, Ib | 30 (19) |
| IIa, IIb | 64 (42) |
| III | 10 (7) |
| IV | 14 (9) |
| Unknown | 36 (23) |
| Median overall survival | |
| Months | 7 |
| Range | 1–134 |
| Primary surgical treatment | |
| Curative resection | 62 (40) |
| Palliative surgery | 62 (40) |
| Inoperable | 30 (20) |
| Dkk‐3 pos. vessels | |
| MVD < 25 | 77 (50) |
| MVD > 25 | 77 (50) |
Immunohistochemical determination of DKK 3 and CD31. Sections were deparaffinized and endogenous peroxidase was blocked with 2% H2O2 (Scharlau, Barcelona, Spain). Microwave treatment in 10 mM citrate buffer (pH 6.0; Dako, Vienna, Austria) was done for antigen retrieval. Non‐specific binding was blocked by incubating the sections with 3% bovine serum albumin (BSA) buffer (Sigma, Vienna, Austria) for 1 h at room temperature before primary and secondary antibody incubation. The primary antibodies goat‐antihuman Dkk‐3 (1 µg/mL; R&D Systems) and mouse antihuman CD31 (1:40; DakoCytomation, Glostrup, Denmark) were incubated for 1 h at 37°C in a humidified chamber. Biotinylated donkey‐antigoat IgG (1:150; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and biotinylated horse‐antimouse IgG (1:200; Vector Laboratories, Burlingame, CA, USA) were used as secondary antibodies. Signal detection was performed with the Vectastain Elite ABC‐Kit (Vector Laboratories) and 3,3′diaminobenzidine (DAB) as substrate (Sigma‐Aldrich, Steinheim, Germany). Slides were counterstained with hematoxylin II (Merck, Darmstadt, Germany).
Blocking controls were performed identically, except for the addition of native Dkk‐3 protein( 13 ) to the primary antibody (10:1), 1 h before application of the antibody solution. Negative controls without primary antibody were included in each run. Antigen expression was defined as the presence of specific staining of cytoplasm of endothelial cells for Dkk‐3 and CD31, respectively. The intratumor microvessel density (MVD) was determined using 3–6 high power fields from each tissue section (magnification 200×). Each field was photographed and stained microvessels with detectable lumen (“lumen method”) were included in the microvessel count as described earlier.( 15 ) The intratumor microvessel counts were assessed by two independent investigators (A.D. and D.P.) without knowledge of patient outcomes. The mean from at least three fields was taken as the final count for that slide and as representative for statistical analysis.
Immunofluorescence and confocal microscopy. After deparaffination the sections were washed in PBS, and non‐specific binding was blocked with PBS/3% BSA for 1 h at room temperature. The following primary antibodies were used: goat‐antihuman Dkk‐3 (1:100; R&D Systems) and guinea pig‐antihuman CD31 (1:80; Abcam, Cambridge, UK). Diluted in 1% BSA/PBS, the antibodies were incubated for 1 h at 37°C in a humidified chamber. The sections were washed and blocked again. The secondary antibodies were incubated for 1 h at 37°C: Rabbit‐antigoat‐rhodamine (1:100; Chemicon, Temecula, CA, USA) and rabbit‐antiguinea pig‐FITC (1:40; Dako). Confocal microscopy was performed with an UltraVIEW RS (Perkin Elmer, Wellesey, MA, USA) mounted on an Olympus IX‐70 inverse microscope (Olympus, Nagano, Japan). The mean vessel count from at least three high power fields was taken as the final count for that slide and as representative for statistical analysis.
Statistical methods. All calculations and statistical analyses were performed using SPSS for Windows. A contingency table χ2‐test was performed to determine a possible association between Dkk‐3, CD31 expression and age, sex, nodal status, stage, and histological grade of tumor. Kaplan–Meier curves were plotted to assess overall survival. Different survival curves were compared using the log‐rank test. Follow‐up time was censored if the patient was lost to follow‐up. Cox regression multivariate analysis was performed to examine whether Dkk‐3 was an independent prognostic factor. Analysis of the dose–response curve was done with Prism 5 for windows, and for comparison of dose–response curves the exact sum‐of‐square F‐test was used. For all analyses, a P‐value < 0.05 was defined as statistically significant.
Results
Expression of Dkk‐3 and CD31. The expression pattern of Dkk‐3 and CD31 was investigated on parallel tissue sections, and 154 samples were evaluated for Dkk‐3 and 108 samples for CD31 expression. Based on the analysis of Dkk‐3 expression, patients were categorized at a cut‐off point of the median of 25 vessels per high power field (n = 77, each group). Tumors with >25 vessels per high power field were classified as having Dkk‐3 high and <25 as having Dkk‐3 low vascularity. Using CD31 staining, the median MVD cut‐off point was 44 positive vessels per high power field (n = 54, each group). The median was chosen as the cut‐off point to ensure sufficient samples in each group for valid statistical analysis.
Dkk‐3 was strongly expressed in vessels of tumor tissue and no staining was found in vessels of tumor adjacent normal tissue or in healthy normal pancreas tissue. Representative immunohistochemical staining results are shown in Figure 1.
Figure 1.
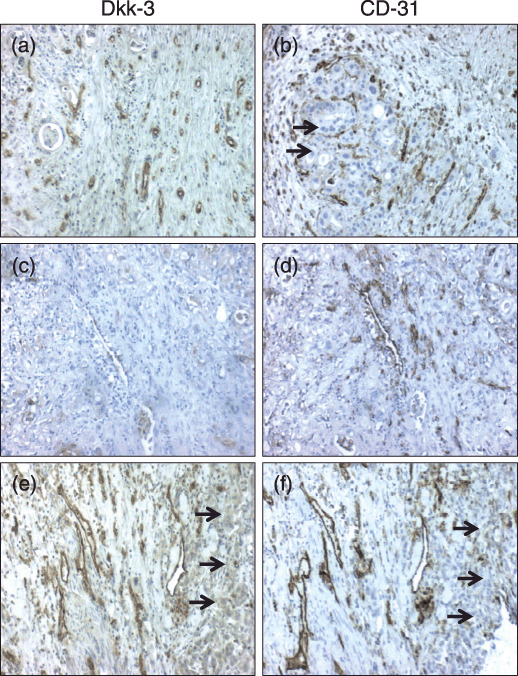
Immunohistochemical validation of Dkk‐3 and CD‐31 presence on pancreatic adenocarcinoma. Shown are representative tissue sections stained with antibodies against Dkk‐3 (a,c,e) and CD‐31 (b,d,f). Tumor vessels stained positive for Dkk3 and CD31 are shown in (a) and (b). However, not all CD31‐positive vessels were also Dkk‐3‐positive and vice versa (c,d). Tumor epithelial tissue was positive for Dkk‐3 (e, arrows) but not for CD31 staining (a,f, arrows).
Moreover, we found that not all CD31‐positive vessels were also positive for Dkk‐3 (Fig. 1c,d). Additionally, in tumor epithelial cells we found Dkk‐3 expression in 26 cases (16.9%) (Fig. 1e; arrows), but no CD31 expression (Fig. 1a,f). However, tumor Dkk‐3 expression was not significantly correlated to clinicopathological parameters (data not shown). Additionally, and as shown previously,( 13 ) vessels of normal pancreatic tissue were negative for Dkk‐3 but not for CD31 staining (not shown).
An interesting observation was the higher MVD for CD31 than for Dkk‐3 (45.99 ± 24.02 vs 31.98 ± 26.05; mean ± SD) (Fig. 2a). Correlation analysis performed between the two markers showed no significant combination (Fig. 2b), which may indicate different endothelial cell (EC) phenotypes or EC functions which can be distinguished by these markers. In order to determine the correlation between CD31‐ and Dkk‐3‐positive tumor vessels, more accurate double immunofluorescence staining on randomly selected cases of pancreatic cancer tissue sections (n = 38) was performed. This analysis revealed that CD31 and Dkk‐3 colocalize in double positive vessels and are expressed in the cytosol. Additionally, CD31 was expressed at the EC surface as indicated by the green signal at the luminal side of the blood vessels (arrows in Fig. 3c). Single positive blood vessels for Dkk‐3 (Fig. 3d,e) or CD31 (not shown) were found in tumor tissue, whereas CD31‐positive vessels were exclusively found in normal pancreas tissue (Fig. 3e,f). Correlation analysis performed on this data set showed no significant correlation between CD31 and Dkk‐3 vessel number (R 2 = 0.061; P = 0.167) supporting the data of vessel counts obtained from parallel sections.
Figure 2.
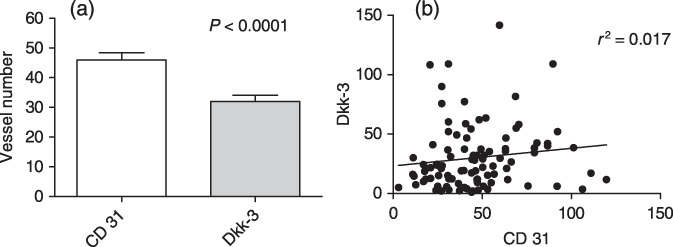
Comparison of Dkk‐3‐ and CD31‐positive vessel counts in pancreas carcinoma. (a) Vessel number per high power field was higher in CD31 stained tumors than in Dkk‐3 stained tumors (45.99 ± 24.02 vs 31.98 ± 26.05; mean ± SD). (b) Correlation analysis of CD31‐ and Dkk‐3‐positive vessel number, as determined on parallel tissue sections, revealed no significant correlation between these two markers.
Figure 3.
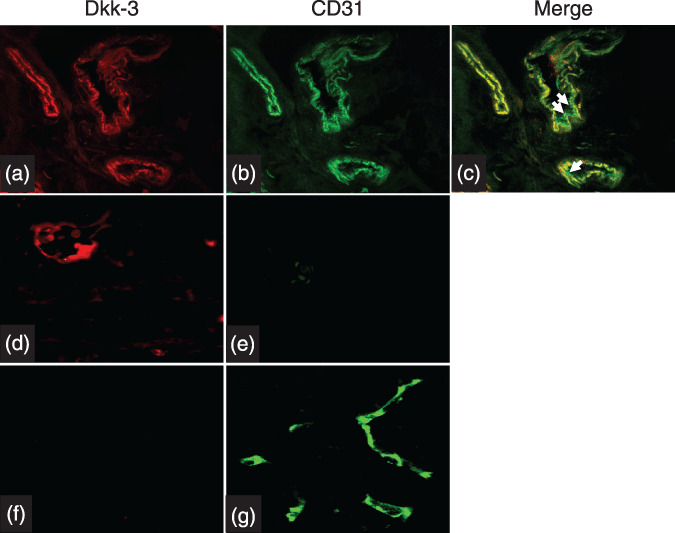
Double immunofluorescence staining was performed on 38 randomly selected pancreatic cancer tissue sections. This analysis revealed that CD31 and Dkk‐3 colocalize in double positive vessels and are expressed in the cytosol. Additionally, CD31 was expressed at the endothelial cell surface as indicated by the green signal at the luminal side of the blood vessels (arrows in Fig. 3a–c). Single positive blood vessels for Dkk‐3 (Fig. 3d,e) were found in tumor tissue, whereas CD31‐positive vessels were exclusively found in normal pancreas tissue (Fig. 3e,f).
Prognostic significance of Dkk‐3 and CD31 expression. Dkk‐3 expression was not associated with clinical stage (P = 0.15), age (P = 0.48), sex (P = 0.37), and nodal status (P = 0.25). Interestingly, the Dkk‐3‐positive vessel count was significantly correlated with tumor grading (P = 0.006). The proportion of high Dkk‐3 MVD was 73.7% in well, 52.7% in moderate, and 30.9% in poor differentiated tumors (Table 2).
Table 2.
Correlation of Dkk‐3 and CD31 expression with conventional clinicopathological parameters in patients with pancreatic carcinoma
| Dkk‐3 expression † | CD31 expression † | |||||||
|---|---|---|---|---|---|---|---|---|
| Patients n = 154 | <25 vessels n | >25 vessels n | P‐values ‡ | Patients n = 108 | <25 vessels n | >25 vessels n | P‐values ‡ | |
| Age at diagnosis | ||||||||
| ≤ 67 | 78 | 36 | 42 | 0.476 | 57 | 14 | 43 | 0.108 |
| > 67 | 76 | 32 | 43 | 51 | 18 | 33 | ||
| Sex | ||||||||
| Male | 83 | 39 | 44 | 56 | 17 | 39 | ||
| Female | 72 | 29 | 41 | 0.370 | 52 | 17 | 35 | 0.822 |
| Differentiation | ||||||||
| Well | 19 | 5 | 14 | 17 | 4 | 13 | ||
| Moderate | 93 | 44 | 49 | 0.006 | 65 | 20 | 45 | 0.482 |
| Poor | 42 | 29 | 13 | 26 | 9 | 17 | ||
| UICC stage | ||||||||
| I, II | 24 | 15 | 9 | 53 | 9 | 44 | ||
| III, IV | 94 | 39 | 55 | 0.107 | 19 | 5 | 14 | 0.489 |
| Unknown § | 36 | 36 | ||||||
Number of positive vessels per high power field.
χ2‐test.
Not included in statistical analysis.
Kaplan–Meier curves were plotted to visualize the influence of Dkk‐3 expression on overall survival time. A clear difference was observed between patients with low and high Dkk‐3‐positive MVD (P = 0.0013; log‐rank test) (Fig. 3a). Median survival time was 7 months (95% confidence interval [CI]: 4.84–9.16) for patients with low Dkk‐3 MVD expression compared to 15 months (95% CI: 9.7–20.3) for patients with high Dkk‐3 MVD expression. Furthermore, Kaplan–Meier analysis revealed no significant correlation between CD31 expression and overall survival (Fig. 3b). Additionally, Dkk‐3 expression in tumor cells was not significantly associated with overall survival (not shown).
A subset analysis including patients treated with chemotherapy (n = 66) demonstrated a significantly better overall survival time for Dkk‐3 high tumors (median overall survival 20; CI: 13.312–26.688) when compared with Dkk‐3 low tumors (9; CI: 3.87–14.13) (P = 0.009) (Fig. 4c). The influence of CD31 on survival time in patients receiving chemotherapy was not significant (P = 0.662) (Fig. 4d).
Figure 4.
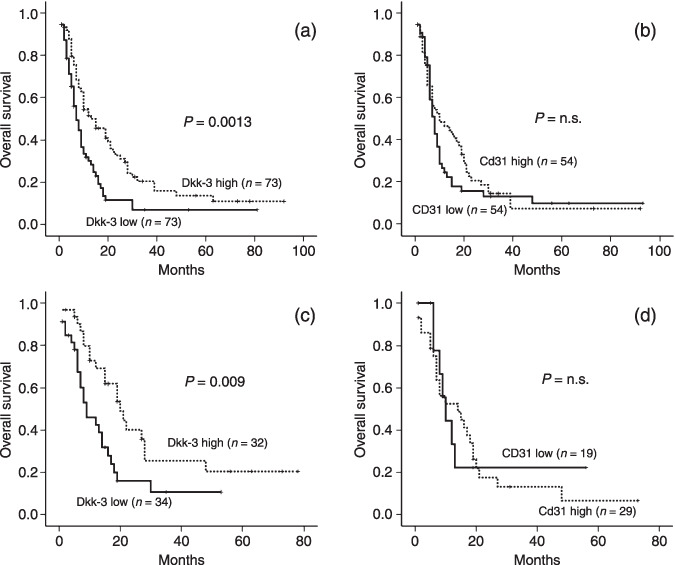
Association of Dkk‐3 and CD31 expression with survival time in pancreas carcinoma. (a) Median survival time was 15 months for patients with tumors of high Dkk‐3 microvessel density (MVD) compared with only 7 months for patients with tumors of low Dkk‐3 MVD. The difference was significant by log‐rank test (P = 0.0013). (b) Kaplan–Meier analysis demonstrated that CD31 as vessel marker is not a prognostic marker for overall survival. Subset analysis including patients treated with chemotherapy (n = 66) demonstrated a significantly better overall survival time for Dkk‐3 high tumors when compared with Dkk‐3 low tumors (P = 0.009) (Fig. 4c). The influence of CD31 MVD on survival time in patients receiving chemotherapy was not significant (P = 0.662) (Fig. 4d).
Furthermore, the established clinical parameters of stage (P < 0.001) and histological grade (P = 0.009) were significantly associated with overall survival time. Survival analysis including patients receiving chemotherapy demonstrated that stage (P < 0.001) but not grade (P = 0.132) was significantly associated with survival. This analysis showed Dkk‐3 as a more superior prognostic marker for overall survival than tumor grade in pancreas carcinomas. Univariate analysis of CD31 expression demonstrated no significant correlation with clinical stage (P = 0.49), tumor grade (P = 0.48), age (P = 0.11), and sex (P = 0.82) (Table 2).
The multivariate proportional hazard model was used to analyze the prognostic impact of Dkk‐3 adjusted for well‐established prognostic factors: clinical stage and histological grade (Table 3). The analysis revealed that Dkk‐3 expression, tumor stage, and tumor grade were of prognostic relevance (Table 3). Additionally, for clinical stage (P < 0.0001) and tumor grade 3 (P = 0.015) Dkk‐3 expression also turned out to be a prognostic factor in our study population (P = 0.024) (Table 3).
Table 3.
Multivariate analysis (Cox regression) of different prognostic parameters in patients with pancreatic cancer (n = 114)
| Overall survival | |||
|---|---|---|---|
| P‐values | Relative risk | 95% Confidence interval | |
| Stage (UICC) | |||
| I, II vs III, IV | 0.000 | 2.503 | 1.521–4.119 |
| Grading | 0.015 | 1.517 | 1.094–2.332 |
| Dkk‐3 high vs low | 0.024 | 0.610 | 0.396–0.938 |
Sensitivity of Dkk‐3‐positive endothelial cells and pancreatic cancer cell lines to 5‐fluorouracil (5‐FU) and gemcitabine. The observation of a good prognosis in patients with carcinomas of high Dkk‐3 MVD forced us to analyze the role of Dkk‐3 expression on chemoresistance. To determine the role of Dkk‐3 overexpression in EC on the effects of 5‐FU and gemcitabine on cell growth, a dose–response curve was determined and a comparison of the IC50 values was performed. For 5‐FU the antiproliferative IC50 values were 0.08 µg/mL for Dkk‐3 overexpressing cells and 0.49 µg/mL for control cells (P = 0.005) (Fig. 5a). For gemcitabine the IC50 values were, 2.94 nmol/L for Dkk‐3‐positive cells and 5.87 nmol/L for control cells (P = 0.0015) (Fig. 5b). The results showed a significantly lower IC50 in the Dkk‐3 overexpressing EC than in control cells. At the end of the dose–response experiments, Dkk‐3 expression was investigated in EC from parallel cultures. Western blot revealed a high level of Dkk‐3 expression in the Dkk‐3‐transfected EC and a weak endogenous Dkk‐3 expression in control, Green Fluorescent Protein (GFP)‐transfected EC (Fig. 5c).
Figure 5.
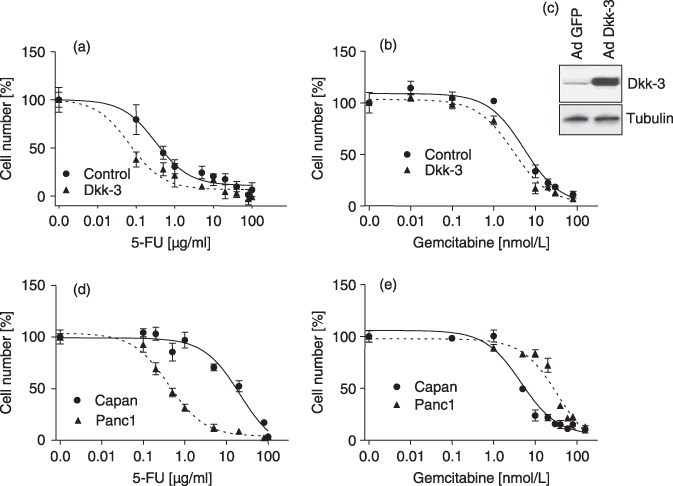
Dose–response analysis of HUVEC after (a) 5‐fluorouracil (5‐FU) and (b) gemcitabine treatment, respectively. Dkk‐3‐overexpressing endothelial cells and Green Fluorescent Protein (GFP)‐control cells were treated with different concentrations of 5‐FU or gemcitabine for 48 h. Thereafter, cell number was determined and expressed as percentage of control. Dkk‐3 overexpression significantly sensitizes endothelial cells to 5‐FU and gemcitabine treatment. Using 5‐FU, IC50 was 0.08 µg/mL for Dkk‐3 overexpressing cells and 0.49 µg/mL for control cells, respectively (P = 0.005). For gemcitabine IC50 values were 2.94 nmol/L and 5.87 nmol/L, respectively (P = 0.0015). (c) Western blot analysis of Dkk‐3 in EC transfected with GFP‐ (Ad‐GFP) or Dkk‐3 (Ad‐Dkk‐3) adenovirus. High Dkk‐3 expression was found only in Dkk‐3‐transfected cells. Tubulin was used as internal loading control. (d,e) Dose–response analyses of pancreatic carcinoma cell lines were done in a similar manner to that for EC. IC50 for 5‐FU treatment was 0.42 µg/mL for Panc‐1 and 21.13 µg/mL for the Capan‐1 cell line. After gemcitabine application the IC50 was 4.62 nmol/L for Capan‐1 and 45.86 nmol/L for the Panc‐1 cell line. Comparison of IC50 between Dkk‐3‐transfected EC and cancer cell lines demonstrated a significant lower IC50 for EC than for Panc‐1 after 5‐FU treatment (P = 0.0092) and a significant lower IC50 compared to Capan‐1 after gemcitabine treatment (P = 0.012).
In order to determine if the dose range of 5‐FU and gemcitabine in pancreatic cancer cell lines is of the same or different magnitude than for EC, cancer cell lines were cultured in the presence of increasing concentrations of the drugs. As shown in Figure 5(d), the antiproliferative IC50 for 5‐FU was 0.42 µg/mL for Panc‐1 and 21.13 µg/mL for the Capan‐1 cell line. After gemcitabine treatment the IC50 was 4.62 nmol/L for Capan‐1 and 45.86 nmol/L for Panc‐1 (Fig. 5e). Statistical analysis revealed that IC50 values were significantly higher in pancreatic cancer cell lines compared to Dkk‐3‐positive HUVEC (EC vs Panc‐1 after 5‐FU treatment P = 0.0092; and EC vs Capan‐1 after gemcitabine treatment P = 0.012). In contrast IC50 of Dkk‐3‐negative endothelial cells was not significantly different compared to Capan‐1 cells after gemcitabine treatment, and to Panc‐1 cells after 5‐FU treatment (Fig. 5d,e), indicating an enhanced sensitivity of Dkk‐3‐positive endothelial cells to chemotherapy.
Discussion
The aim of the present study was to analyze Dkk‐3 expression in tumor endothelial cells as a marker for angiogenesis in pancreatic cancer. Interestingly, patients with high Dkk‐3‐positive MVD had a significantly better overall survival than patients with low Dkk‐3 MVD expression. Moreover, Dkk‐3 expression in tumor endothelial cells in vitro resulted in a significantly enhanced growth inhibition after chemotherapy compared to control EC.
Antiangiogenic targeting of the neovasculature within tumors is considered to be one of the most promising strategies. Different attempts have been undertaken to assess the degree of vascularization in solid tumors. Tumor angiogenesis quantified by microvessel counting has been correlated with poor prognosis in several human cancer entities, including breast cancer,( 16 ) malignant melanoma,( 17 ) prostate cancer,( 18 ) lung cancer,( 19 ) and colorectal cancer.( 20 , 21 ) Additionally, MVD is also an independent prognostic factor in invasive breast carcinomas.( 22 )
In pancreatic cancer, however, the data regarding the prognostic value of MVD are conflicting. Using factor VIII as marker for MVD, Ellis et al. found that vessel counts as well as Vascular Endothelial Growth Factor (VEGF) expression are not a predictor of survival,( 23 ) whereas two other groups could clearly demonstrate a significant correlation for high‐MVD and a poor outcome in pancreatic cancer.( 24 , 25 ) Comparable data were described in other studies using CD34 as a marker for vessel staining.( 26 , 27 ) However, a possible limitation of these studies’ ability to draw more general conclusions is the low number of analyzed tumor samples (n = 21–41). Fujioka et al. reported that MVD was correlated to overall and relapse‐free survival, as examined by CD34 in 104 patients with pancreatic adenocarcinoma.( 28 ) Analyzing a comparable number of cases we found no correlation of MVD to overall survival and to clinicopathological parameters. Two obvious reasons for this difference can be mentioned: First we used CD31 as marker for vessel staining since in our hand this antibody gave the strongest vessel staining compared to CD34 and factor VIII, and second only microvessels with a detectable lumen were counted in our study. In contrast Fujioka et al. counted stained microvessels with detectable lumen as well as single brown immunostained cells without detectable lumen,( 28 ) which could result in an overestimation of blood vessels since single CD31‐positive infiltrating cells could also originate from infiltrating macrophages.( 29 )
In our study Dkk‐3 was not expressed in blood vessels in the normal human pancreas. Similar results were also found in other tumors entities where Dkk‐3 was highly expressed in vessels of gliomas, non‐Hodgkin lymphomas, melanomas, and colorectal carcinomas, and not in vessels of matched normal tissue,( 12 , 30 ) indicating Dkk‐3 as a specific marker of tumor vessels in general. In contrast to these findings in tumor vessels, Dkk‐3 was described as a candidate suppressor gene in various tumors, since it is down‐regulated in a number of cancer cells such as osteosarcoma, hepatocellular carcinoma, lung cancer, and prostate cancer.( 31 , 32 , 33 , 34 ) Furthermore, overexpression of Dkk‐3 suppresses tumor cell growth and induces apoptosis.( 35 ) Just recently, we found that Dkk‐3 overexpression did not change EC growth, or induce apoptosis in vitro, but Dkk‐3 seems to be important for tube formation.( 12 ) This probably indicates different functions of Dkk‐3 in cancer cells and in EC.
The surprising observation in our study was that Dkk‐3 high MVD correlated with good prognosis in overall survival in pancreatic carcinoma patients. Good prognosis was also found if only patients who received gemcitabine‐based chemotherapy were included in the analysis. Additionally, Dkk‐3 MVD turned out to be a better prognostic marker for survival than tumor grading in our sample group. The significant positive correlation of Dkk‐3 high MVD and well‐differentiated tumors suggests Dkk‐3 to be a new EC marker in pancreas carcinoma indicating early changes in the tumor microenvironment. It is well established that during tumor progression blood vessels are destabilized and EC acquire new characteristics distinct from their normal counterparts.( 36 )
With regard to these findings, data presented here demonstrated that Dkk‐3 high expression sensitize endothelial cells to 5‐FU and gemcitabine treatment, indicating a possible explanation for the good prognosis found for patients with Dkk‐3 high MVD tumors. However, it is known that adenovirus exerts biological effects on transfected cells which can interfere with cell growth assays. Therefore, EC were also transfected with recombinant adenovirus enabling overexpression of GFP and used as controls. Moreover, a low multiplicity of infection (virus to cells ratio) was used to ensure a moderate expression of Dkk‐3 than in GFP transfected cells. This should avoid the overload of the endoplasmatic reticulum with recombinant protein and thus minimize an unfolded protein response and apoptosis of transfected cells. However, as shown previously, transfected cells showed no reduction in DNA synthesis and cell growth as determined by BrdU‐incorporation assays and Water Soluble Tetrazolium (WST)‐1 cell viability assays.( 12 )
Furthermore, Dkk‐3‐positive EC are significantly more sensitive to 5‐FU and gemicitabine treatment than pancreatic cancer cell lines in vitro. This observation may be an explanation of the significant better overall survival of patients with tumors with Dkk‐3 high MVD. However, whether Dkk‐3 expression in tumor blood vessels also leads to more EC sensitivity to chemotherapy in vivo as found in normal EC in vitro is not known.
At present, single‐agent gemcitabine is the standard chemotherapy in advanced pancreatic cancer. However, the clinical response to gemcitabine with an overall response rate of about 12% remains modest and seems to be improvable.( 37 , 38 , 39 ) Therefore, there is an urgent need for novel biomarkers to predict better response to therapy and minimize unnecessary treatment‐related toxicities. Based on the prognostic value of Dkk‐3 expression in pancreatic cancer vessels, Dkk‐3 may practically be used as marker for selecting patients who might benefit from chemotherapy.
In summary, data presented here suggest that Dkk‐3 characterizes an EC phenotype, which sensitizes them to growth inhibition after chemotherapeutic treatment. Furthermore, the usages of Dkk‐3 as a predictive marker in pancreatic carcinoma to select patients who will benefit from chemotherapy have to be analyzed in prospective studies.
Acknowledgments
The authors would like to thank Ms. Ines Brosch for her excellent technical assistance. This work was supported by grants from the Österreichischen Krebshilfe/Tirol. We gratefully acknowledge the support provided by Allianz Elementar Versicherungs‐AG.
References
- 1. Warshaw AL, Fernandez‐del CC. Pancreatic carcinoma. N Engl J Med 1992; 326: 455–65. [DOI] [PubMed] [Google Scholar]
- 2. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007; 57: 43–66. [DOI] [PubMed] [Google Scholar]
- 3. Klapman J, Malafa MP. Early detection of pancreatic cancer: why, who, and how to screen. Cancer Control 2008; 15: 280–7. [DOI] [PubMed] [Google Scholar]
- 4. Berlin JD, Rothenberg ML. Chemotherapeutic advances in pancreatic cancer. Curr Oncol Report 2003; 5: 219–26. [DOI] [PubMed] [Google Scholar]
- 5. Bodner WR, Hilaris BS, Mastoras DA. Radiation therapy in pancreatic cancer: current practice and future trends. J Clin Gastroenterol 2000; 30: 230–3. [DOI] [PubMed] [Google Scholar]
- 6. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29: 15–8. [DOI] [PubMed] [Google Scholar]
- 7. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64. [DOI] [PubMed] [Google Scholar]
- 8. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 9. St. Croix B, Rago C, Velculescu V et al . Genes expressed in human tumor endothelium. Science 2000; 289: 1197–202. [DOI] [PubMed] [Google Scholar]
- 10. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006; 25: 7469–81. [DOI] [PubMed] [Google Scholar]
- 11. Barrantes IB, Montero‐Pedrazuela A, Guadano‐Ferraz A et al . Generation and characterization of dickkopf3 mutant mice. Mol Cell Biol 2006; 26: 2317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Untergasser G, Steurer M, Zimmermann M et al . The Dickkopf‐homolog 3 is expressed in tumor endothelial cells and supports capillary formation. Int J Cancer 2008; 122: 1539–47. [DOI] [PubMed] [Google Scholar]
- 13. Hermann M, Pirkebner D, Draxl A et al . Dickkopf‐3 is expressed in a subset of adult human pancreatic beta cells. Histochem Cell Biol 2007; 127: 513–21. [DOI] [PubMed] [Google Scholar]
- 14. Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997; 80: 1803–4. [DOI] [PubMed] [Google Scholar]
- 15. Hall NR, Fish DE, Hunt N, Goldin RD, Guillou PJ, Monson JR. Is the relationship between angiogenesis and metastasis in breast cancer real? Surg Oncol 1992; 1: 223–9. [DOI] [PubMed] [Google Scholar]
- 16. Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 17. Srivastava A, Laidler P, Hughes LE, Woodcock J, Shedden EJ. Neovascularization in human cutaneous melanoma: a quantitative morphological and Doppler ultrasound study. Eur J Cancer Clin Oncol 1986; 22: 1205–9. [DOI] [PubMed] [Google Scholar]
- 18. Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol 1993; 143: 401–9. [PMC free article] [PubMed] [Google Scholar]
- 19. Macchiarini P, Fontanini G, Hardin MJ, Squartini F, Angeletti CA. Relation of neovascularisation to metastasis of non‐small‐cell lung cancer. Lancet 1992; 340: 145–6. [DOI] [PubMed] [Google Scholar]
- 20. Choi HJ, Hyun MS, Jung GJ, Kim SS, Hong SH. Tumor angiogenesis as a prognostic predictor in colorectal carcinoma with special reference to mode of metastasis and recurrence. Oncology 1998; 55: 575–81. [DOI] [PubMed] [Google Scholar]
- 21. Lindmark G, Gerdin B, Sundberg C, Pahlman L, Bergstrom R, Glimelius B. Prognostic significance of the microvascular count in colorectal cancer. J Clin Oncol 1996; 14: 461–6. [DOI] [PubMed] [Google Scholar]
- 22. Gasparini G, Weidner N, Bevilacqua P et al . Tumor microvessel density, p53 expression, tumor size, and peritumoral lymphatic vessel invasion are relevant prognostic markers in node‐negative breast carcinoma. J Clin Oncol 1994; 12: 454–66. [DOI] [PubMed] [Google Scholar]
- 23. Ellis LM, Takahashi Y, Fenoglio CJ, Cleary KR, Bucana CD, Evans DB. Vessel counts and vascular endothelial growth factor expression in pancreatic adenocarcinoma. Eur J Cancer 1998; 34: 337–40. [DOI] [PubMed] [Google Scholar]
- 24. Karademir S, Sokmen S, Terzi C et al . Tumor angiogenesis as a prognostic predictor in pancreatic cancer. J Hepatobiliary Pancreat Surg 2000; 7: 489–95. [DOI] [PubMed] [Google Scholar]
- 25. Takagi K, Takada T, Amano H. A high peripheral microvessel density count correlates with a poor prognosis in pancreatic cancer. J Gastroenterol 2005; 40: 402–8. [DOI] [PubMed] [Google Scholar]
- 26. Khan AW, Dhillon AP, Hutchins R et al . Prognostic significance of intratumoural microvessel density (IMD) in resected pancreatic and ampullary cancers to standard histopathological variables and survival. Eur J Surg Oncol 2002; 28: 637–44. [DOI] [PubMed] [Google Scholar]
- 27. Ikeda N, Adachi M, Taki T et al . Prognostic significance of angiogenesis in human pancreatic cancer. Br J Cancer 1999; 79: 1553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fujioka S, Yoshida K, Yanagisawa S, Kawakami M, Aoki T, Yamazaki Y. Angiogenesis in pancreatic carcinoma: thymidine phosphorylase expression in stromal cells and intratumoral microvessel density as independent predictors of overall and relapse‐free survival. Cancer 2001; 92: 1788–97. [DOI] [PubMed] [Google Scholar]
- 29. Bittinger F, Klein CL, Skarke C et al . PECAM‐1 expression in human mesothelial cells: an in vitro study. Pathobiology 1996; 64: 320–7. [DOI] [PubMed] [Google Scholar]
- 30. Zitt M, Untergasser G, Amberger A et al . Dickkopf‐3 as a new potential marker for neoangiogenesis in colorectal cancer: expression in cancer tissue and adjacent non‐cancerous tissue. Dis Markers 2008; 24: 101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kawano Y, Kitaoka M, Hamada Y, Walker MM, Waxman J, Kypta RM. Regulation of prostate cell growth and morphogenesis by Dickkopf‐3. Oncogene 2006; 25: 6528–37. [DOI] [PubMed] [Google Scholar]
- 32. Kobayashi K, Ouchida M, Tsuji T et al . Reduced expression of the REIC/Dkk‐3 gene by promoter‐hypermethylation in human tumor cells. Gene 2002; 282: 151–8. [DOI] [PubMed] [Google Scholar]
- 33. Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res 2005; 65: 4218–27. [DOI] [PubMed] [Google Scholar]
- 34. Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y, Namba M. A REIC gene shows down‐regulation in human immortalized cells and human tumor‐derived cell lines. Biochem Biophys Res Commun 2000; 268: 20–4. [DOI] [PubMed] [Google Scholar]
- 35. Abarzua F, Sakaguchi M, Takaishi M et al . Adenovirus‐mediated overexpression of REIC/Dkk‐3 selectively induces apoptosis in human prostate cancer cells through activation of c‐Jun – NH2‐kinase. Cancer Res 2005; 65: 9617–22. [DOI] [PubMed] [Google Scholar]
- 36. McDonald DM, Baluk P. Significance of blood vessel leakiness in cancer. Cancer Res 2002; 62: 5381–5. [PubMed] [Google Scholar]
- 37. Burris HA III, Moore MJ, Andersen J et al . Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–13. [DOI] [PubMed] [Google Scholar]
- 38. Crino L, Mosconi AM, Calandri C et al . Gemcitabine in advanced pancreatic cancer: a phase II trial. Am J Clin Oncol 2001; 24: 296–8. [DOI] [PubMed] [Google Scholar]
- 39. Heinemann V. Gemcitabine: progress in the treatment of pancreatic cancer. Oncology 2001; 60: 8–18. [DOI] [PubMed] [Google Scholar]


