Abstract
Several studies have suggested that lactoferrin administration may decrease the serum level of hepatitis C virus (HCV) RNA in patients with chronic hepatitis C. The aim of the present study was to confirm the efficacy of orally administered bovine lactoferrin (bLF) in patients with chronic hepatitis C. The patients with chronic hepatitis C randomly received either oral bLF at a dose of 1.8 g daily for 12 weeks, or an oral placebo. The primary endpoint was the virologic response, defined as a 50% or greater decrease in serum HCV RNA level at 12 weeks compared with the baseline. The secondary endpoint was the biochemical response, which was defined as a 50% or greater decrease in the serum alanine aminotransferase (ALT) level at 12 weeks compared with the baseline. One hundred and ninety‐eight of 199 patients were evaluable for efficacy and safety. bLF treatment was well tolerated and no serious toxicities were observed. A virologic response was achieved in 14 of 97 patients (14.4%) in the bLF group, and 19 of 101 (18.8%) in the placebo group. There was no significant difference in virologic response rates between the two groups (−4.4%, 95% confidence interval −14.8, 6.1). In addition, bLF intake did not have any favorable effect on the serum ALT level. The virologic responses were not different between two groups in any subgroup analysis. In conclusion, orally administered bLF does not demonstrate any significant efficacy in patients with chronic hepatitis C. (Cancer Sci 2006; 97: 1105–1110)
Abbreviations:
- ALT
alanine aminotransferase
- bLF
bovine lactoferrin
- CI
confidence interval
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IL
interleukin
- NK
natural killer.
Hepatitis C virus is a leading cause of chronic liver disease in Japan, and nearly two million people are estimated to be infected.( 1 ) It is well known that HCV infection frequently causes chronic hepatitis, and that chronic hepatitis eventually progresses to liver cirrhosis and HCC approximately 30 years after HCV infection.( 2 ) In Japan, more than 30 000 people die of HCC annually, and approximately 80% of HCC patients are infected with HCV.( 3 ) Therefore, effective anti‐HCV therapy is necessary to reduce the number of patients suffering from cirrhosis or HCC. To date, interferon‐based therapy is the only effective treatment used clinically for chronic hepatitis C. A sustained complete virologic response (loss of detectable serum HCV RNA) occurs in 15–20% of patients with chronic hepatitis C after interferon therapy.( 4 ) Moreover, recent studies have demonstrated that interferon with ribavirin or peginterferon with ribavirin improves the sustained complete virologic response rate by up to 40–50%.( 5 , 6 ) However, because more than half of patients do not respond to interferon therapy, and because interferon therapy sometimes induces strong adverse effects, further developments in the treatment of chronic hepatitis C are required.
Lactoferrin, a member of the transferrin family of iron‐binding glycoproteins, is present mainly in breast milk and other exocrine secretions. Several biological activities of lactoferrin have been demonstrated, including regulation of iron absorption in the intestine and modulation of immunoreactions.( 7 ) Lactoferrin also plays an important role in human innate defense mechanisms against bacteria, fungi and viruses.( 8 ) In vitro studies to date have shown that lactoferrin has antiviral effects against human immunodeficiency virus‐1 and human cytomegalovirus.( 9 ) Recent experimental studies have suggested that lactoferrin has antiviral effect against HCV.( 10 , 11 , 12 ) Yi et al. have reported that lactoferrin binds to HCV envelope proteins in vitro.( 10 ) Ikeda et al. have reported that lactoferrin prevents HCV infection in cultured human hepatocytes, and suggested that the anti‐HCV activity of lactoferrin might be related to its direct binding to viral surfaces.( 11 , 12 ) In addition, recent clinical studies have demonstrated the potential efficacy of lactoferrin against chronic hepatitis C.( 13 , 14 ) Tanaka et al. reported that 8‐week oral administration of bLF at a dose of 1.8 or 3.6 g/day decreased the serum level of HCV RNA markedly in three of four patients with a low pretreatment HCV RNA level (<100 Kcopy/mL).( 13 ) Iwasa et al. administered bLF (3.6 g/day) orally to 15 patients with high viral loads (100 KIU/mL), and reported that the mean serum HCV RNA level decreased significantly from 1106 KIU/mL at entry to 612 KIU/mL after 6 months of treatment (P < 0.01).( 14 ) Based on these promising findings, we planned to investigate the efficacy of orally administered bLF in patients with chronic hepatitis C. First, we conducted a dose‐finding study in 45 patients with chronic hepatitis C.( 15 ) In that study, three dose levels of bLF (1.8, 3.6 and 7.2 g/day) were scheduled, and 15 patients at each dose level received the determined dose of bLF for 8 weeks. bLF treatment was well tolerated up to 7.2 g/day, and no serious adverse events were observed. Although no relationship between bLF dose and efficacy was recognized, a 50% or greater decrease in the serum HCV RNA level was seen in four of 45 patients (8.9%). Furthermore, the HCV RNA level was decreased by 50% or more in eight patients (17.8%) at week 8 after the end of treatment. These results encouraged us to conduct further investigations, and the present randomized trial was designed to clarify the anti‐HCV activity of bLF in patients with chronic hepatitis C.
Patients and Methods
Patients. Each patient was required to meet the following eligibility criteria: 20–74 years of age; positivity for anti‐HCV antibody; an HCV RNA level of 0.5–850 KIU/mL evaluated within 1 month before entry; a sustained elevation of serum ALT level for at least 6 months; a serum ALT level of at least twice the upper normal limit evaluated within 1 month before entry; no evidence of HCC on the basis of ultrasonography or computed tomography carried out within 3 months before entry; and adequate bone marrow function (white blood cell count 4000/mm3, platelet count 100 000/mm3, and hemoglobin level 11 g/dL), liver function (total bilirubin level 2.0 mg/dL, serum albumin level 3.5 g/dL, and serum aspartate aminotransferase and ALT level 200 IU/L) and renal function (normal serum creatinine and blood urea nitrogen levels).
The exclusion criteria were: positivity for hepatitis B surface antigen; interferon therapy within 6 months before entry; immunomodulatory or corticosteroid therapy within 3 months before entry; intravenous glycyrrhizin therapy within 1 month before entry; past or present history of bLF tablet intake; pregnant or lactating females; severe hepatic disease (e.g. autoimmune hepatitis and primary biliary cirrhosis); other serious medical conditions (e.g. gastrointestinal bleeding, active infection, severe pulmonary disease and psychiatric disorders).
Methods. This double‐blind, placebo‐controlled phase III trial was conducted at 11 centers in Japan. The study was approved by the institutional review board at each center, and all the participants provided written informed consent. Eligible participants were assigned randomly to one of two treatment groups in equal proportions using permutation blocks stratified by centers. A randomization list was drawn up using the SAS random number generator at the data center (Quintiles Transnational Japan K. K. Tokyo, Japan). The treatments consisted of bLF at a dose of 1.8 g/day or a placebo, administered orally twice daily for 12 weeks. In the current study, bLF at 1.8 g/day was selected on the basis of the previous dose‐finding study, which indicated that there was no significant relationship between bLF dose (range, 1.8–7.2 g/day) and anti‐HCV activity.( 15 ) After the treatment allocation, the data center sent a numbered container of bLF or placebo tablets to a participant. During treatment, combined use of interferon, immunomodulatory therapy, corticosteroid and intravenous glycyrrhizin was prohibited. bLF (450 mg/tablet) and placebo tablets were provided by Morinaga Milk Industries (Tokyo, Japan).
In the current study, we tested the hypothesis that oral administration of bLF would: (1) reduce the serum HCV RNA level; and (2) reduce the serum ALT level in patients with chronic hepatitis C. In addition, we investigated the influence of orally administered bLF on systemic immune response in a small group of participants. The participants were evaluated every 4 weeks as outpatients until 4 weeks after completion of treatment. Serum HCV RNA level and serum ALT level were measured before treatment, during treatment at weeks 4, 8 and 12, and at 4 weeks after treatment. Serum HCV RNA level was determined by reverse transcription–polymerase chain reaction using the Amplicor‐HCV monitor V 2.0 kit with a sensitivity of 0.5 KIU/mL (Roche Diagnostics, Tokyo, Japan). Anti‐HCV antibody was determined by chemiluminescent enzyme immunoassay (Ortho‐Clinical Diagnostics, Tokyo, Japan). HCV serotyping was carried out as described previously.( 16 ) HCV serotype 1 corresponds to genotypes 1a and 1b of the Simmonds classification, and HCV serotype 2 corresponds to genotypes 2a and 2b.( 17 ) Serum concentration of IL‐18 was measured in participants at two institutions (National Cancer Center Hospital and Osaka Red Cross Hospital), and the percentage of CD4+, CD8+, CD16+ and CD56+ peripheral blood lymphocytes was measured in participants at the National Cancer Center Hospital. IL‐18 and all lymphocytes were measured before treatment, during treatment at weeks 4, 8 and 12, and at 4 weeks after completion of treatment. Serum concentration of IL‐18 was assayed with a human IL‐18 enzyme‐linked immunosorbent assay kit (Medical and Biological Laboratories, Nagoya, Japan). Lymphocyte surface phenotypes of CD4, CD8, CD16 and CD56 were determined by flow cytometry.
Adverse events were graded for severity according to the Japan Society for Cancer Therapy criteria,( 18 ) which are similar to the National Cancer Institute Common Toxicity criteria. During treatment, participants were asked to record in a daily journal both compliance and any adverse events they experienced.
Assessment of efficacy and statistical analysis. Analyses were carried out on an intention to treat basis. The primary endpoint was a virologic response. In the current study, we defined a virologic response as a 50% or greater decrease in the serum HCV RNA level at 12 weeks compared with the baseline. Secondary endpoints were a biochemical response, as were changes in serum HCV RNA level and serum ALT level. If the serum ALT level at 12 weeks showed both a 50% decrease compared with the baseline and was twice the upper normal limit, we considered it a biochemical response. Response rate was calculated as the number of responders divided by the total number in each group. Participants whose HCV RNA (or ALT) data at 12 weeks were missing were included only in the denominator. Change in HCV RNA level (or ALT level) was calculated as the logarithm of the HCV RNA level (or ALT level) at 12 weeks minus the logarithm of these at the baseline. Differences in the virologic or biochemical response rates between two groups were analyzed using a test for the difference between two proportions. Differences in the change in HCV RNA level or ALT level between two groups were analyzed using a test for the difference between two means. In addition to the above planned analyses, subgroup analyses for virologic response were carried out based on pretreatment variables including age, serum HCV RNA level and HCV serotype. In a small group of participants, change in the serum concentration of IL‐18 and changes in the percentage of CD4+, CD8+, CD16+ and CD56+ peripheral blood lymphocytes during the study period were investigated. Analyses were carried out using JMP4.0 and PC SAS Release v.8.02 (SAS Institute Japan Ltd, Tokyo, Japan). All P‐values are two‐tailed, and differences at P < 0.05 were regarded as statistically significant.
We estimated that a total of 250 participants would be the maximum to enroll for a 2‐year enrollment period. Subsequent power analysis revealed that 125 participants per group would have 75% power to detect a 10% difference in the virologic response rate (15 vs 5%) at the 5% level of significance. An interim analysis by the independent data monitoring committee was planned after the first 125 participants had been enrolled. All trial personnel and participants were blinded to treatment assignment for the duration of the trial. Only the trial statistician and the independent data monitoring committee saw unblinded data. In the interim analysis of the primary endpoint, the O’Brien–Fleming method was used.( 19 )
Results
Patients. Enrollment began at seven institutions in April 2001. Because 250 participants were not enrolled for the 2 years planned originally, we extended the registration period for one more year and increased the number of participating institutions from seven to 11. An interim analysis was carried out in March 2004 with the data from the first 125 participants. Because the results of the interim analysis indicated that it was highly unlikely that a significant difference in treatment efficacy between the two groups would be observed with the planned full enrollment of 250 participants, the data monitoring committee recommended discontinuation of further enrollment. Therefore, enrollment was stopped on 31 March 2004, at which point 199 participants had been enrolled. Because one patient refused to participate in the study before randomization, efficacy and safety were analyzed in the remaining 198 participants (97 bLF and 101 placebo) (Fig. 1). Although three participants in the bLF group discontinued treatment for reasons other than an adverse event, the remaining 195 participants completed the scheduled 12 weeks of treatment. The baseline characteristics of the 198 participants are shown in Table 1. There was no significant difference between the bLF and placebo groups regarding the pretreatment characteristics including age, sex, serum ALT level and serum HCV RNA level.
Figure 1.
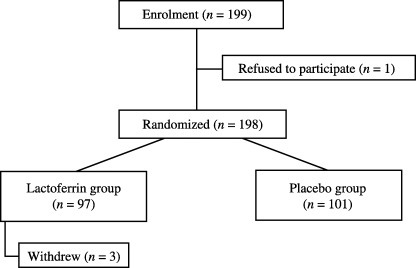
Flow diagram of participant enrolment.
Table 1.
Baseline characteristics of the patients
| Characteristic | Bovine lactoferrin | Placebo |
|---|---|---|
| No. patients | 97 | 101 |
| Age (years) † | 61 (29–74) | 58 (31–74) |
| Sex (male/female) | 53/44 | 55/46 |
| History of interferon therapy | 25 | 29 |
| ALT level (IU/L) † | 91 (41–340) | 98 (27–250) |
| HCV RNA level (KIU/mL) † | 378 (8.8–960) | 452 (8.0–1560) |
| HCV serotype (1/2/ND) | 78/17/1 | 76/22/3 |
Median (range). ALT, alanine aminotransferase; HCV, hepatitis C virus; ND, not determined.
Virologic efficacy. Virologic response, the primary endpoint, was assessed in all 198 participants who received at least one dose of treatment. Virologic response was observed in 14 of 97 participants (14.4%) in the bLF group, and in 19 of 101 (18.8%) in the placebo group (Table 2). No complete virologic response (loss of detectable serum HCV RNA) was seen in either of the groups. There was no significant difference in the virologic response rate with bLF treatment in comparison with the placebo (−4.4%, 95% CI −14.8, 6.1). Change in the HCV RNA level at 12 weeks compared with the baseline was assessed in 190 participants (93 bLF group, 97 placebo group), excluding eight participants for whom HCV RNA data at 12 weeks were lacking. The change in the mean logarithm of the HCV RNA level was −0.09 in the bLF group and −0.09 in the placebo group, indicating no significant difference between the groups (P = 1.00).
Table 2.
Virologic and biochemical efficacy
| Characteristic | Bovine Lactoferrin | Placebo | Difference (95% CI) | P‐value |
|---|---|---|---|---|
| Virologic efficacy | ||||
| Response rate (%) | 14.4 | 18.8 | −4.4 (−14.8, 6.1) | |
| Change in HCV RNA level † | −0.09 | −0.09 | 1.00 | |
| Biochemical efficacy | ||||
| Response rate (%) | 6.2 | 4.0 | 2.2 (−3.9, 8.3) | |
| Change in ALT level † | −0.085 | −0.080 | 0.93 | |
Mean logarithm. ALT, alanine aminotransferase; CI, confidence interval; HCV, hepatitis C virus.
Biochemical efficacy. Biochemical response was assessed in 198 participants. Biochemical response was seen in six of 97 participants (6.2%) in the bLF group, and in four of 101 participants (4.0%) in the placebo group (Table 2). No significant difference in the biochemical response rate was seen between the groups (2.2%, 95% CI −3.9, 8.3). Change in the serum AST level was assessed in 192 participants (93 bLF group, 99 placebo group), excluding six participants for whom ALT data at 12 weeks were lacking. The change in the mean logarithm of the ALT level was −0.085 in the bLF group and −0.080 in the placebo group, indicating no significant difference (P = 0.93).
Subgroup analysis. The rates of virologic response with respect to pretreatment variables are presented in Table 3. Among participants with a low HCV RNA level (<100 KIU/mL), the virologic response rate was 29.4% in the bLF group and 15.4% in the placebo group, indicating no significant difference between the groups (14.0%, 95% CI −15.2, 43.2). The virologic responses were also not different between two groups in other subgroup analyses such as age, sex and HCV serotype.
Table 3.
Virologic response rate as a function of baseline variables
| Variable | Bovine lactoferrin (n = 97) | Placebo (n = 101) | Difference | |||
|---|---|---|---|---|---|---|
| Response/total | % | Response/total | % | % | 95% CI | |
| Age | ||||||
| <65 years | 12/62 | 19.4 | 14/77 | 18.2 | 1.2 | −11.9, 14.2 |
| ≥65 years | 2/35 | 5.7 | 5/24 | 20.8 | −15.1 | −33.1, 2.9 |
| Sex | ||||||
| Male | 10/53 | 18.9 | 10/55 | 18.2 | 0.7 | −14.0, 15.3 |
| Female | 4/44 | 9.1 | 9/46 | 19.6 | −10.5 | −24.7, 3.8 |
| ALT level | ||||||
| <100 IU/L | 6/57 | 10.5 | 7/51 | 13.7 | −3.2 | −15.6, 9.2 |
| ≥100 IU/L | 8/40 | 20.0 | 12/50 | 24.0 | −4.0 | −21.1, 13.1 |
| HCV RNA level | ||||||
| <100 KIU/mL | 5/17 | 29.4 | 2/13 | 15.4 | 14.0 | −15.2, 43.2 |
| ≥100 KIU/mL | 9/80 | 11.3 | 17/88 | 19.3 | −8.0 | −18.8, 2.7 |
| HCV serotype † | ||||||
| 1 | 11/78 | 14.1 | 16/76 | 21.1 | −7.0 | −18.9, 5.0 |
| 2 | 3/18 | 16.7 | 2/22 | 9.1 | 7.6 | −31.4, 28.6 |
Hepatitis C virus serotype was not measured in four patients. ALT, alanine aminotransferase; CI, confidence interval; HCV, hepatitis C virus.
Analysis of IL‐18 and lymphocytes. The serum concentration of IL‐18 was measured in 73 participants enrolled at the National Cancer Center Hospital and Osaka Red Cross Hospital (36 bLF, 37 placebo). Figure 2 shows the changes in the mean IL‐18 levels in the bLF group and placebo group. The mean IL‐18 levels in the bLF and placebo groups were 293.9 pg/mL and 309.9 pg/dL at the baseline and 280.7 pg/mL and 291.5 pg/mL at 12 weeks, respectively. The corresponding changes in the mean IL‐18 level at 12 weeks were −14.5 pg/mL and −15.9 pg/mL, respectively, indicating no significant difference between the groups (P = 0.91). Similarly, there were no significant differences between the groups at any other points during the study period. The percentage of lymphocyte was measured in 46 participants at the National Cancer Center Hospital (bLF 23, placebo 23), and the results are shown in Fig. 3. The percentage of CD4+, CD8+, CD16+ and CD56+ peripheral blood lymphocytes remained almost unchanged throughout the study in both groups, and the differences between them were not significant.
Figure 2.
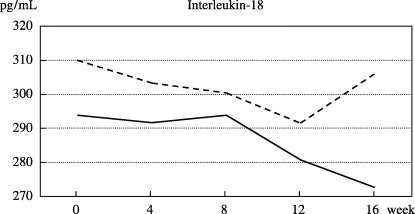
Changes in the mean serum concentration of interleukin‐18 in the bovine lactoferrin group (straight line, n = 36) and the placebo group (dotted line, n = 37).
Figure 3.
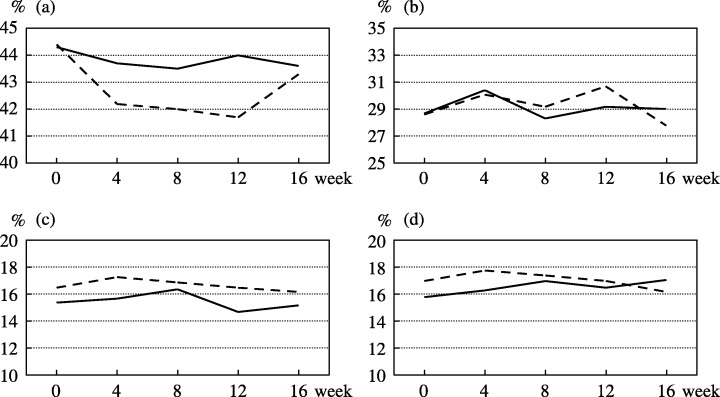
Changes in the mean percentages of (a) CD4+, (b) CD8+, (c) CD16+ and (d) CD56+ peripheral blood lymphocytes in the bovine lactoferrin group (straight line, n = 23) and the placebo group (dotted line, n = 23).
Safety. Safety was assessed in 198 participants who received at least one dose of bLF or placebo during the study. The bLF treatment was well tolerated, and no serious complications occurred during the treatment. Although minor adverse events including neutropenia, γ‐GTP elevation and hyperglycemia were observed in participants treated with bLF, their frequency and intensity did not differ from those in the placebo group. HCC was detected in one participant in the bLF group and in one participant in the placebo group during the study period.
Discussion
The present study was carried out to confirm the anti‐HCV activity of orally administered bLF in patients with chronic hepatitis C. A virologic response (a 50% or greater decrease in the serum level of HCV RNA at 12 weeks compared with the baseline) was observed in 14 of 97 participants (14.4%) in the bLF group, and 19 of 101 (18.8%) in the placebo group, the difference between the groups being non‐significant. The virologic responses were not different between two groups in any subgroup analysis. Furthermore, bLF intake did not have any favorable effect on the serum ALT level. On the basis of these results, we concluded that orally administered bLF did not have any efficacy, including anti‐HCV activity, in patients with chronic hepatitis C.
The virologic response rate of 14.4% observed in the bLF group was somewhat higher than that reported in the previous dose‐finding study,( 15 ) in which four of 45 patients (8.9%) showed a virologic response at the end of bLF treatment. Nevertheless, the current study failed to demonstrate any anti‐HCV activity of bLF, because a similar virologic response rate to that in the bLF group was seen in the placebo group. Having designed this randomized study, we assumed that a virologic response rate of around 5% would be seen in the placebo group due to spontaneous remission of viral activity. However, contrary to our expectation, 19 of 101 participants (18.8%) in the placebo group showed a 50% decrease in the HCV RNA level at 12 weeks, indicating that our assumption was inappropriate. Our results suggested that in order to assess the reduction of the HCV RNA level, periodic evaluation would be necessary to exclude the influence of spontaneous fluctuation of HCV RNA.
Several experimental studies have suggested that lactoferrin has some activity against HCV. Yi et al.( 10 ) reported that lactoferrin binds to the HCV E1 and E2 envelope proteins in vitro, and Ikeda et al.( 11 , 12 ) reported that lactoferrin prevents HCV infection in cultured human hepatocytes. They suggested that the anti‐HCV activity of lactoferrin might be due to a neutralizing efficacy, in which the administered lactoferrin became bound directly to the HCV virion, thus inhibiting adsorption of the HCV‐lactoferrin complex into human hepatocytes. Therefore, intravenous administration of lactoferrin might improve the viremic state in patients with chronic hepatitis C. However, for practical application, administration of lactoferrin directly into blood does not seem to be a suitable approach because lactoferrin is a large glycoprotein molecule (80 kDa) that may cause allergic reactions. Therefore, oral administration of bLF was selected for the present study, even though the metabolism and mechanism of ingested lactoferrin are yet to be clarified. As to absorption, it has been reported that intact lactoferrin and its fragments are present in the urine of human milk‐fed preterm infants.( 20 ) However, in adult rats, lactoferrin and its fragments are not detectable in portal blood after bLF ingestion,( 21 ) and in adult humans, the serum lactoferrin level does not increase after oral administration of recombinant human lactoferrin.( 22 ) However, several studies have suggested that orally administered lactoferrin might enhance immune responses via cytokine production.( 23 , 24 ) It has been reported that oral administration of bLF to mice enhances the production of IL‐18 and interferon‐γ in the mucosa of the small intestine, and increases the number of CD4+, CD8+ and NK cells in the small‐intestinal epithelium.( 25 , 26 ) Varadhachary et al. reported that oral administration of recombinant human lactoferrin to mice stimulates IL‐18 production from gut enterocytes, and augments the NK activity of spleen cells and production of blood CD8+ cells.( 27 ) Furthermore, a recent clinical study has demonstrated that oral administration of bLF (0.6 g/day) for 3 months in 36 patients with chronic hepatitis C increased the serum IL‐18 level significantly compared with the baseline.( 28 ) However, our study found no evidence that oral administration of bLF influences the serum concentration of IL‐18 or the percentage of CD4+, CD8+, CD16+ and CD56+ lymphocytes. Further investigations are required to clarify the peripheral and systemic effects of orally administered lactoferrin. In addition, as many in vitro studies have suggested that lactoferrin has direct binding neutralizing efficacy against HCV,( 29 , 30 , 31 ) further investigations are needed to devise a means of delivering lactoferrin or its fragment into the bloodstream safely and effectively.
Recently, several studies have investigated the value of adding lactoferrin to interferon therapy for chronic hepatitis C. Hirashima et al. randomly assigned 21 patients with chronic hepatitis C to either a consensus interferon plus oral lactoferrin (3.0 g/day) group or a consensus interferon monotherapy group.( 32 ) Three of 10 patients in the consensus interferon plus lactoferrin group showed a sustained complete virologic response, as did four of 11 patients in the consensus interferon group, indicating no statistically significant difference between the groups. Ishibashi et al. conducted a randomized controlled trial to investigate the efficacy of interferon α‐2b and ribavirin plus oral lactoferrin (0.6 g/day) compared with interferon α‐2b and ribavirin plus placebo in 36 patients with chronic hepatitis C.( 33 ) A sustained complete virologic response was seen in six of 18 patients in the lactoferrin group and in five of 18 patients in the placebo group, there being no statistically significant difference between the groups (P = 0.7). Although the numbers of patients recruited in the two randomized trials were small, these results suggested that the additional value of oral lactoferrin combined with interferon therapy would be negative for the treatment of chronic hepatitis C.
In summary, oral administration of bLF at a dose of 1.8 g/day for 12 weeks showed an acceptable safety profile in patients with chronic hepatitis C. However, there was no significant difference in the virologic responses between patients who received oral bLF and those receiving placebo. In addition, bLF intake did not have any favorable effect on the serum ALT level. These findings do not support the practical use of oral bLF in patients with chronic hepatitis C.
Acknowledgments
This article is dedicated to the memory of the late Dr S. Okada, a principal investigator, and the late Dr N. Okazaki, the chairman of the data monitoring committee. We thank Drs M. Kato, Y. Inaba and K. Hayashi as members of the data monitoring committee. We are grateful to Dr H. Tsuda as a scientific contributor. We also thank Ms K. Kondo and Dr H. Nakajima for assistance in data management, and Morinaga Milk Industries for providing the bLF tablets and placebo. This work was supported by Grants‐in‐Aid for Cancer Research for the Second‐ and Third‐Term Comprehensive 10‐Year Strategy for Cancer Control from the Ministry of Health, Labor and Welfare of Japan.
References
- 1. Higuchi M, Tanaka E, Kiyosawa K. Epidemiology and clinical aspects on hepatitis C. Jpn J Infect Dis 2002; 55: 69–77. [PubMed] [Google Scholar]
- 2. Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349: 825–32. [DOI] [PubMed] [Google Scholar]
- 3. Yoshizawa H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology 2002; 62: 8–17. [DOI] [PubMed] [Google Scholar]
- 4. Poynard T, Leroy V, Cohard M et al. Meta‐analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology 1996; 24: 778–89. [DOI] [PubMed] [Google Scholar]
- 5. Poynard T, Marcellin P, Lee SS et al. Randomised trial of interferon α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Ther Group (IHIT) Lancet 1998; 352: 1426–32. [DOI] [PubMed] [Google Scholar]
- 6. Manns MP, McHutchison JG, Gordon SC et al. Peginterferon α‐2b plus ribavirin compared with interferon α‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958–65. [DOI] [PubMed] [Google Scholar]
- 7. Lonnerdal B, Iyer S. Lactoferrin: molecular structure and biological function. Annu Rev Nutr 1995; 15: 93–110. [DOI] [PubMed] [Google Scholar]
- 8. Farnaud S, Evans RW. Lactoferrin − a multifunctional protein with antimicrobial properties. Mol Immunol 2003; 40: 395–405. [DOI] [PubMed] [Google Scholar]
- 9. Florisa R, Recio I, Berkhout B, Visser S. Antibacterial and antiviral effects of milk proteins and derivatives thereof. Curr Pharm Des 2003; 9: 1257–75. [DOI] [PubMed] [Google Scholar]
- 10. Yi M, Kaneko S, Yu DY, Murakami S. Hepatitis C virus envelope proteins bind lactoferrin. J Virol 1997; 71: 5997–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ikeda M, Sugiyama K, Tanaka T et al. Lactoferrin markedly inhibits hepatitis C virus infection in cultured human hepatocytes. Biochem Biophys Res Commun 1998; 245: 549–53. [DOI] [PubMed] [Google Scholar]
- 12. Ikeda M, Nozaki A, Sugiyama K et al. Characterization of antiviral activity of lactoferrin against hepatitis C virus infection in human cultured cells. Virus Res 2000; 66: 51–63. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka K, Ikeda M, Nozaki A et al. Lactoferrin inhibits hepatitis C: virus viremia in patients with chronic hepatitis C: a pilot study. Jpn J Cancer Res 1999; 90: 367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iwasa M, Kaito M, Ikoma J et al. Lactoferrin inhibits hepatitis C virus viremia in chronic hepatitis C patients with high viral loads and HCV genotype 1b. Am J Gastroenterol 2002; 97: 766–7. [DOI] [PubMed] [Google Scholar]
- 15. Okada S, Tanaka K, Sato T et al. Dose–response trial of lactoferrin in patients with chronic hepatitis C. Jpn J Cancer Res 2002; 93: 1063–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanaka T, Tsukiyama‐Kohara K, Yamaguchi K et al. Significance of specific antibody assay for genotyping of hepatitis C virus. Hepatology 1994; 19: 1347–53. [PubMed] [Google Scholar]
- 17. Simmonds P, McOmish F, Yap PL et al. Sequence variability in the 5′ non‐coding region of hepatitis C virus: identification of a new virus type and restrictions on sequence diversity. J General Virol 1993; 74: 661–8. [DOI] [PubMed] [Google Scholar]
- 18. Japan Society for Cancer Therapy. Criteria for the evaluation of the clinical effects of solid cancer chemotherapy. J Jpn Soc Cancer Ther 1993; 28: 101–30. [Google Scholar]
- 19. O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979; 35: 549–56. [PubMed] [Google Scholar]
- 20. Hutchens TW, Henry JF, Yip TT et al. Origin of intact lactoferrin and its DNA‐binding fragments found in the urine of human milk‐fed preterm infants. Evaluation by stable isotopic enrichment. Pediatr Res 1991; 29: 243–50. [DOI] [PubMed] [Google Scholar]
- 21. Wakabayashi H, Kuwata H, Yamauchi K, Teraguchi S, Tamura Y. No detectable transfer of dietary lactoferrin or its functional fragments to portal blood in healthy adult rats. Biosci Biotechnol Biochem 2004; 68: 853–60. [DOI] [PubMed] [Google Scholar]
- 22. Hayes TG, Falchook GF, Varadhachary GR et al. Phase I trial of oral talactoferrin alfa in refractory solid tumors. Invest New Drugs 2006; 24: 233–40. [DOI] [PubMed] [Google Scholar]
- 23. Teraguchi S, Wakabayashi H, Kuwata H, Yamauchi K, Tamura Y. Protection against infections by oral lactoferrin: evaluation in animal models. Biometals 2004; 17: 231–4. [DOI] [PubMed] [Google Scholar]
- 24. Wakabayashi H, Kurokawa M, Shin K, Teraguchi S, Tamura Y, Shiraki K. Oral lactoferrin prevents body weight loss and increases cytokine responses during herpes simplex virus type 1 infection of mice. Biosci Biotechnol Biochem 2004; 68: 537–44. [DOI] [PubMed] [Google Scholar]
- 25. Kuhara T, Iigo M, Itoh T et al. Orally administered lactoferrin exerts an antimetastatic effect and enhances production of IL‐18 in the intestinal epithelium. Nutr Cancer 2000; 38: 192–9. [DOI] [PubMed] [Google Scholar]
- 26. Wang WP, Iigo M, Sato J, Sekine K, Adachi I, Tsuda H. Activation of intestinal mucosal immunity in tumor‐bearing mice by lactoferrin. Jpn J Cancer Res 2000; 91: 1022–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varadhachary A, Wolf JS, Petrak K et al. Oral lactoferrin inhibits growth of established tumors and potentiates conventional chemotherapy. Int J Cancer 2004; 111: 398–403. [DOI] [PubMed] [Google Scholar]
- 28. Ishii K, Takamura N, Shinohara M et al. Long‐term follow‐up of chronic hepatitis C patients treated with oral lactoferrin for 12 months. Hepatol Res 2003; 25: 226–33. [DOI] [PubMed] [Google Scholar]
- 29. Nozaki A, Ikeda M, Naganuma A et al. Identification of a lactoferrin‐derived peptide possessing binding activity to hepatitis C virus E2 envelope protein. J Biol Chem 2003; 278: 10 162–73. [DOI] [PubMed] [Google Scholar]
- 30. Matsuura Y, Tani H, Suzuki K et al. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology 2001; 286: 263–75. [DOI] [PubMed] [Google Scholar]
- 31. Tamura K, Oue A, Tanaka A et al. Efficient formation of vesicular stomatitis virus pseudotypes bearing the native forms of hepatitis C virus envelope proteins detected after sonication. Microbes Infect 2005; 7: 29–40. [DOI] [PubMed] [Google Scholar]
- 32. Hirashima N, Orito E, Ohba K et al. A randomized controlled trial of consensus interferon with or without lactoferrin for chronic hepatitis C patients with genotype 1b and high viral load. Hepatol Res 2004; 29: 9–12. [DOI] [PubMed] [Google Scholar]
- 33. Ishibashi Y, Takeda K, Tsukidate N et al. Randomized placebo‐controlled trial of interferon α‐2b plus ribavirin with and without lactoferrin for chronic hepatitis C. Hepatol Res 2005; 32: 218–23. [DOI] [PubMed] [Google Scholar]


