Abstract
Epstein–Barr virus (EBV) infection is a risk factor for Hodgkin lymphoma (HL). To test whether the frequency of HL subtypes and their association with EBV has shifted with rising socioeconomic status in Taiwan, we compared the pathological features and EBV status, detected by in situ hybridization, of HL diagnosed between 1996 and 2007 (99 cases) and 1982 and 1995 (74 cases). The male‐to‐female ratio was 121:52 (2.3:1) and the mean age at presentation was 41.5 years. The overall EBV positivity rate was 50% (86/173 cases). Comparing the distribution of HL cases diagnosed at two different time periods, we found an increased frequency of the nodular sclerosis (NS) subtype (53 vs 68%, P = 0.045), a decreased frequency of the mixed cellularity subtype (35 vs 13%, P < 0.001), a reduced male‐to‐female ratio (2.9:1 compared to 1.4:1) and mean age (42.4 vs 36.6 years) in the NS subtype, and a significant decrease in EBV positivity rates among the NS and lymphocyte‐depletion subtypes (61 vs 39%, P = 0.03). These data indicate shifts in the frequency of histological subtype and EBV association for HL in Taiwan over the last decade, with a trend closer to that seen in Western countries and Japan. (Cancer Sci 2008; 99: 345–349)
Early acquisition of Epstein–Barr virus (EBV) infection is prevalent in developing countries.( 1 ) Previous studies have demonstrated that acute EBV infection (or infectious mononucleosis) is a risk factor for Hodgkin lymphoma (HL),( 2 , 3 ) and its infectious manifestations, especially age at infection, are affected by socioeconomic status.( 4 , 5 ) Among the five subtypes of HL, nodular lymphocyte predominance (NLP), nodular sclerosis (NS), mixed cellularity (MC), lymphocyte‐rich classical (LRC), and lymphocyte depletion (LD), the MC subtype is most frequently associated with EBV worldwide, ranging from 72 to 86%, whereas the NLP subtype is almost always EBV negative in Western countries.( 6 , 7 , 8 ) However, we and others have previously found that EBV is associated with all histological subtypes of HL in Vietnamese and southern American children, especially the NLP subtype, which showed a 100% positivity rate.( 6 , 9 , 10 ) The differences in EBV association between HL in developed and developing countries and with different histological subtypes may be reconciled by the ‘two‐disease hypothesis’, which proposes that the HL seen in the younger group is infectious in nature whereas that in older persons has causes similar to other types of lymphomas.( 11 , 12 )
Epidemiological studies have revealed three patterns of HL distribution. Pattern 1, seen in developing countries and in patients of low socioeconomic status, shows an early childhood peak with tumors that are predominantly MC subtype and EBV positive. Pattern 3, seen in developed countries and in patients of high socioeconomic status, shows a peak incidence in the third decade with tumors that are mainly NS subtype and EBV negative. Pattern 2, seen in countries with transitional economies, has an early childhood peak and a second decade peak with an equal frequency of the MC and NS subtypes.( 2 , 13 , 14 ) It is therefore intriguing to observe whether there exists a change in the frequency of HL subtypes and EBV association with time in countries like Taiwan, where socioeconomic status has improved over the past decades. The changing pattern of EBV association in HL may furthermore shed light on the role of EBV in HL tumorigenesis.
In the present study, by comparing the frequency of HL subtypes and EBV association in two medical centers in northern and southern Taiwan during two time periods, we demonstrated a shift toward the distribution of HL seen in Western countries, including an increased frequency of NS subtype, a decreased frequency of MC subtype, and a decreased overall EBV positivity rate, especially for the NS and LD subtypes. These findings clearly depict the trend toward EBV‐independent pathogenesis for HL in Taiwan and indicate that, with the improvement of public health status, the role of viral pathogens (e.g. EBV) in tumor generation may decrease, further supporting the theory of ‘two‐disease hypothesis’ in HL.
Materials and Methods
Hodgkin lymphoma cases. A total of 173 cases of HL were studied, including 53 cases from the National Cheng Kung University Hospital (NCKUH), and 120 cases from the Veterans General Hospital (VGH)‐Taipei, of which 70 cases have been published previously.( 15 ) Studies were carried out under a laboratory protocol approved by the institutional review board. The cases from VGH‐Taipei were submitted by using a high‐density tissue array technique as described previously.( 16 ) Some of the 70 cases published previously were reviewed, including all cases of NLP subtype. To study the changing patterns in the frequency of HL subtypes and EBV association over time, these cases were divided into two time periods: one was from 1982 to 1995 and the other was from 1996 to April 2007, based on a previous study.( 15 ) The specimens were fixed in 10% neutral formalin solution. All of the cases were reviewed and classified by two hematopathologists according to the World Health Organization (WHO) classification criteria in May 2007.( 17 ) The diagnosis made by the first pathologist was confirmed by the second reviewer. Clinical data, including sex, age, presenting features, and tumor site, were obtained by chart review.
Immunohistochemical staining. For subtype classification, immunohistochemical staining was carried out on deparaffinized tissue sections of formalin‐fixed material after microwave‐enhanced epitope retrieval. Detection was done with streptavidin‐biotinylated peroxidase‐conjugated reagents (LSAB+ kit; Dako, Carpinteria, CA, USA) with 3‐amino‐9‐ethyl carbazole as the chromogen and hematoxylin as the counterstain. The primary antibodies and working dilutions were as follows: ALK‐1 (1:25; Dako, Glostrup, Denmark), CD3 (PC3/188 A, 1:100; Dako, Glostrup), CD15 (Leu‐M1, 1:50; Becton Dickinson, San Jose, CA, USA), CD20 (L‐26, 1:50; Dako, Glostrup), CD21 (1F8, 1:50; Dako, Glostrup), CD30 (Ki‐1, Ber‐H2, 1:40; Dako, Glostrup), CD45 (leukocyte common antigen, 1:50; Dako, Glostrup), epithelial membrane antigen (EMA, 1:50; Dako, Glostrup), and κ (1:50) and λ (1:75) light chains (Dako, Glostrup).
Epstein–Barr virus detection. In situ hybridization studies were carried out for all cases of HL to detect EBV‐encoded early RNA (EBER1) using a polymerase chain reaction‐derived digoxigenin‐labeled DNA probe, as in our previous study.( 9 , 18 ) Specimens of nasopharyngeal carcinoma and tonsil were used as positive and negative controls, respectively. Only unequivocal tumor cells were evaluated for EBER1 signal expression, and EBV positivity was defined as EBER1 signals expressed in at least one unequivocal Reed‐Sternberg or Hodgkin cell, as described previously.( 19 )
Statistical analysis. Appropriate statistical tests were used to examine the relationships and correlations between variables, including the χ2‐test, and paired and unpaired t‐tests. The P‐value referred to was two sided and the analyses were carried using SPSS 13.0 statistical software (SPSS, Chicago, IL, USA).
Results
Relative frequency and clinicopathological features. There was a total of 173 cases of HL at the NCKUH and VGH centers over the years 1982–2007, with a male‐to‐female ratio of 121:52 (55:19 during 1982–1995 and 66:33 during 1996–2007), with a mean age at diagnosis of 41.5 years (41.9 years during 1982–1995 and 41.3 years during 1996–2007). Using a standard immunohistochemical panel (Fig. 1) and the morphological features outlined in the current WHO scveme for classification, the subtype distribution of 173 HL cases is summarized in Table 1. NLP accounted for 7% of cases, NS for 61%, MC for 23%, LRC for 4%, LD for 2%, and unclassified HL for 3%. Among the cases from NCKUH during the period 1996 through 2007, HL comprised 49 of the 636 (8%) malignant lymphoma cases diagnosed, which was similar to the 8% reported during 1982–1995, as detailed in the study by Liu et al. in northern Taiwan.( 15 )
Figure 1.
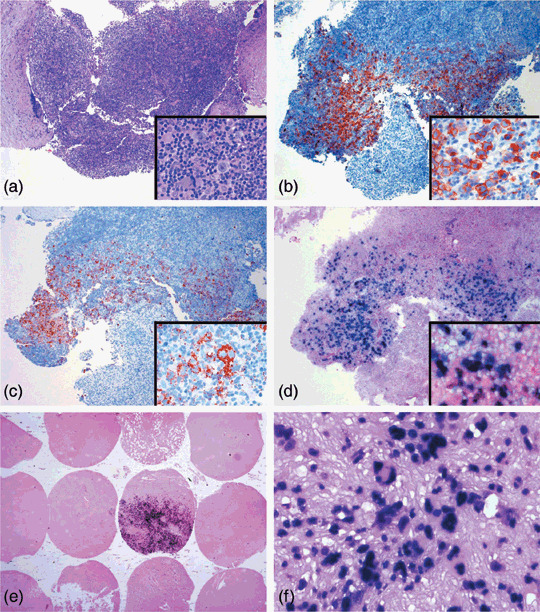
Epstein–Barr virus (EBV)‐encoded early RNA (EBER1) in situ hybridization for Hodgkin lymphoma (HL) cases shows positive signals in tumor cells with larger nuclear appearance. (a–d) The tumor cells of this nodular sclerosis Hodgkin's lymphoma case best appreciated by (a) hematoxylin–eosin (HE) section are positive for (b) CD30 and (c) CD15. (d) The distribution of EBER‐positive tumor cells corresponds to that seen in the HE section. (e) Sections of tissue microarray for cases from Veterans General Hospital Taipei show positive signals mainly in large nucleated tumor cells, (f) although a few inflammatory cells show weaker staining. (a–d) Original magnification 40×, inset 400×; (e) original magnification 10×; (f) original magnification 200×.
Table 1.
Hodgkin lymphoma, by subtype, diagnosed in Taiwan at National Cheng Kung University Hospital and Veterans General Hospital Taipei between 1982 and 2007
| Subtype | 1982–95/1996–2007 | EBER1‐positive (1982–95/1996–2007) | Male‐to‐female ratio | Mean age (years) | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Nodular lymphocyte predominance | 12 (6/6) | 7 | 1 (1/0) | 8 | 9/3 | 52.9 |
| Nodular sclerosis | 106 (36/67) | 61 | 50 (23/27) | 47 | 68/38 | 38.7 |
| Mixed cellularity | 39 (26/13) | 23 | 27 (16/11) | 69 | 32/7 | 40.6 |
| Lymphocyte‐rich classical | 7 (1/6) | 4 | 2 (0/2) | 29 | 3/4 | 53.1 |
| Lymphocyte depletion | 4 (2/2) | 2 | 2 (2/0) | 50 | 4/0 | 44.5 |
| Unclassified | 5 (0/5) | 3 | 4 (0/4) | 80 | 5/0 | 62.6 |
| Total | 173 (74/99) | 100 | 86 (42/44) | 50 | 121/52 | 41.5 |
EBER1, Epstein–Barr virus‐encoded early RNA.
Epstein–Barr virus in situ hybridization. Epstein–Barr virus‐encoded early RNA was tested by in situ hybridization in all cases, and positivity was seen only in those tissue areas involving HL (Fig. 1a–d; Table 1). The overall EBER positivity rate was 50% (86/173), and was 8% for NLP (1/12), 47% for NS (50/106), 69% for MC (27/39), 29% for LRC (2/7), 50% for LD (2/4), and 80% for unclassified HL (4/5). In EBV‐positive cases, the Reed–Sternberg or Hodgkin cells showed positive signals in nuclear regions (Fig. 1d–f). A few background non‐neoplastic lymphocytes were positive for EBV, although the signal was weaker than the tumor cells (Fig. 1f). There were a total of 12 cases that were double positive for EBER1 and CD20 (7%, 12/173) with 7% each for the two time periods (5/74 during 1982–1995 and 7/99 during 1996–2007). Among the 12 cases, 67% (8/12) were elderly patients (>60 years).
Shifts in the frequencies of HL subtype and EBV association from 1982 to 2007. The incidence of HL subtype frequency and EBV association in the last decade (1996–2007) were compared with those from Taiwan during 1982–1995 and a summary of series from Western countries (Table 2). Comparing our series to the earlier one from Taiwan, the relative frequencies of the NS subtype increased from 53 to 68% (P = 0.045), and the LRC subtype increased from 1 to 6%, whereas the incidence of the MC subtype decreased from 35 to 13% (P < 0.001). The frequencies of the NLP subtype (8 vs 6%) and the LD subtype (3 vs 2%) were similar. Taken as a whole, the changes in the overall frequency of subtypes between two periods (1982–1995 and 1996–1997) was highly statistically significant (P = 0.004, χ2‐test).
Table 2.
Comparison of Hodgkin lymphoma subtypes and Epstein–Barr virus (EBV) association over time in Taiwan and in previous studies from Western countries
| Subtype | Taiwan, 1982–1995 | Taiwan, 1996–2007 | Studies from Western countries | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | EBER+ (%) | M/F | Mean age (years) | % | EBER+ (%) | M/F | Mean age (years) | P‐value* | % | EBER+ (%) | M/F | Mean age (years) | Reference | |
| Nodular lymphocyte predominance | 8 | 17 | 4/2 | 55.0 | 6 | 0 | 5/1 | 50.8 | 0.60 | 5 | 0 | MP | 30–50 | ( 17 ) |
| Nodular sclerosis | 53 | 59 | 29/10 | 42.4 | 68 | 40 | 39/28 | 36.6 | 0.045 | 70 | 10–40 | 1/1 | 28 | ( 6, 8, 17 ) |
| Mixed cellularity | 35 | 62 | 20/6 | 36.8 | 13 | 85 | 12/1 | 48.2 | <0.001 | 20–25 | 75 (72–85%) | 7/3 | 37 | ( 6, 8, 17 ) |
| Lymphocyte‐rich classical | 1 | 0 | 0/1 | 32.0 | 6 | 33 | 3/3 | 56.7 | 0.12 | 5 | 57 | 7/3 | >40–50 | ( 17, 33 ) |
| Lymphocyte depletion | 3 | 100 | 2/0 | 64.0 | 2 | 0 | 2/0 | 25.0 | 0.77 | <5 | 12–50 | 3/1 | 37 | ( 8, 17, 32 ) |
| Unclassified | 0 | – | – | – | 5 | 80 | 5/0 | 62.6 | – | 4 | 33–100 | NA | NA | ( 6, 8 ) |
| Total | 57 | 55/19 | 41.9 | 44 | 66/33 | 41.3 | 0.004 | 30–50 | ( 20 ) | |||||
P‐value for difference in each subtype frequency in Taiwan. EBER, EBV‐encoded early RNA; M/F, male‐to‐female ratio; MP, male predominance; NA, not available.
The overall male‐to‐female (M/F) ratios of the HL cases in Taiwan during 1982–1995 and 1996–2007 were 55/19 (2.9:1) and 66/33 (2.0:1), respectively, with a decrease of borderline significance in the proportion of men with the NS subtype from 29/10 (2.9:1) to 39/28 (1.4:1) (P = 0.09, χ2‐test). The mean presenting ages for each subtype during 1982–1995 and 1996–2007 were 55.0 and 50.8 years for NLP, 42.4 and 36.6 for NS, 36.8 and 48.2 for MC, 32.0 and 56.7 for LRC, and 64.0 and 25.0 for LD, respectively. Although the overall mean age between the two time periods was similar (range 2–75 years, mean 41.9 years vs 4–82 years, mean 41.3 years), in each subtype the mean age increased (or decreased) along with the increase (or decrease) in EBER1 positivity rate (Table 2). The frequency of EBV‐positive HL in elderly patients (>60 years) was slightly increased from 71% for the period 1982–1995 to 76% for the period 1996–2007 (Table 3).
Table 3.
Shift in frequency of Epstein–Barr virus (EBV)‐positive Hodgkin lymphoma (HL) toward older patients
| Age (years) | 1982–1995 | 1996–2007 | P‐value (χ2) | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| <15 | 4/7 † | 57 | 5/9 | 56 | 0.95 |
| 15–29 | 9/18 | 50 | 8/29 | 28 | 0.12 |
| 30–44 | 6/14 | 43 | 4/20 | 20 | 0.15 |
| 45–59 | 6/11 | 55 | 5/12 | 42 | 0.54 |
| ≥60 | 17/24 | 71 | 22/29 | 76 | 0.68 |
| Total | 42/74 | 57 | 44/99 | 44 | 0.10 |
Portion of EBV‐positive cases to total HL cases in the indicated age group.
There were also changes in the incidence of EBV positivity in HL subtypes, with a decrease in the NLP, NS, and LD subtypes and an increase in the MC and LRC subtypes. Overall, the EBER1 positivity decreased from 57 to 44% (P = 0.1, χ2‐test), with a more significant decrease in the NS and LD subtypes (0.06 and 0.04 for each, and 0.03 for the combination, χ2‐test). The decreased EBER1 positivity for NLP and increased positivity for MC and LRC was not significant (P = 0.3, 0.14, and 0.49, respectively).
Discussion
The comparison of HL cases from the same geographic area during different time periods provides an opportunity to observe the influence of global environmental factors, such as EBV infection and socioeconomic shifts, on disease patterns in HL. In the present study, by comparing the distribution of HL subtypes and EBV association in the last decade with that in 1982–1995, we found an increased frequency of the NS subtype and a decreased frequency of the MC subtype, a reduced mean age and M/F ratio for the NS subtype, and a decreased overall EBV‐positive rate, particularly for the NS and LD subtypes. These data indicate a shift in Taiwan from pattern 2 of HL toward pattern 3 of HL, as reported previously in Western countries. One may be concerned whether there existed patient selection bias or changes in diagnostic criteria to affect the comparison of the earlier and current Taiwan datasets. Because we recruited consecutive HL cases in two general hospitals, the patient selection bias could be minimized if present. The diagnostic criteria for HL is similar in the WHO classification and Rye classification schemes.( 14 , 17 ) The major difference between the two schemes is the amendment of NLP, especially the diffuse subtype in the Rye classification into LRC.( 14 ) Immunophenotyping of HL cells, as we have done in the present study, also plays an important role in correct classification of HL.
For each HL subtype in the present study, the mean age changed in the same direction as the change in EBV positivity rate over the study period. In previous studies it was found that there are two age peaks in EBV‐positive HL cases, namely less than 10 years old and more than 45 years old.( 20 , 21 ) However, this biphasic pattern possibly represents two distinct phenomena, one related to age of EBV acquisition and the other to the decline in immune function, each of which will likely predominate in different populations. For example, we noted in Vietnamese children that there was a strong association of EBV with all histological subtypes of HL,( 9 ) consistent with a younger age of EBV seroconversion in that country. It appears that, with improved public health status in Taiwan over the last two decades,( 22 , 23 ) EBV positivity in HL is becoming associated with the older age group (Table 3). These findings support the hypothesis that HL may have different etiologies in different age groups and indicate that the association of EBV with HL will likely become more common in older patients as the age of primary EBV infection rises in any given country.( 21 ) Interestingly, this trend of decreased EBV positivity rate in HL over time has been similarly observed in Japanese patients and was thought to reflect an increase in the incidence of EBV‐negative NS subtype along with the progress in improvement of living standards.( 24 )
The oncogenic role of EBV in HL remains to be fully clarified. Previous studies have shown that EBV‐positive HL cells demonstrate a type II latency phenotype and that these cells are of germinal‐center B‐cell origin.( 17 , 25 , 26 ) One model suggests that EBV initially infects naïve B cells, which become activated and transform into germinal‐center B cells by antigen selection, but then persist due to establishment of the EBV latent transcription program. EBV‐encoded latent membrane protein (LMP1) plays an important role in tumorigenesis as it can prevent B cells from undergoing tumor necrosis factor‐mediated apoptosis by activating a variety of signaling molecules, such as nuclear factor κB (NFκB).( 27 , 28 ) Constitutive expression of EBV nuclear antigen‐1 and LMP1/2A may also block further differentiation of these infected B cells, allowing time for accumulation of additional acquired mutations that lead to neoplastic transformation.( 26 , 29 )
Constitutive activation of the NFκB pathway is a common feature of both EBV‐positive and EBV‐negative HL. As discussed above, in the EBV‐positive subset, LMP1 overexpression appears to be critical whereas in the EBV‐negative subset, activation can be due to IκBα gene mutation.( 30 ) These distinct pathogenetic features are linked to differing clinical features in that EBV‐positive HL occurs more often in children, is more common in men, and may bear a worse prognosis.( 20 , 31 ) In developing countries where EBV infection occurs earlier in life when the immune system is relatively underdeveloped, viral initiation of transformation may predominate in HL. In developed areas, EBV has a more minor role in initiating transformation in younger patients and instead drives transformation in older patients with impaired immune status. This ‘two‐disease hypothesis’ linking pathogenesis with population‐based features is supported by the present study. We demonstrate shifts in the incidence of subtype and EBV association in HL over a decade that coincide with improvements in public health in Taiwan.( 22 , 23 )
In conclusion, by comparing the frequency of HL subtypes and EBV association during two different time periods, we have shown a shift in HL subtypes in Taiwan to a pattern that is more similar to that in Western countries, indicating a decreasing role for direct EBV transformation. These findings suggest that environmental factors can have profound effects on the role of pathogens in tumor development and provide insights into how strategies for improvement of public health can greatly influence cancers.
Acknowledgments
This work was supported by a grant from the National Cheng Kung University Hospital, Taiwan (NCKUH‐96‐004) to Dr K. C. Chang.
References
- 1. Lehane DE. A seroepidemiologic study of infectious mononucleosis. The development of EB virus antibody in a military population. JAMA 1970; 212: 2240–2. [DOI] [PubMed] [Google Scholar]
- 2. Gutensohn N, Cole P. Childhood social environment and Hodgkin's disease. N Engl J Med 1981; 304: 135–40. [DOI] [PubMed] [Google Scholar]
- 3. Hjalgrim H, Askling J, Rostgaard K et al . Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med 2003; 349: 1324–32. [DOI] [PubMed] [Google Scholar]
- 4. Hesse J, Ibsen KK, Krabbe S, Uldall P. Prevalence of antibodies to Epstein–Barr virus (EBV) in childhood and adolescence in Denmark. Scand J Infect Dis 1983; 15: 335–8. [DOI] [PubMed] [Google Scholar]
- 5. Sumaya CV, Henle W, Henle G, Smith MH, LeBlanc D. Seroepidemiologic study of Epstein–Barr virus infections in a rural community. J Infect Dis 1975; 131: 403–8. [DOI] [PubMed] [Google Scholar]
- 6. Ambinder RF, Browning PJ, Lorenzana I et al . Epstein–Barr virus and childhood Hodgkin's disease in Honduras and the United States. Blood 1993; 81: 462–7. [PubMed] [Google Scholar]
- 7. Kanavaros P, Sakalidou A, Tzardi M et al . Frequent detection of Epstein–Barr virus (EBV), EBER transcripts and latent membrane protein‐1 (LMP‐1) in tumor cells in Hodgkin's disease arising in childhood. Pathol Res Pract 1994; 190: 1026–30. [DOI] [PubMed] [Google Scholar]
- 8. Weinreb M, Day PJ, Murray PG et al . Epstein–Barr virus (EBV) and Hodgkin's disease in children: incidence of EBV latent membrane protein in malignant cells. J Pathol 1992; 168: 365–9. [DOI] [PubMed] [Google Scholar]
- 9. Chang KC, Khen NT, Jones D, Su IJ. Epstein–Barr virus is associated with all histological subtypes of Hodgkin lymphoma in Vietnamese children with special emphasis on the entity of lymphocyte predominance subtype. Hum Pathol 2005; 36: 747–55. [DOI] [PubMed] [Google Scholar]
- 10. Quintanilla‐Martinez L, Gamboa‐Domnquez A, Gamez‐Ledesma I, Angeles‐Angeles A, Mohar A. Association of Epstein–Barr virus latent membrane protein and Hodgkin's disease in Mexico. Mod Pathol 1995; 8: 675–9. [PubMed] [Google Scholar]
- 11. Gutensohn NM. Social class and age at diagnosis of Hodgkin's disease: new epidemiologic evidence for the ‘two‐disease hypothesis’. Cancer Treat Rep 1982; 66: 689–95. [PubMed] [Google Scholar]
- 12. MacMahon B. Epidemiology of Hodgkin's disease. Cancer Res 1966; 26: 1189–201. [PubMed] [Google Scholar]
- 13. Correa P, O’Conor GT. Epidemiologic patterns of Hodgkin's disease. Int J Cancer 1971; 8: 192–201. [DOI] [PubMed] [Google Scholar]
- 14. Harris NL. The many faces of Hodgkin's disease around the world: what have we learned from its pathology? Ann Oncol 1998; 9 (Suppl 5): S45–56. [DOI] [PubMed] [Google Scholar]
- 15. Liu SM, Chow KC, Chiu CF, Tzeng CH. Expression of Epstein–Barr virus in patients with Hodgkin's disease in Taiwan. Cancer 1998; 83: 367–71. [DOI] [PubMed] [Google Scholar]
- 16. Pan CC, Chen PC, Chiang H. An easy method for manual construction of high‐density tissue arrays. Appl Immunohistochem Mol Morph 2004; 12: 370–2. [DOI] [PubMed] [Google Scholar]
- 17. Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Pathology and genetics of tumours of haematopoietic and Lymphoid tissues. World Health Organization Classification of Tumours. Lyon: IARC Press, 2001. [Google Scholar]
- 18. Tsai ST, Jin YT, Wu TC. Synthesis of PCR‐derived, digoxigenin‐labeled DNA probes for in situ detection of Epstein–Barr early RNAs in Epstein–Barr virus‐infected cells. J Virol Meth 1995; 54: 67–74. [DOI] [PubMed] [Google Scholar]
- 19. Gulley ML, Glaser SL, Craig FE et al . Guidelines for interpreting EBER in situ hybridization and LMP1 immunohistochemical tests for detecting Epstein–Barr virus in Hodgkin lymphoma. Am J Clin Pathol 2002; 117: 259–67. [DOI] [PubMed] [Google Scholar]
- 20. Gandhi MK, Tellam JT, Khanna R. Epstein–Barr virus‐associated Hodgkin's lymphoma. Br J Haematol 2004; 125: 267–81. [DOI] [PubMed] [Google Scholar]
- 21. Jarrett RF, Gallagher A, Jones DB et al . Detection of Epstein–Barr virus genomes in Hodgkin's disease: relation to age. J Clin Pathol 1991; 44: 844–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin JD, Chao TC, Sun JH, Ho C, Weng HF. Trends in the clinical characteristics of patients with papillary thyroid carcinoma in Taiwan. Oncology 2000; 58: 280–5. [DOI] [PubMed] [Google Scholar]
- 23. Wang SM, Liu CC, Huang YS, Yang YJ, Lei HY. Change in hepatitis A virus seroepidemiology in southern Taiwan: a large percentage of the population lack protective antibody. J Med Virol 2001; 64: 104–8. [DOI] [PubMed] [Google Scholar]
- 24. Takeuchi K, Morishita Y, Fukayama M, Mori S. Marked decrease in the Epstein–Barr virus positivity rate in nodular sclerosis subtype Hodgkin's disease in Tokyo: trend between 1955 and 1999. Br J Haematol 2001; 113: 429–31. [DOI] [PubMed] [Google Scholar]
- 25. Knecht H, Berger C, Rothenberger S, Odermatt BF, Brousset P. The role of Epstein–Barr virus in neoplastic transformation. Oncology 2001; 60: 289–302. [DOI] [PubMed] [Google Scholar]
- 26. Thorley‐Lawson DA, Gross A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N Engl J Med 2004; 350: 1328–37. [DOI] [PubMed] [Google Scholar]
- 27. Asso‐Bonnet M, Feuillard J, Ferreira V et al . Relationship between IκBα constitutive expression, TNFα synthesis, and apoptosis in EBV‐infected lymphoblastoid cells. Oncogene 1998; 17: 1607–15. [DOI] [PubMed] [Google Scholar]
- 28. Devergne O, Hatzivassiliou E, Izumi KM et al . Association of TRAF1, TRAF2, and TRAF3 with an Epstein–Barr virus LMP1 domain important for B‐lymphocyte transformation: role in NF‐κB activation. Mol Cell Biol 1996; 16: 7098–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thorley‐Lawson DA. Epstein–Barr virus: exploiting the immune system. Nat Rev Immunol 2001; 1: 75–82. [DOI] [PubMed] [Google Scholar]
- 30. Bargou RC, Leng C, Krappmann D et al . High‐level nuclear NF‐κB and Oct‐2 is a common feature of cultured Hodgkin/Reed–Sternberg cells. Blood 1996; 87: 4340–7. [PubMed] [Google Scholar]
- 31. Jarrett RF, Stark GL, White J et al . Impact of tumor Epstein–Barr virus status on presenting features and outcome in age‐defined subgroups of patients with classic Hodgkin lymphoma: a population‐based study. Blood 2005; 106: 2444–51. [DOI] [PubMed] [Google Scholar]
- 32. Fellbaum C, Hansmann ML, Niedermeyer H et al . Influence of Epstein–Barr virus genomes on patient survival in Hodgkin's disease. Am J Clin Pathol 1992; 98: 319–23. [DOI] [PubMed] [Google Scholar]
- 33. Quinones‐Avila Mdel P, Gonzalez‐Longoria AA, Admirand JH, Medeiros LJ. Hodgkin lymphoma involving Waldeyer ring: a clinicopathologic study of 22 cases. Am J Clin Pathol 2005; 123: 651–6. [PubMed] [Google Scholar]


