Abstract
Although chemokines have been thought of primarily as leukocyte attractants, a growing body of evidence indicates that they also contribute to a number of tumor‐related processes, such as tumor cell growth, angiogenesis/angiostasis, local invasion, and metastasis. The current knowledge of the possible involvement of chemokines and their receptors in these cellular events are reviewed here. The operating mechanism of chemokines in relation to metastatic processes in vivo are also discussed. (Cancer Sci 2005; 96: 317–322)
Chemokines, their receptors and nomenclature. Chemokines represent a family of small‐molecular‐weight (8–14 kDa) chemotactic cytokines that bind to G‐protein‐coupled heptahelical receptors.( 1 ) Structurally, chemokines are categorized into four major subfamilies (CXC, CC, CX3C, and C), based on the arrangement of four conserved N‐terminal cysteine residues in the mature proteins. Between the first two cysteine residues, the CC, CXC, and CX3C chemokines have 0, 1, and 3 non‐conserved amino‐acid residues, respectively, while the C chemokines lack the first and third of the four conserved residues. There are approximately 50 chemokine family members, and the CC and CXC chemokines represent the majority (Table 1). There are at least 18 chemokine receptors. Each receptor binds ligands from only one of the four structural subfamilies described above, and hence, the receptors are also grouped into four subfamilies (Table 1).
Table 1.
Chemokines and their receptors. Chemokines consisting of four major subfamilies (CXC, CC, XC, CX3C) are listed here together with their original names. Major receptors for each chemokine are also shown, although some chemokines may bind other receptors. Chemokines and their receptors identified in humans are listed here
| New official name | Original name (other names may exist) | Receptor(s) |
|---|---|---|
| CXCL1 | GROα– growth related oncogene α | CXCR2 > CXCR1 |
| CXCL2 | GROβ– growth related oncogene β | CXCR2 |
| CXCL3 | GROγ– growth related oncogene γ | CXCR2 |
| CXCL4 | PF‐4 – platelet factor 4 | Unknown |
| CXCL5 | ENA‐78 – epithelial cell derived neutrophil activating factor 78 | CXCR2 |
| CXCL6 | GCP‐2 – granulocyte chemoattractant protein 2 | CXCR1, CXCR2 |
| CXCL7 | NAP‐2 – neutrophil activating protein 2 | CXCR1, CXCR2 |
| CXCL8 | IL‐8 – interleukin 8 | CXCR1, CXCR2 |
| CXCL9 | MIG – monokine induced by interferon‐γ | CXCR3 |
| CXCL10 | IP‐10 –γ interferon inducible ptrotein 10 | CXCR3 |
| CXCL11 | I‐TAC – interferon inducible T cell α‐chemoattractant | CXCR3 |
| CXCL12 | SDF‐1 – stromal cell derived factor 1 | CXCR4 |
| CXCL13 | BCA‐1–B cell activating chemokine 1 | CXCR5 |
| CXCL14 | BRAK – breast and kidney chemokine | Unknown |
| CXCL15 | Unknown | Unknown |
| CXCL16 | SR‐PSOX – scavenger receptor that binds phosphatidylserine and oxidized lipoprotein | CXCR6 |
| CCL1 | I‐309 | CCR8 |
| CCL2 | MCP‐1 – monocyte chemoattractant protein 1 | CCR2 |
| CCL3 | MIP‐1α– macrophage inflammatory protein 1α | CCR1, CCR5 |
| CCL4 | MIP‐1β– macrophage inflammatory protein 1β | CCR5 |
| CCL5 | RANTES – regulated on activation, normally T cell expressed and secreted | CCR1, CCR3, CCR5 |
| CCL6 | Unknown | CCR1, CCR2, CCR3 |
| CCL7 | MCP‐3 – monocyte chemoattractant protein 3 | CCR1, CCR2, CCR3 |
| CCL8 | MCP‐2 – monocyte chemoattractant protein 2 | CCR2, CCR3, CCR5 |
| CCL9/10 | Unknown | CCR1 |
| CCL11 | Eotaxin | CCR3 |
| CCL12 | Unknown | CCR2 |
| CCL13 | MCP‐4 – monocyte chemoattractant protein 4 | CCR1, CCR2, CCR3 |
| CCL14 | HCC‐1 – hemofiltrate CC chemokine | CCR1 |
| CCL15 | Lkn‐1 – leukotactin 1 | CCR1, CCR3 |
| CCL16 | LEC – liver expressed chemokine | CCR1 |
| CCL17 | TARC – thymus and activation regulated chemokine | CCR4 |
| CCL18 | PARC – pulmonary and activation regulated chemokine | Unknown |
| CCL19 | ELC – Epstein‐Barr virus induced receptor ligand chemokine | CCR7 |
| CCL20 | LARC – liver and activation regulated chemokine | CCR6 |
| CCL21 | SLC – secondary lymphoid tissue chemokine | CCR7 |
| CCL22 | MDC – macrophage derived chemokine | CCR4 |
| CCL23 | MPIF‐1 – myeloid progenitor inhibitory factor 1 | CCR1 |
| CCL24 | MPIF‐2 – myeloid progenitor inhibitory factor 2 | CCR3 |
| CCL25 | TECK – thymus expressed chemokine | CCR9 |
| CCL26 | Eotaxin‐3 | CCR3 |
| CCL27 | ESkine | CCR3, CCR2, CCR10 |
| CCL28 | MEC – mucosae‐associated epithelial chemokine | CCR10, CCR3 |
| XCL1 | Lymphotactin‐α | XCR1 |
| XCL2 | Lymphotactin‐β | XCR1 |
| CX3CL1 | Fractalkine | CX3CR1 |
As the chemokines were discovered, they were named by the individual laboratories that identified and/or characterized them; in fact, a single chemokine often had multiple names, which resulted in significant confusion. Therefore, as summarized in Table 1, a new nomenclature system was developed several years ago, in which the chemokine structural code (CXC, CC, CX3C, or C) is followed by the letter ‘L’ (ligand) for each chemokine (as in CXCL1) or by the letter ‘R’ (receptor) for each receptor (as in CXCR1).( 1 ) This simple numbering system was adopted for the almost 50 chemokines and 20 receptors. However, it is often difficult, even for those who are actively working in this area, to correlate the rather nondescript numbered code names with known chemokines or their receptors. To alleviate this problem, we will provide both the new and old names of chemokines when they appear in the text.
Chemokines are also classified into two main groups based on their function and pattern of expression: inflammatory and homeostatic.( 1 ) The inflammatory chemokines are not constitutively expressed but are inducible and up‐regulated by inflammatory stimuli, at which point they recruit leukocytes to the sites of the inflammatory stimulus. Their expression is under the tight control of the local proinflammatory cytokine milieu. In contrast, the homeostatic chemokines are constitutively expressed in certain cell types or tissues, and play a vital role in the development and maintenance (homeostasis) of the hemopoietic and immune systems. These chemokines are often called lymphoid chemokines, because they act preferentially on lymphocytes, although some of them act on dendritic cells (DC) as well. Although the functional classification of ‘inflammatory’ versus ‘homeostatic’ has been simple and extremely useful for understanding the functional significance of each chemokine, it is becoming clear that a few chemokines do not belong in either of these groups, but rather share characteristics of both. These are now called ‘dual‐function’ chemokines.( 2 ) They are up‐regulated upon exposure to inflammatory stimuli, and play an important role in the recruitment of various lymphocyte subsets to the relevant tissues during immune responses. A salient feature of both the dual‐function and homeostatic chemokines is that each of these chemokines binds to a single receptor, expressed mainly on lymphoid cells, whereas the inflammatory chemokines bind to multiple receptors. Furthermore, the receptors for inflammatory chemokines bind to multiple chemokines (receptor promiscuity).
Chemokine‐induced biological events. The binding of a cognate chemokine ligand to its receptor modifies the tertiary structure of the receptor, such that its cytoplasmic portion can bind and activate heterotrimeric G proteins. The activated G‐protein subunits subsequently stimulate multiple signal transduction pathways involving phospholipase Cβ (PLCβ) isoforms, phosphoinositide‐3 kinases (PI3K), and various Src family kinases; the pathway details outlined in Fig. 1 vary slightly, depending on the cellular context in which the relevant receptor is expressed. For instance, the activation of PI3Kγ, but not PLCβ activity, is required for myeloid cell chemotaxis,( 3 ) whereas PI3Kγ activation is dispensable for lymphocyte chemotaxis.( 4 ) In neutrophils, PI3K activation results in the generation of phophatidylinositol‐3,4,5‐triphosphate (PIP3), and both PI3K and PIP3 translocate to the leading edge of the chemotaxing cell, where they activate the small GTPase Rac, which then induces local actin polymerization in the leading edge. Protein kinase B (PKB) also translocates to the leading edge, where it contributes to local actin polymerization.( 5 , 6 ) In lymphocytes, the translocation of PI3K and PIP3 has not been documented,( 7 ) and activation of a PI3K‐independent pathway leads to Rac activation, in which the scaffold protein DOCK‐2 appears to play a critical role.( 8 ) Indeed, in DOCK‐2‐deficient mice, in which chemokine‐induced Rac activation is severely decreased in lymphocytes but not in monocytes, lymphocyte chemotaxis is preferentially abrogated, whereas monocyte chemotaxis is uncompromised,( 8 ) validating the idea that lymphocytes use biochemical pathways for directional cell migration that are distinct from those used by monocytes.( 7 ) In the leading edge of monocytes, another small GTPase, Cdc42, is recruited and activated locally, and appears to be essential for directional cell migration, because the absence of Cdc42 results in non‐directional cell migration.( 6 , 9 ) In neutrophils, Cdc42 has an essential role in the exclusion from the leading edge of a PIP3‐phosphatase, PTEN, which is a negative regulator of PIP3.( 10 ) Thus, upon chemokine stimulation, PI3K localizes anteriorly, whereas PTEN localizes posteriorly in neutrophils,( 11 ) and the spatial and temporal regulation of the PI3K‐dependent pathways as well as of Rac and Cdc42 GTPases appears essential for determining the internal polarity (particularly the ‘frontness’) of a migrating cell.
Figure 1.
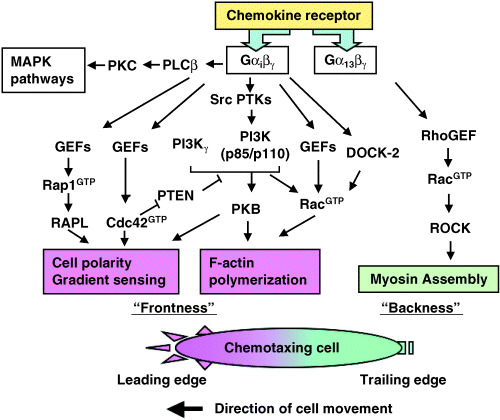
The chemokine receptor signaling network. Chemokine receptors can bind and activate heterotrimeric G proteins, including Gαißγ and Gα13βγ. In a chemotaxing cell, signaling components responsible for the formation of cell polarity, directional sensing and F‐actin polymerization, such as PI3K, Rac and Cdc42, are preferentially recruited to the leading edge, whereas the mediators of actomyosin contraction, which are downstream of Rho, are recruited to the trailing edge. PTEN is excluded from the leading edge, helping to restrict PI3K signaling to the leading edge. Thus, the spatially regulated localization of signaling components plays a vital role in determining the ‘frontness’ and ‘backness’ in a chemotaxing cell. The details of the chemokine signaling pathways appear to vary slightly depending on the cellular context.
Another small GTPase, Rho, is involved in the regulation of cellular morphology through its effects on the actin cytoskeleton. Upon chemokine stimulation, Rho preferentially localizes to the trailing edge of a migrating cell and is activated by Rho guanine nucleotide exchange factors (GEF) in non‐lymphoid cells.( 6 ) Activated Rho then induces the formation of actin and myosin complexes, resulting in the retraction of the trailing edge of the cell. Thus, Rho appears to be directly involved in determining the ‘backness’ of at least non‐lymphoid cells. Interestingly, the polarity signals are transmitted by different G‐protein subunits in non‐lymphoid cells, in which frontness is controlled by Gβγ subunits, which coordinate the activation of PI3K and Rac and of actin organization at the leading edge, whereas backness is controlled by the Gα12/13 subunit, which activates Rho, Rho‐dependent kinase, and myosin II.( 11 ) The role of Rho in lymphoid cells is currently unclear.( 7 )
In lymphocytes, Rap1 appears be important in both cell polarity determination and integrin activation by chemokines. Rap1 activation occurs rapidly and transiently upon chemokine stimulation in a Gαi‐dependent manner.( 12 ) Following Rap1 activation, a Rap1‐binding protein (RAPL) rapidly associates with a β2 integrin (LFA‐1) and translocates with LFA‐1 to the leading edge,( 13 ) which is essential for the activation of cell‐surface LFA‐1 and its adhesion to intercellular adhesion molecule‐1 (ICAM‐1). The expression of a dominant‐active form of Rap1 promotes shear‐resistant cell adhesion to LFA‐1 ligands such as ICAM‐1 and promotes a polarized morphology in lymphoid cells.( 12 ) Inhibition of Rap1 activation by the forced expression of a Rap GTPase‐activating protein, Spa‐1, inhibits lymphoid chemotaxis,( 12 ) and a lack of RAPL results in remarkably decreased levels of integrin‐mediated cell adhesion and chemotaxis.( 13 , 14 ) These results strongly indicate that Rap1 plays an essential role in integrin activation and cell polarity determination through the action of RAPL in lymphocytes, although the exact biochemical pathways leading to Rap1 activation remain to be fully explored.
Roles of chemokines and their receptors in tumor biology. Although chemokines were initially characterized as attractants of leukocytes, it is now widely recognized that any cell type can express chemokines and chemokine receptors. In particular, their expression by tumor cells has attracted much attention, and the prevailing hypothesis implicates chemokines as road signs for tumor cell invasion and metastasis. However, chemokines appear to function more than as attractants, and increasing evidence indicates that interactions between chemokines and their receptors are important in virtually every step of tumor development, including tumor growth, progression, and metastasis, as reviewed here.
Chemokines and tumor transformation, survival, and growth. Emerging evidence indicates that chemokines are directly involved in the transformation, survival and growth of tumor cells. For instance, several chemokines, including CXCL1/Groα/MGSAα and CXCL8/IL‐8, bind not only to their natural receptor, CXCR2, but also to the G‐protein‐coupled receptors encoded by tumorigenic viruses such as Kaposi's sarcoma‐associated herpes virus‐8 (HHV‐8). Transgenic expression of the HHV8‐encoded receptor results in the development of angioproliferative lesions resembling Kaposi's sarcoma in mice,( 15 ) indicating that chronic and excessive signaling through the CXCR2‐like GPCR promotes oncogenic cellular transformation.
Furthermore, CXCR2 transmits an autocrine cell growth signal in several tumor cell types. A few of the established melanoma cell lines produce CXCL1/Groα/MGSAα( 16 ) and CXCL8/IL‐8( 17 ) and express their common receptor CXCR2 constitutively,( 18 ) and blocking either the ligands or their receptor inhibits cell growth.( 17 , 19 ) Similar observations have been made in other tumor cell types (reviewed in Arya et al.).( 20 ) These results indicate that chemokines produced by tumor cells can function as autocrine and/or paracrine growth factors under certain conditions.
In addition, chemokines provide survival signals to tumor cells in some instances. For example, CXCL12/SDF‐1α and CXCL9/MIG can enhance the survival of CXCR4‐expressing( 21 ) and CXCR3‐expressing( 22 ) cells, respectively, grown in suboptimal conditions, such as a low serum concentration.
Chemokines and angiogenesis/angiostasis. Chemokines can affect tumor growth not only directly but also indirectly by promoting or inhibiting angiogenesis. Among the CXC chemokines, those bearing an ELR (Glu‐Leu‐Arg) motif at their NH2 terminus (ELR+ chemokines) are angiogenic,( 23 ) stimulating endothelial cell chemotaxis, whereas those lacking the ELR motif (ELR− chemokines) are angiostatic, inhibiting endothelial cell chemotaxis. Accumulating evidence strongly indicates that the ELR+ chemokines promote tumor growth by acting as angiogenic factors in vivo. In particular, CXCL8/IL‐8 and CXCL1/GROα have been reported to contribute to tumor‐derived angiogenic activity in a variety of human tumors. In prostate cancer, significant levels of CXCL8 are observed in tumor cells, but not in normal or benign hyperplastic cells.( 24 ) CXCL8 serum concentrations( 25 ) and CXCL8 mRNA levels in radical prostatectomy specimens are positively correlated with an advanced pathologic stage.( 26 ) Interestingly, anti‐CXCL8 antibodies effectively inhibit the tumorigenesis and tumor‐related angiogenesis of a CXCL8‐producing prostate cancer cell line, PC‐3, in severe combined immunodeficiency (SCID) mice, whereas anti‐CXCL1 antibodies inhibit those of a CXCL1‐producing prostate cancer cell line, Du145, in the same model.( 27 ) Lung carcinoma cells also often secrete CXCL8, and its neutralization by a specific antibody abrogates the tumor‐related angiogenic activity( 28 ) and tumor growth in SCID mice,( 29 ) supporting the hypothesis that angiogenic chemokines play a role in promoting tumor growth in vivo.
CXCL10/IP‐10 is an angiostatic ELR− chemokine produced at high levels by human non‐small cell lung cancer cells.( 30 ) In SCID mice, the production of CXCL10 is inversely correlated with the tumorigenesis of lung cancer cells, and the intratumoral injection of CXCL10 attenuates the growth and neovascularization of the tumors, whereas the functional depletion of CXCL10 by the systemic administration of neutralizing antibodies augments the tumor growth and neovascularization.( 30 ) The forced expression of murine CXCL10 in human Burkitt lymphoma cells also inhibits subcutaneous tumor growth in nude mice, causing capillary damage and tumor necrosis.( 31 )
Chemokines and tumor‐leukocyte interactions. The local production of inflammatory chemokines by tumor cells and/or stromal cells would be expected to cause the recruitment of various types of leukocytes into the tumor tissue. Indeed, there is ample evidence for the association of various types of inflammatory cells with cancer. Because these inflammatory cells secrete a variety of biologically active molecules, including cytokines, chemokines, proteases, and lipid mediators, they are likely to regulate neoplastic processes that affect the growth and spread of tumor cells. In breast cancer, tumor cells produce CCL5/RANTES, and the level of expression correlates with the extent of macrophage infiltration and lymph node metastasis.( 32 ) In a mouse model, the long‐term administration of a CCL5 antagonist, Met‐CCL5, significantly reduces the subcutaneous growth of CCL5‐producing syngeneic mouse breast cancer cells without affecting their proliferative ability, but concurrently inhibits leukocyte infiltration into the tumor.( 33 ) In esophageal carcinoma, CCL2/MCP‐1 expression is positively correlated with the level of macrophage infiltration, tumor angiogenesis, and invasion.( 34 ) These results indicate that tumor‐infiltrating macrophages may in fact facilitate tumor growth and progression. Enforcing this idea, genetic studies by Lin et al. ( 35 , 36 ) clearly demonstrate the tumor‐growth‐promoting activity of infiltrating macrophages and implicate colony‐stimulating factor (CSF) as a tumor‐derived macrophage growth factor that exacerbates macrophage infiltration, thereby promoting subsequent tumor progression and metastasis. However, there are conflicting reports on the role of tumor‐associated leukocytes. Monti et al. reported that CCL2/MCP‐1 is secreted by pancreatic carcinoma cells and that inflammatory cytokines such as IFNγ, TNFα, and IL‐1β synergistically up‐regulate its expression.( 37 ) The tumor‐associated CCL2 is apparently released into the circulation of tumor‐bearing patients, and interestingly, those with high circulating levels of CCL2 have significantly higher survival than do low CCL2 producers. In addition, serum CCL2 levels are positively correlated with intratumoral macrophage infiltration and inversely correlated with tumor cell proliferative activity, while these tumor cells do not express functional receptors for CCL2.( 37 )
A variety of DC subsets are also found in tumor tissues. In breast carcinoma, immature DC that are likely to be of myeloid origin are found preferentially within the tumor bed where CCL20/LARC/MIP‐3α is highly expressed; in contrast, more mature DC are confined to peritumoral areas where clusters of T cells are often found.( 38 ) Plasmacytoid DC are also found in certain tumors, and chemokines have been implicated in their recruitment.( 39 ) As with intratumoral macrophages, the available data are conflicting as to the role of these DC subsets, and it is unclear whether the presence of these DC is correlated with a beneficial or adverse outcome. It is also unclear whether their presence within tumor tissues signifies an active host response or a subversive effect of the tumor.( 40 )
Chemokines and tumor invasion/metastasis. Accumulating data show that some but not all tumor cells express chemokine receptors and respond to chemokine gradients in vitro. Furthermore, in vivo data also indicate that certain chemokines can serve as tissue‐specific attractant molecules for tumor cells, promoting tumor‐cell migration to particular sites, as reviewed here.
The concept that a particular chemokine‐receptor pair may promote organ‐specific tumor metastasis was first experimentally addressed by Muller et al. ( 41 ) They showed that a chemokine receptor, CXCR4, is more highly expressed in mammary carcinoma tissue than in normal mammary tissue and that its ligand CXCL12/SDF‐1α is expressed in a variety of tissues including lymph nodes, bone marrow, and lungs, where mammary carcinoma cells preferentially metastasize.( 41 ) They then showed that certain mammary carcinoma cell lines can undergo chemotactic migration to CXCL12 in vitro and that experimental metastases to lymph nodes and lungs induced by intravenous or orthotopic injection of one of these cell lines into SCID mice are inhibited significantly by treating the mice with a neutralizing monoclonal antibody against CXCR4. In fact, CXCR4 is the chemokine receptor most commonly found in human and murine cancer cells,( 42 , 43 ) and its involvement in metastasis has been suggested in a variety of tumors, including small‐cell lung cancers, pancreatic cancers, astrogliomas, myelomas, B cell lymphomas, and chronic lymphocytic leukemias.( 42 ) However, it may be too simplistic to envision that the mere ability of CXCL12 to induce chemotaxis explains why this chemokine plays an important role in tissue‐specific tumor metastasis, because CXCL12 is expressed in a wide variety of tissues and can perform functions other than chemotaxis, such as the promotion of tumor cell growth/survival and cytokine secretion.( 43 , 44 )
Furthermore, in a mouse metastasis model, CCR7, which is the receptor for two major chemokines, CCL19/ELC and CCL21/SLC, that are expressed in lymph nodes, plays an important role in tumor metastasis. Wiley et al. demonstrated that the functional expression of CCR7 enhances the metastasis of B16 murine melanoma cells to regional lymph nodes compared with control melanoma cells, and this metastasis is blocked by neutralizing anti‐CCL21 antibodies but not by control IgG.( 45 ) Because CCR7 ligands are expressed in both high endothelial venule cells and lymphatic endothelial cells in lymph nodes,( 46 ) Wiley et al. examined whether CCR7‐B16 melanoma cells metastasize to draining lymph nodes through blood vessels or lymphatic vessels. Intravenous administration of CCR7‐B16 or control B16 cells yielded comparable numbers of metastatic nodules in the lungs without forming visible metastasis in the lymph nodes, indicating that CCR7 expression specifically enhances the metastatic ability of B16 melanoma cells through a lymph‐mediated but not a blood‐borne pathway.( 45 ) Because the CCR7‐dependent lymphatic migration of tumor cells is reminiscent of the physiological migration of DC into draining lymph nodes,( 47 ) an interesting possibility is that tumor cells co‐opt physiological mechanisms of lymph node immigration during metastasis.( 45 ) Supporting this idea, CCR7‐positive gastric carcinoma cells have a high incidence of lymph node metastasis, and patients with CCR7‐positive tumors have a significantly poorer prognosis than those with CCR7‐negative tumors.( 48 ) Similar observations have been made in esophageal carcinoma patients.( 49 )
Other chemokine receptors have been implicated in chemokine‐dependent tumor cell attraction to certain tissues. CCR10 is expressed in melanoma cells,( 41 ) and its ligand, CCL27/CTACK, is produced constitutively by keratinocytes in the skin, to which melanomas often metastasize. CCR4 is often expressed in adult T‐cell leukemias that preferentially invade the skin, where one of the CCR4 ligands, CCL17/TARC, can be expressed.( 50 ) CCR3 is expressed in CD30+ cutaneous lymphomas, and its ligand CCL11/eotaxin is often expressed in the tumor cells and tumor‐associated skin lesions.( 51 ) Although chemotaxis has been invoked as a possible mechanism for the organ‐specific metastasis of tumor cells in these studies, this idea is based mainly on inference and remains to be proven in vivo. It is also questionable whether the metastatic migration of tumor cells is governed over long distances by the chemotactic gradient of a single chemokine. Metastasis is a complex phenomenon that involves the ability of tumor cells both to migrate to a particular site and to become established and proliferate. Thus, the prevailing view of chemokines as mere attractants for tumor cells may be an oversimplification.( 43 , 44 )
Operating mechanisms of chemokines in vivo. While there is sufficient evidence to suggest that chemokines can stimulate tumor motility and attract them to sites of production, it must be kept in mind that chemokines are soluble and subject to diffusion from their source as a result of body fluid flow in vivo. Thus, for chemokines to function locally in the body, they would have to be immobilized on tissue components upon secretion. In this regard, it is notable that chemokines can bind to glycosaminoglycans, such as heparan sulfate( 52 ) and chondroitin sulfates( 53 ) that decorate proteins expressed broadly in various tissues (i.e. proteoglycans). Vascular endothelial cells express some proteoglycans and can bind certain chemokines on the luminal cell surface, which is apparently a prerequisite for leukocyte adhesion to and extravasation through blood vessels.( 54 ) Endothelial cell‐immobilized chemokines can activate integrins on leukocytes, enabling them to adhere to the endothelial cells and subsequently to extravasate into tissues. The involvement of a similar mechanism has been speculated for the extravasation of tumor cells.( 43 )
In addition, certain chemokines can bind to non‐proteoglycan‐type ECM proteins, some of which are abundantly expressed in the basal lamina of small blood vessels. CXCL12 is reported to bind fibronectin preferentially,( 55 ) although our preliminary results indicate that CXCL12 can bind to other vascular ECM components as well, including collagen IV and laminins (BG Yang & T Tanaka, unpubl. data, 2005). In addition, CCL21, CCL5/RANTES, and CXCL10/IP‐10 bind to mac25/angiomodulin expressed on the basal lamina of small venules in lymph nodes.( 56 ) Interestingly, chemokines immobilized on either of the above‐mentioned ECM components can effectively induce the chemotaxis of receptor‐expressing cells, in the absence of soluble chemokine (Bai et al., unpubl. data, 2005), suggesting that immobilized and not soluble chemokines accomplish their necessary functions in vivo.
In considering the mode of action of chemokines in vivo, it must also be noted that chemokines do not always induce the migration of cells along a positive chemical gradient. CXCL12 has been shown to induce the repulsion of human T cells at high concentrations but attraction at low ones.( 57 ) In our study, at various concentrations used, CXCL12 tends to induce mainly a random‐walk type of movement in a majority of murine peripheral T cells and thymocytes, whereas CCL21 tends to induce mainly positive migration along a chemokine gradient in these cells (Z Bai & Y Srinoulprasert, unpubl. data, 2005). Thus, certain chemokines may provide a multidirectional rather than unidirectional cue to receptor‐expressing cells, and the notion of chemokines as directional migration inducers or simple tissue‐specific attractants in tumor metastasis should be considered with caution.
Therapeutic implications. Given that chemokines play important roles in multiple steps of tumor progression and metastasis, chemokines and their receptors are now regarded as attractive molecular targets for the treatment of malignant tumors. In particular, monoclonal antibodies against chemokine receptors have been useful for inhibiting the growth and/or spread of malignant tumor cells in experimental settings. For instance, an anti‐CXCR4 monoclonal antibody significantly inhibits the metastasis of human breast carcinoma cells to the lymph nodes of SCID mice.( 41 ) Pretreatment of non‐Hodgkin lymphoma cells with an anti‐CXCR4 antibody also inhibits subsequent growth of the cells in immunodeficient mice.( 58 ) However, whether the inhibitory effects observed with these antibodies are caused only by the inhibition of chemotaxis remains unclear, because antibody‐bound tumor cells are likely to be subject to Fc‐mediated trapping by the liver and/or lung and to Fc‐mediated killing by macrophages.
Expectations are growing that chemokine receptors will be subject to therapeutic intervention using small molecule inhibitors. A specific antagonist of CXCR4, AMD3100, which is also a potent blocker of human immunodeficiency virus cell entry, was shown to inhibit the growth of brain tumors by inducing apoptotic cell death in the tumor cells.( 59 ) However, because the CXCL12‐CXCR4 system plays an important role in a variety of homeostatic processes, such as the development and migration of hemopoietic stem cells, primitive germ cells, and neural precursors, clinical applications of CXCR4 inhibitors must be approached with extreme caution.( 43 )
Conclusion
Because chemokines and chemokine receptors are important in tumor progression and metastasis, as summarized in the present review, disrupting these interactions may prove to be a useful approach for treating cancers. However, given the complex nature of carcinogenesis and metastasis formation, let alone the heterogeneity of different cancers, it is not likely that any single inhibitor or functional modulator of chemokines or chemokine receptors will become a ‘cure’ for cancer. It is more probable that, when used in conjunction with other therapeutic regimens, newly discovered chemokine‐ or chemokine‐receptor‐based agents will contribute significantly to the control of tumor cell invasion and metastasis. Such an approach may lead to many cancers becoming dormant and clinically manageable. An increased understanding of the mode of action of chemokines on tumor cells and their microenvironment will help us meet this goal.
Acknowledgments
We thank Drs T. Hirata and M.H. Jang for critical reading of the manuscript and Ms. S. Yamashita and M. Komine for secretarial assistance. The authors’ own studies were supported in part by a grant for Advanced Research on Cancer from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Grant‐in‐Aid of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity 2000; 12: 121–7. [DOI] [PubMed] [Google Scholar]
- 2. Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol 2004; 25: 75–84. [DOI] [PubMed] [Google Scholar]
- 3. Hirsch E, Katanaev VL, Garlanda C et al. Central role for G protein‐coupled phosphoinositide 3‐kinase γ in inflammation. Science 2000; 287: 1049–53. [DOI] [PubMed] [Google Scholar]
- 4. Curnock AP, Sotsios Y, Wright KL, Ward SG. Optimal chemotactic responses of leukemic T cells to stromal cell‐derived factor‐1 requires the activation of both class IA and IB phosphoinositide 3‐kinases. J Immunol 2003; 170: 4021–30. [DOI] [PubMed] [Google Scholar]
- 5. Servant G, Weiner OD, Herzmark P, Balla T, Sedat JW, Bourne HR. Polarization of chemoattractant receptor signaling during neutrophil chemotaxis. Science 2000; 287: 1037–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meili R, Firtel RA. Two poles and a compass. Cell 2003; 114: 153–6. [DOI] [PubMed] [Google Scholar]
- 7. Ward SG. Do phosphoinositide 3‐kinases direct lymphocyte navigation? Trends Immunol 2004; 25: 67–74. [DOI] [PubMed] [Google Scholar]
- 8. Fukui Y, Hashimoto O, Sanui T et al. Haematopoietic cell‐specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature 2001; 412: 826–31. [DOI] [PubMed] [Google Scholar]
- 9. Weber KS, Klickstein LB, Wever PC, Weber C. Chemokine‐induced monocyte transmigration requires cdc42‐mediated cytoskeletal changes. Eur J Immunol 1998; 28: 2245–51. [DOI] [PubMed] [Google Scholar]
- 10. Li Z, Hannigan M, Mo Z et al. Directional sensing requires G βγ‐mediated PAK1 and PIX α‐dependent activation of Cdc42. Cell 2003; 114: 215–27. [DOI] [PubMed] [Google Scholar]
- 11. Xu J, Wang F, Keymeulen AV et al. Divergent signals and cytoskeletal assemblies regulate self‐organizing polarity in neutrophils. Cell 2003; 114: 201–14. [DOI] [PubMed] [Google Scholar]
- 12. Shimonaka M, Katagiri K, Nakayama T et al. Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow. J Cell Biol 2003; 161: 417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katagiri K, Maeda A, Shimonaka M, Kinashi T. RAPL, a Rap1‐binding molecule that mediates Rap1‐induced adhesion through spatial regulation of LFA‐1. Nat Immunol 2003; 4: 741–8. [DOI] [PubMed] [Google Scholar]
- 14. Katagiri K, Ohnishi N, Kabashima K et al. Critical functions of the Rap1 effector molecule RAPL in lymphocyte and dendritic cell trafficking. Nat Immunol 2004; 5: 1045–51. [DOI] [PubMed] [Google Scholar]
- 15. Yang TY, Chen SC, Leach MW et al. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J Exp Med 2000; 191: 445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Payne AS, Cornelius L. A, The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol 2002; 118: 915–22. [DOI] [PubMed] [Google Scholar]
- 17. Singh RK, Gutman M, Radinsky R, Bucana CD, Fidler IJ. Expression of interleukin 8 correlates with the metastatic potential of human melanoma cells in nude mice. Cancer Res 1994; 54: 3242–7. [PubMed] [Google Scholar]
- 18. Varney ML, Li A, Dave BJ, Bucana CD, Johansson SL, Singh RK. Expression of CXCR1 and CXCR2 receptors in malignant melanoma with different metastatic potential and their role in interleukin‐8 (CXCL‐8)‐mediated modulation of metastatic phenotype. Clin Exp Metastasis 2003; 20: 723–31. [DOI] [PubMed] [Google Scholar]
- 19. Norgauer J, Metzner B, Schraufstatter I. Expression and growth‐promoting function of the IL‐8 receptor β in human melanoma cells. J Immunol 1996; 156: 1132–7. [PubMed] [Google Scholar]
- 20. Arya M, Patel HR, Williamson M. Chemokines: key players in cancer. Curr Med Res Opin 2003; 19: 557–64. [DOI] [PubMed] [Google Scholar]
- 21. Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem 2002; 277: 49481–7. [DOI] [PubMed] [Google Scholar]
- 22. Kawada K, Sonoshita M, Sakashita H et al. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res 2004; 64: 4010–7. [DOI] [PubMed] [Google Scholar]
- 23. Strieter RM, Polverini PJ, Kunkel SL et al. The functional role of the ELR motif in CXC chemokine‐mediated angiogenesis. J Biol Chem 1995; 270: 27348–57. [DOI] [PubMed] [Google Scholar]
- 24. Ferrer FA, Miller LJ, Andrawis RI et al. Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology 1998; 51: 161–7. [DOI] [PubMed] [Google Scholar]
- 25. Veltri RW, Miller MC, Zhao G et al. Interleukin‐8 serum levels in patients with benign prostatic hyperplasia and prostate cancer. Urology 1999; 53: 139–47. [DOI] [PubMed] [Google Scholar]
- 26. Uehara H, Troncoso P, Johnston D et al. Expression of interleukin‐8 gene in radical prostatectomy specimens is associated with advanced pathologic stage. Prostate 2005; 64: 40–9. [DOI] [PubMed] [Google Scholar]
- 27. Moore BB, Arenberg DA, Stoy K et al. Distinct CXC chemokines mediate tumorigenicity of prostate cancer cells. Am J Pathol 1999; 154: 1503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith DR, Polverini PJ, Kunkel SL et al. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 1994; 179: 1409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Strieter RM, Polverini PJ, Arenberg DA et al. Role of C‐X‐C chemokines as regulators of angiogenesis in lung cancer. J Leukoc Biol 1995; 57: 752–62. [DOI] [PubMed] [Google Scholar]
- 30. Arenberg DA, Kunkel SL, Polverini PJ et al. Interferon‐γ‐inducible protein 10 (IP‐10) is an angiostatic factor that inhibits human non‐small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med 1996; 184: 981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sgadari C, Angiolillo AL, Cherney BW et al. Interferon‐inducible protein‐10 identified as a mediator of tumor necrosis in vivo. Proc Natl Acad Sci USA 1996; 93: 13791–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luboshits G, Shina S, Kaplan O et al. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res 1999; 59: 4681–7. [PubMed] [Google Scholar]
- 33. Robinson SC, Scott KA, Wilson JL, Thompson RG, Proudfoot AE, Balkwill FR. A chemokine receptor antagonist inhibits experimental breast tumor growth. Cancer Res 2003; 63: 8360–5. [PubMed] [Google Scholar]
- 34. Ohta M, Kitadai Y, Tanaka S et al. Monocyte chemoattractant protein‐1 expression correlates with macrophage infiltration and tumor vascularity in human esophageal squamous cell carcinomas. Int J Cancer 2002; 102: 220–4. [DOI] [PubMed] [Google Scholar]
- 35. Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony‐stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001; 193: 727–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin EY, Gouon‐Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF‐1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia 2002; 7: 147–62. [DOI] [PubMed] [Google Scholar]
- 37. Monti P, Leone BE, Marchesi F et al. The CC chemokine MCP‐1/CCL2 in pancreatic cancer progression: regulation of expression and potential mechanisms of antimalignant activity. Cancer Res 2003; 63: 7451–61. [PubMed] [Google Scholar]
- 38. Bell D, Chomarat P, Broyles D et al. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med 1999; 190: 1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zou W, Machelon V, Coulomb‐L’Hermin A et al. Stromal‐derived factor‐1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med 2001; 7: 1339–46. [DOI] [PubMed] [Google Scholar]
- 40. Vicari AP, Caux C. Chemokines in cancer. Cytokine Growth Factor Rev 2002; 13: 143–54. [DOI] [PubMed] [Google Scholar]
- 41. Muller A, Homey B, Soto H et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–6. [DOI] [PubMed] [Google Scholar]
- 42. Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol 2004; 14: 171–9. [DOI] [PubMed] [Google Scholar]
- 43. Balkwill F. Cancer and the chemokine network. Nat Rev Cancer 2004; 4: 540–50. [DOI] [PubMed] [Google Scholar]
- 44. Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol 2004; 14: 181–5. [DOI] [PubMed] [Google Scholar]
- 45. Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor‐7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst 2001; 93: 1638–43. [DOI] [PubMed] [Google Scholar]
- 46. Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 2004; 4: 360–70. [DOI] [PubMed] [Google Scholar]
- 47. Cavanagh LL, Von Andrian UH. Travellers in many guises: the origins and destinations of dendritic cells. Immunol Cell Biol 2002; 80: 448–62. [DOI] [PubMed] [Google Scholar]
- 48. Mashino K, Sadanaga N, Yamaguchi H et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res 2002; 62: 2937–41. [PubMed] [Google Scholar]
- 49. Ding Y, Shimada Y, Maeda M et al. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res 2003; 9: 3406–12. [PubMed] [Google Scholar]
- 50. Ishida T, Utsunomiya A, Iida S et al. Clinical significance of CCR4 expression in adult T‐cell leukemia/lymphoma: its close association with skin involvement and unfavorable outcome. Clin Cancer Res 2003; 9: 3625–34. [PubMed] [Google Scholar]
- 51. Kleinhans M, Tun‐Kyi A, Gilliet M et al. Functional expression of the eotaxin receptor CCR3 in CD30+ cutaneous T‐cell lymphoma. Blood 2003; 101: 1487–93. [DOI] [PubMed] [Google Scholar]
- 52. Kuschert GS, Coulin F, Power CA et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 1999; 38: 12959–68. [DOI] [PubMed] [Google Scholar]
- 53. Kawashima H, Atarashi K, Hirose M et al. Oversulfated chondroitin/dermatan sulfates containing GlcAβ1/IdoAα1–3GalNAc (4,6‐O‐disulfate) interact with L‐ and P‐selectin and chemokines. J Biol Chem 2002; 277: 12921–30. [DOI] [PubMed] [Google Scholar]
- 54. Rot A, Von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol 2004; 22: 891–928. [DOI] [PubMed] [Google Scholar]
- 55. Pelletier AJ, Van Der Laan LJ, Hildbrand P et al. Presentation of chemokine SDF‐1 alpha by fibronectin mediates directed migration of T cells. Blood 2000; 96: 2682–90. [PubMed] [Google Scholar]
- 56. Nagakubo D, Murai T, Tanaka T et al. A high endothelial venule secretory protein, mac25/angiomodulin, interacts with multiple high endothelial venule‐associated molecules including chemokines. J Immunol 2003; 171: 553–61. [DOI] [PubMed] [Google Scholar]
- 57. Poznansky MC, Olszak IT, Foxall R, Evans RH, Luster AD, Scadden DT. Active movement of T cells away from a chemokine. Nat Med 2000; 6: 543–8. [DOI] [PubMed] [Google Scholar]
- 58. Bertolini F, Dell’Agnola C, Mancuso P et al. CXCR4 neutralization, a novel therapeutic approach for non‐Hodgkin's lymphoma. Cancer Res 2002; 62: 3106–12. [PubMed] [Google Scholar]
- 59. Rubin JB, Kung AL, Klein RS et al. A small‐molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Nat Acad Sci USA 2003; 100: 13513–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


