Abstract
The purpose of this study was to analyze socioeconomic differences in cervical and corpus cancer survival, and to investigate if the differences are due to differences in age, cancer stage, histology and treatment. A total of 14 055 cases with cervical cancer and 3113 cases with corpus cancer were obtained from the Osaka Cancer Registry. Municipality‐based SES measurements were obtained from the System of Social and Demographic Statistics. Survival analysis was carried out with Kaplan–Meier survival curves. Three types of Cox proportional hazards regression models were tested to assess survival differences among groups and effects of SES on survival, controlling for clinical factors. SES was related to age and cancer stage for cervical and corpus cancer patients, and histology for cervical cancer patients. Differences were observed in cumulative 5‐year survival for cervical cancer patients among low, middle and high unemployment municipalities (68.9%, 64.3% and 50.9%, respectively, P < 0.0001). Differences in cumulative 5‐year survival for cervical cancer patients were also observed among high, middle and low education municipalities (65.1%, 62.2% and 56.1%, respectively, P < 0.0001). Similar patterns in 5‐year survival were also found for corpus cancer patients. After adjusting for age, cancer stage, histology and treatment, survival differences between patients from high and low SES areas still remained. In conclusion, our population‐based analysis of a metropolitan representative sample in Japan has demonstrated, for the first time in Japan, SES differences in survival following cervical and corpus cancer. (Cancer Sci 2006; 97: 283 –291)
Abbreviations
- ICD
International Classification of Diseases
- SES
socioeconomic status.
Social inequalities in cancer survival need to be taken into consideration, especially in terms of equal provision for cancer detection and treatment.( 1 , 2 , 3 , 4 , 5 ) Differences in cancer survival among patients from different socioeconomic backgrounds may be due to inequalities in access to quality treatment as well as variations in cancer stage at presentation.( 6 ) Survival differences in cervical and corpus cancer by SES have been studied in different countries.( 4 , 5 , 7 , 8 , 9 , 10 , 11 , 12 , 13 )
In Japan, cancer has been a leading cause of death since 1981, with one in three persons dying from cancer. Cancer mortality rates for males and females in 2002 were 298.8 and 187.1 per 100 000, respectively (ICD, 10th revision, codes C00 and C97).( 14 ) Age‐standardized incidence rates (standard; world population) for cancer in 1999 estimated by the Research Group for Population‐based Cancer Registration in Japan were 271.1 for males and 168.6 for females per 100 000.( 15 ) The mortality rate for cervical, corpus cancer and uterine cancer not otherwise specified (NOS) (ICD, 10th revision, codes C53‐55) mortality rate was 8.3 per 100 000 females.( 14 ) The estimated cervical cancer (ICD, 10th revision, codes C53) incidence rate has decreased by approximately 50% from 13.4 in 1975 to 6.6 in 1999 per 100 000 females, however, the rate among young females has increased. By contrast, corpus cancer (ICD, 10th revision, code C54) incidence rates increased from 1.4 in 1975 to 5.4 in 1999 per 100 000 females.( 15 , 16 ) Relative 5‐year survival following cervical and corpus cancer reached a plateau of around 70% after 1980.( 17 )
In March 2005, the Japanese Ministry of Health, Labour and Welfare Special Committee on the Equation of Cancer Control released its national cancer control strategy. One of the programs of the national strategy focuses on enhancing the provision of cancer prevention and treatment efforts targeted at women. Despite the launch of the national comprehensive cancer control strategy, however, few studies in Japan have addressed socioeconomic disparities in cancer survival.
We sought to analyze socioeconomic differences in cervical and corpus cancer survival in Osaka, a major metropolitan area of Japan, and to investigate if the disparities were due to differences in age, cancer stage, histology or treatment.
Materials and Methods
Data on newly diagnosed uterine cancer cases (ICD, 10th revision, codes C53 and C54) between January 1975 and December 1997 were extracted from the Osaka Cancer Registry (OCR). Death certificate only registrations were excluded. We analyzed 14 055 cases of cervical cancer and 3113 cases of corpus cancer. The OCR is one of the largest and longest‐running population‐based cancer registries in Japan. The validity and procedures of the OCR have been described elsewhere.( 18 )
Variables extracted age, cancer stage, histology, treatment, area‐based SES and cumulative 5‐year survival. Cancer stage at diagnosis was classified into three groups: localized, regional and distant stage. Localized stage cancer was limited to the original organ. Regional stage cancers were those that had spread to regional lymph nodes and/or adjacent tissues, and distant stage cancers were those that had metastasized to distant organs. Histology was categorized using Berg's classification used by the International Agency for Research on Cancer for identifying groups of malignant neoplasms considered to be histologically distinct for the purpose of classifying tumors in their Recommendations for Coding Multiple Primaries.( 19 ) The histology of cervical cancer was categorized as squamous carcinomas, adenocarcinomas, other specific carcinomas and ‘other’. The histology of corpus cancer was categorized as squamous carcinomas, adenocarcinomas, other specific carcinomas, sarcomas and soft tissue tumors, and ‘other’. Treatment was categorized into nine groups: surgery alone, radiation alone, chemotherapy alone, surgery and radiation, surgery and chemotherapy, radiation and chemotherapy, combined surgery/radiation/chemotherapy, other treatments, and unknown.
Because of lack of individual socioeconomic data in the OCR, we used municipality‐based SES as a proxy, drawing on the percentages of male unemployment, and college or graduate school graduates within the 67 municipalities of the Osaka area. The percentage of unemployment in 1995 and college or graduate school graduates in 1990 were obtained from the System of Social and Demographic Statistics (SSDS) provided by the Ministry of Internal Affairs and Communications, drawn from the national census. The 67 municipalities were categorized into three socioeconomic groups: 22 municipalities with low percentages of unemployment (2.54–5.37%), or high proportions of college or graduate school graduates (13.98–25.04%) (‘high SES municipalities’); 23 municipalities with middle percentages of unemployment (5.41–6.82%) or college or graduate school graduates (10.54–13.96%) (‘middle SES municipalities’); and 22 municipalities with high percentages of unemployment (7.13–17.4%) or low proportions of college or graduate school graduates (6.22–10.34%) (‘low SES municipalities’).
Survival analysis was carried out with Kaplan–Meier survival curves with follow‐up for 5 years. The survival time was defined as the time from the date of first diagnosis to the date of death from any causes. Log–rank tests were used to determine the significance of differences between survival curves by SES and other prognostic factors. Three sets of sequential Cox proportional hazards regression models were carried out to assess survival differences among SES groups, controlling for clinical factors. In the first model, we controlled for age as a confounder. In the second model we controlled for age, plus biological factors (cancer stage and histology of cervical cancer, cancer stage of corpus cancer). In the third model, we controlled for all of the variables in the second model, plus treatment type. The statistical significance of differences in distributions of clinical factors was determined by χ2 tests for categorical variables and by Kruskal–Wallis tests for continuous variables. STATA (version 7.0) was used for statistical analyses.
Results
The characteristics of cervical and corpus cancer patients by SES area are shown in 1, 2. Histology for all cases in ‘sarcomas and soft tissue tumors’ (according to Berg's classification) was sarcoma. Significant SES differences were found in the age of patients as well as cancer stage for cervical and corpus cancer patients. The distribution of histology for corpus cancer patients did not differ by SES. Proportions of localized cervical (59.2%) and corpus (68.7%) cancers were higher among patients from low unemployment areas compared to patients from middle (57.2%, 61.1%) or high (51.6%, 61.0%) unemployment municipalities. Similarly, the proportions of localized cervical (57.6%) and corpus (67.1%) cancers in high education municipalities were higher compared to middle (56.8%, 62.1%) and low (51.7%, 59.4%) education municipalities. The proportion of squamous carcinomas in cervical cancer in low unemployment municipalities (82.2%) was lower compared to middle (84.9%) and high (85.6%) unemployment municipalities. We observed significant differences in the distribution of treatment for both cervical and corpus cancer patients. The proportions of cervical cancer patients who underwent surgery alone were higher among patients from low (32.4%) and middle (30.2%) unemployment municipalities compared to patients from high unemployment municipalities (25.5%). Surgery alone was more common among patients from high (30.6%) and middle (30.0%) education municipalities compared to those from low education municipalities (25.9%).
Table 1.
Characteristics of cervical cancer patients by area‐based socioeconomic status (SES)
| Factor | Percentage of male unemployment in each municipality | Percentage of college or graduate school graduates in each municipality | ||||||
|---|---|---|---|---|---|---|---|---|
| Low | Middle | High | P‐value | High | Middle | Low | P‐value | |
| No. of patients | 3308 | 5838 | 4909 | 4265 | 6100 | 3690 | ||
| Mean age (years) | 54 | 54 | 56 | 0.086 | 54 | 54 | 55 | 0.054 |
| Age (years) | <0.0001 | 0.026 | ||||||
| Under 40 | 477 (14.4) | 875 (15.0) | 608 (12.4) | 580 (13.6) | 914 (15.0) | 466 (12.6) | ||
| 40–49 | 908 (27.5) | 1453 (24.9) | 1129 (23.0) | 1107 (26.0) | 1485 (24.3) | 898 (24.3) | ||
| 50–59 | 786 (23.8) | 1411 (24.2) | 1231 (25.1) | 1039 (24.4) | 1464 (24.0) | 925 (25.1) | ||
| 60–69 | 653 (19.7) | 1162 (19.9) | 1113 (22.6) | 881 (20.7) | 1232 (20.2) | 815 (22.1) | ||
| 70–79 | 388 (11.7) | 724 (12.4) | 657 (13.4) | 510 (12.0) | 794 (13.0) | 465 (12.6) | ||
| 80+ | 96 (2.9) | 213 (3.6) | 171 (3.5) | 148 (3.5) | 211 (3.5) | 121 (3.3) | ||
| Cancer stage | <0.0001 | <0.0001 | ||||||
| Localized | 1957 (59.2) | 3338 (57.2) | 2534 (51.6) | 2458 (57.6) | 3462 (56.8) | 1909 (51.7) | ||
| Regional | 930 (28.1) | 1725 (29.5) | 1585 (32.3) | 1217 (28.5) | 1844 (30.2) | 1179 (32.0) | ||
| Distant | 107 (3.2) | 229 (3.9) | 212 (4.3) | 158 (3.7) | 225 (3.7) | 165 (4.5) | ||
| Unknown | 314 (9.5) | 546 (9.4) | 578 (11.8) | 432 (10.2) | 569 (9.3) | 437 (11.8) | ||
| Histology | <0.0001 | <0.0001 | ||||||
| Squamous carcinomas | 2719 (82.2) | 4955 (84.9) | 4201 (85.6) | 3508 (82.2) | 5221 (85.6) | 3146 (85.3) | ||
| Adenocarcinomas | 263 (8.0) | 356 (6.1) | 277 (5.6) | 337 (7.9) | 348 (5.7) | 211 (5.7) | ||
| Other specific carcinomas | 97 (2.9) | 117 (2.0) | 62 (1.3) | 110 (2.6) | 113 (1.9) | 53 (1.4) | ||
| Other | 229 (6.9) | 410 (7.0) | 369 (7.5) | 310 (7.3) | 418 (6.8) | 280 (7.6) | ||
| Treatment | <0.0001 | <0.0001 | ||||||
| Surgery alone | 1073 (32.4) | 1765 (30.2) | 1254 (25.5) | 1307 (30.6) | 1830 (30.0) | 955 (25.9) | ||
| Radiation alone | 494 (14.9) | 96 (16.6) | 80 (22.8) | 734 (17.2) | 1084 (17.8) | 764 (20.7) | ||
| Chemotherapy alone | 48 (1.5) | 96 (1.6) | 80 (1.6) | 66 (1.6) | 84 (1.4) | 74 (2.0) | ||
| Surgery + radiation | 647 (19.6) | 972 (16.7) | 861 (17.5) | 800 (18.8) | 1022 (16.7) | 658 (17.8) | ||
| Surgery + chemotherapy | 236 (7.1) | 397 (6.8) | 371 (7.6) | 308 (7.2) | 431 (7.1) | 265 (7.2) | ||
| Radiation + chemotherapy | 275 (8.3) | 593 (10.2) | 439 (8.9) | 357 (8.4) | 615 (10.1) | 335 (9.1) | ||
| Surgery, radiation + | 363 (11.0) | 704 (12.1) | 493 (10.0) | 453 (10.6) | 710 (11.6) | 397 (10.7) | ||
| chemotherapy | ||||||||
| Other treatments | 21 (0.6) | 62 (1.1) | 21 (0.4) | 23 (0.5) | 59 (1.0) | 22 (0.6) | ||
| Unknown | 151 (4.6) | 282 (4.8) | 269 (5.5) | 217 (5.1) | 265 (4.3) | 220 (6.0) | ||
Table 2.
Characteristics of corpus cancer patients by area‐based socioeconomic status (SES)
| Factor | Percentage of male unemployment in each municipality | Percentage of college or graduate school graduates in each municipality | ||||||
|---|---|---|---|---|---|---|---|---|
| Low | Middle | High | P‐value | High | Middle | Low | P‐value | |
| No. of patients | 906 | 1225 | 982 | 1127 | 1292 | 694 | ||
| Mean age (years) | 55 | 56 | 58 | 0.357 | 56 | 56 | 57 | 0.385 |
| Age (years) | <0.0001 | 0.039 | ||||||
| Under 40 | 64 (7.1) | 60 (4.9) | 37 (3.8) | 60 (5.3) | 66 (5.1) | 35 (5.0) | ||
| 40–49 | 188 (20.8) | 233 (19.0) | 162 (16.5) | 219 (19.4) | 245 (19.0) | 119 (17.2) | ||
| 50–59 | 355 (39.2) | 527 (43.0) | 374 (38.1) | 449 (39.9) | 539 (41.7) | 268 (38.6) | ||
| 60–69 | 196 (21.6) | 271 (22.1) | 243 (24.7) | 258 (22.9) | 292 (22.6) | 160 (23.0) | ||
| 70–79 | 81 (8.9) | 103 (8.4) | 133 (13.5) | 108 (9.6) | 119 (9.2) | 90 (13.0) | ||
| 80+ | 22 (2.4) | 31 (2.6) | 33 (3.4) | 33 (2.9) | 31 (2.4) | 22 (3.2) | ||
| Cancer stage | 0.005 | 0.009 | ||||||
| Localized | 623 (68.7) | 749 (61.1) | 599 (61.0) | 756 (67.1) | 803 (62.1) | 412 (59.4) | ||
| Regional | 148 (16.3) | 256 (20.9) | 190 (19.3) | 200 (17.8) | 261 (20.2) | 133 (19.1) | ||
| Distant | 63 (7.0) | 104 (8.5) | 85 (8.7) | 77 (6.8) | 102 (7.9) | 73 (10.5) | ||
| Unknown | 72 (8.0) | 116 (9.5) | 108 (11.0) | 94 (8.3) | 126 (9.8) | 76 (11.0) | ||
| Histology | 0.401 | 0.994 | ||||||
| Squamous carcinomas | 12 (1.3) | 23 (1.9) | 12 (1.2) | 16 (1.4) | 20 (1.6) | 11 (1.6) | ||
| Adenocarcinomas | 712 (78.6) | 963 (79.0) | 747 (76.1) | 887 (78.7) | 1002 (78.0) | 532 (76.7) | ||
| Other specific carcinomas | 40 (4.4) | 51 (4.2) | 38 (3.9) | 46 (4.1) | 50 (3.9) | 32 (4.6) | ||
| Sarcomas | 39 (4.3) | 50 (4.1) | 52 (5.3) | 48 (4.3) | 83 (4.6) | 34 (4.9) | ||
| Other | 103 (11.4) | 133 (10.8) | 133 (13.5) | 130 (11.5) | 137 (11.9) | 85 (12.2) | ||
| Treatment | 0.005 | <0.0001 | ||||||
| Surgery alone | 324 (35.8) | 399 (32.6) | 364 (37.1) | 403 (35.8) | 443 (34.3) | 241 (34.7) | ||
| Radiation alone | 16 (1.8) | 27 (2.2) | 26 (2.7) | 23 (2.0) | 23 (1.8) | 23 (3.3) | ||
| Chemotherapy alone | 15 (1.7) | 39 (3.2) | 24 (2.4) | 19 (1.7) | 40 (3.1) | 19 (2.7) | ||
| Surgery + radiation | 59 (6.5) | 108 (8.8) | 84 (8.6) | 79 (7.0) | 122 (9.4) | 50 (7.2) | ||
| Surgery + chemotherapy | 364 (40.2) | 454 (37.1) | 318 (32.4) | 438 (38.9) | 456 (35.3) | 242 (34.9) | ||
| Radiation + chemotherapy | 7 (0.8) | 10 (0.8) | 14 (1.4) | 9 (0.8) | 14 (1.1) | 8 (1.2) | ||
| Surgery, radiation + chemotherapy | 66 (7.3) | 111 (9.1) | 71 (7.2) | 88 (7.8) | 107 (8.3) | 53 (7.6) | ||
| Other treatments | 8 (0.9) | 7 (0.6) | 5 (0.5) | 8 (0.7) | 6 (0.5) | 6 (0.9) | ||
| Unknown | 47 (5.2) | 70 (5.7) | 76 (7.7) | 60 (5.3) | 81 (6.3) | 52 (7.5) | ||
As shown in 1, 2, a difference was observed in the cumulative 5‐year survival for cervical cancer patients from low, middle and high unemployment municipalities: 68.9%, 64.3% and 50.9%, respectively (P < 0.0001). A difference in cumulative 5‐year survival for cervical cancer patients was also observed for high, middle versus low education municipalities: 65.1%, 62.2% and 56.1%, respectively (P < 0.0001). Patients from high unemployment municipalities had a much lower cervical cancer 5‐year survival than patients from middle and high socioeconomic municipalities. The cumulative 5‐year survival differences for corpus cancer by level of SES showed a similar pattern as that for cervical cancer patients (3, 4). However, the SES disparities in corpus cancer cumulative 5‐year survival were even wider than cervical cancer survival differences.
Figure 1.
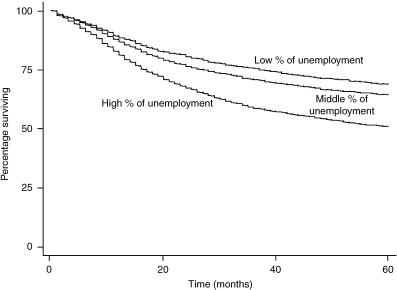
Kaplan–Meier survival curves by socioeconomic status (SES) (unemployment) for cervical cancer patients.
Figure 2.
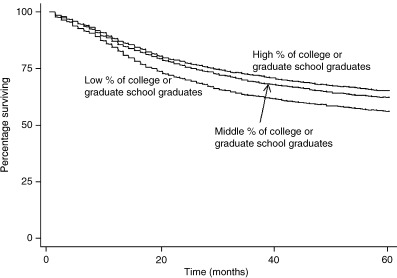
Kaplan–Meier survival curves by socioeconomic status (SES) (education) for cervical cancer patients.
Figure 3.
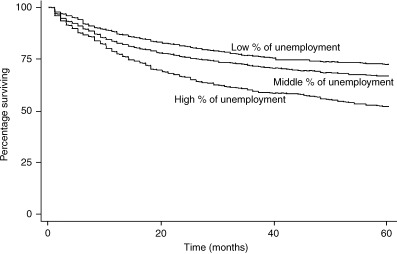
Kaplan–Meier survival curves by socioeconomic status (SES) (unemployment) for corpus cancer patients.
Figure 4.
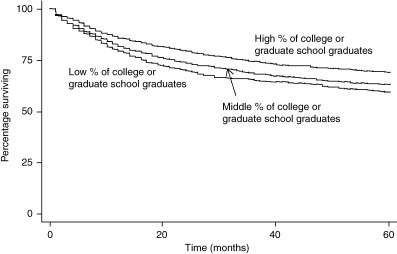
Kaplan–Meier survival curves by socioeconomic status (SES) (education) for corpus cancer patients.
Prognostic factors such as age, cancer stage, histology and treatment were each significantly related to cumulative 5‐year survival for both cervical and corpus cancer patients (P < 0.0001) (Table 3). Cumulative 5‐year survival for younger and older women was broadly comparable for cervical and corpus cancer. Cumulative 5‐year survival for both cervical and corpus cancer patients was worse for distant stage cases compared to more localized stages. The cumulative 5‐year survival of squamous carcinomas (65.1%) and adenocarcinomas (54.8%) for cervical cancer patients differed from survival for corpus cancer patients (48.9% and 69.7%, respectively). The worst 5‐year survival among corpus cancer patients was 29.5% for sarcomas.
Table 3.
Cumulative 5‐year percentage survival by clinical factors for cervical and corpus cancer patients
| Factor | Cervical cancer | Corpus cancer | ||||
|---|---|---|---|---|---|---|
| 5‐year survival (%) | 95% CI | P‐value | 5‐year survival (%) | 95% CI | P‐value | |
| Age (years) | <0.0001 | <0.0001 | ||||
| Under 40 | 81.7 | (79.6–83.5) | 81.4 | (73.6–87.0) | ||
| 40–49 | 73.0 | (71.3–74.7) | 77.5 | (73.6–81.0) | ||
| 50–59 | 62.5 | (60.7–64.2) | 72.8 | (70.0–75.4) | ||
| 60–69 | 56.3 | (54.4–58.2) | 54.2 | (50.3–58.0) | ||
| 70–79 | 41.3 | (38.9–43.7) | 39.1 | (33.6–44.6) | ||
| 80+ | 21.8 | (18.1–25.8) | 21.0 | (12.9–30.4) | ||
| Cancer stage | <0.0001 | <0.0001 | ||||
| Localized | 82.0 | (81.0–82.9) | 81.6 | (79.6–83.3) | ||
| Regional | 38.6 | (37.1–40.1) | 41.8 | (37.6–45.9) | ||
| Distant | 7.3 | (5.3–9.7) | 11.3 | (7.8–15.6) | ||
| Unknown | 53.4 | (50.5–56.2) | 51.9 | (4.5–5.8) | ||
| Histology | <0.0001 | <0.0001 | ||||
| Squamous carcinomas | 65.1 | (64.2–66.1) | 48.9 | (33.1–63.0) | ||
| Adenocarcinomas | 54.8 | (51.0–58.2) | 69.7 | (67.6–71.6) | ||
| Other specific carcinomas | 63.6 | (57.2–69.2) | 67.1 | (58.0–74.7) | ||
| Sarcomas | – | – | 29.5 | (21.8–37.5) | ||
| Other | 28.1 | (25.2–31.0) | 45.0 | (39.5–50.4) | ||
| Treatment | <0.0001 | <0.0001 | ||||
| Surgery alone | 91.4 | (90.3–92.3) | 81.6 | (78.9–84.0) | ||
| Radiation alone | 44.4 | (42.4–46.5) | 36.4 | (25.0–47.9) | ||
| Chemotherapy alone | 7.2 | (4.2–11.1) | 6.5 | (2.4–13.5) | ||
| Surgery + radiation | 66.4 | (64.2–68.4) | 61.6 | (54.7–67.8) | ||
| Surgery + chemotherapy | 73.3 | (70.1–76.2) | 65.4 | (62.3–68.2) | ||
| Radiation + chemotherapy | 31.5 | (29.0–34.2) | 24.3 | (10.7–40.7) | ||
| Surgery, radiation + chemotherapy | 50.9 | (48.3–53.5) | 45.5 | (38.9–51.8) | ||
| Other treatments | 72.2 | (62.2–80.1) | 55.9 | (30.8–75.0) | ||
| Unknown | 50.1 | (46.0–54.0) | 42.0 | (34.4–49.4) | ||
CI, confidence interval.
Table 4 shows cumulative 5‐year survival by cancer stage and SES for cervical cancer patients. Survival differences by area SES were apparent for localized and regional stage cancers. However, SES differences were not seen for survival following distant stage tumors. In contrast, cumulative 5‐year survival differences by SES (education) were not apparent for corpus cancer (Table 4).
Table 4.
Cervical and corpus cancer cumulative 5‐year percentage survival by cancer stage and area‐based socioeconomic status (SES)
| Percentage of male unemployment in each municipality | Percentage of college or graduate school graduates in each municipality | |||||||
|---|---|---|---|---|---|---|---|---|
| Low | Middle | High | P‐value | High | Middle | Low | P‐value | |
| Cervical cancer stage | ||||||||
| Localized | 86.2 | 83.3 | 73.9 | <0.0001 | 84.5 | 81.3 | 79.5 | 0.0004 |
| Regional | 44.3 | 40.1 | 32.9 | <0.0001 | 41.3 | 39.3 | 34.5 | 0.0002 |
| Distant | 7.7 | 8.9 | 5.4 | 0.503 | 7.9 | 9.1 | 4.4 | 0.297 |
| Unknown | 56.3 | 56.7 | 48.0 | 0.027 | 51.3 | 56.4 | 51.7 | 0.239 |
| All stages | 68.9 | 64.3 | 50.9 | <0.0001 | 65.1 | 62.2 | 56.1 | <0.0001 |
| Corpus cancer stage | ||||||||
| Localized | 85.8 | 83.7 | 71.8 | <0.0001 | 83.8 | 81.1 | 78.0 | 0.075 |
| Regional | 44.9 | 48.4 | 29.4 | 0.0002 | 44.8 | 39.1 | 42.4 | 0.447 |
| Distant | 17.5 | 11.7 | 6.2 | 0.063 | 14.3 | 12.2 | 7.0 | 0.117 |
| Unknown | 62.2 | 54.8 | 38.6 | 0.022 | 53.0 | 49.9 | 54.1 | 0.400 |
| All stages | 72.4 | 66.7 | 51.7 | <0.0001 | 69.2 | 62.9 | 59.2 | 0.0001 |
Prognostic factors may confound the association between area SES and survival. Table 5 shows the univariate and multivariate analyses for the effects of area‐based SES on cumulative 5‐year survival for cervical cancer patients. In models 1, 2 and 3, area‐based SES (both unemployment and education) was significantly related to cumulative 5‐year survival for cervical cancer patients, although the effect of SES came with the addition of control variables. However, even after controlling for age, cancer stage, histology and treatment, survival differences between high and low SES still remained (model 3). For example, in model 3 cervical cancer patients in high unemployment municipalities had a 31% higher hazard ratio for mortality compared with patients in low unemployment municipalities. Cervical cancer patients in low education municipalities had a 17% higher hazard ratio compared with patients in high education municipalities. Table 6 shows the univariate and multivariate analyses for the effects of area‐based SES on cumulative 5‐year survival among corpus cancer patients. We did not enter histology as a covariate in models 2 and 3 because the distribution of histology for corpus cancer patients did not vary by area‐based SES. Differences in survival for corpus cancer patients between low and high unemployment municipalities still remained after controlling for age and cancer stage (model 2) as well as after control for age, cancer stage and treatment (model 3).
Table 5.
Effects of area‐based socioeconomic status (SES) on cervical cancer cumulative 5‐year survival estimated by Cox proportional hazards regression models
| Independent variable | Unemployment Univariate Hazard ratio (95%CI) | Model 1 Hazard ratio (95%CI) | Model 2 Hazard ratio (95%CI) | Model 3 Hazard ratio (95%CI) | Education Univariate Hazard ratio (95%CI) | Model 1 Hazard ratio (95%CI) | Model 2 Hazard ratio (95%CI) | Model 3 Hazard ratio (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Area‐based SES † | ||||||||
| High | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Middle | 1.19 (1.10–1.29) | 1.15 (1.07–1.24) | 1.11 (1.23–1.20) | 1.08 (0.99–1.17) | 1.10 (1.03–1.18) | 1.09 (1.02–1.17) | 1.06 (0.99–1.13) | 1.05 (0.95–1.12) |
| Low | 1.80 (1.67–1.95) | 1.59 (1.47–1.72) | 1.39 (1.28–1.50) | 1.31 (1.21–1.42) | 1.36 (1.26–1.47) | 1.29 (1.20–1.40) | 1.21 (1.12–1.31) | 1.17 (1.09–1.27) |
| Age (years) | ||||||||
| Under 40 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 40–49 | 1.56 (1.35–1.79) | 1.56 (1.36–1.79) | 1.31 (1.14–1.50) | 1.24 (1.08–1.43) | 1.56 (1.35–1.79) | 1.55 (1.35–1.78) | 1.31 (1.14–1.50) | 1.24 (1.08–1.43) |
| 50–59 | 2.32 (2.03–2.65) | 2.25 (1.97–2.56) | 1.56 (1.36–1.78) | 1.31 (1.15–1.50) | 2.32 (2.03–2.65) | 2.30 (2.02–2.63) | 1.58 (1.38–1.80) | 1.32 (1.16–1.51) |
| 60–69 | 2.80 (2.46–3.20) | 2.66 (2.33–3.03) | 1.82 (1.59–2.08) | 1.39 (1.22–1.60) | 2.80 (2.46–3.20) | 2.77 (2.43–3.16) | 1.85 (1.62–2.11) | 1.40 (1.23–1.61) |
| 70–79 | 4.29 (3.75–4.90) | 4.61 (3.56–4.65) | 2.57 (2.21–2.90) | 1.75 (2.28–3.17) | 4.29 (3.75–4.90) | 4.24 (3.71–4.85) | 2.58 (2.25–2.95) | 1.77 (1.53–2.03) |
| 80+ | 7.85 (6.71–9.19) | 7.42 (6.34–8.69) | 4.06 (3.46–4.76) | 2.69 (2.77–3.22) | 7.85 (6.71–9.19) | 7.76 (6.63–9.08) | 4.16 (3.54–4.88) | 2.72 (2.31–3.21) |
| Cancer stage | ||||||||
| Localized | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Regional | 4.82 (4.49–5.18) | 4.02 (3.74–4.32) | 2.99 (2.77–3.22) | 4.82 (4.49–5.18) | 4.09 (3.80–4.40) | 3.01 (2.79–3.24) | ||
| Distant | 14.94 (13.42–16.63) | 11.25 (10.09–12.55) | 8.06 (7.21–9.01) | 14.94 (13.42–16.63) | 11.44 (10.25–12.76) | 8.10 (7.25–9.06) | ||
| Unknown | 3.25 (2.93–3.60) | 2.43 (2.19–2.70) | 1.89 (1.70–2.10) | 3.25 (2.93–3.60) | 2.48 (2.23–2.75) | 1.91 (1.71–2.12) | ||
| Histology | ||||||||
| Squamous carcinomas | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Adenocarcinomas | 1.43 (1.28–1.60) | 1.61 (1.44–1.80) | 1.62 (1.45–1.81) | 1.43 (1.28–1.60) | 1.61 (1.44–1.79) | 1.62 (1.45–1.81) | ||
| Other specific | 1.05 (0.86–1.30) | 1.27 (1.03–1.56) | 1.28 (1.04–1.58) | 1.05 (0.86–1.30) | 1.25 (1.01–1.54) | 1.26 (1.02–1.56) | ||
| Carcinomas | ||||||||
| Other | 3.10 (2.85–3.38) | 2.56 (2.35–2.79) | 2.43 (2.22–2.65) | 3.10 (2.85–3.38) | 2.58 (2.36–2.81) | 2.44 (2.24–2.67) | ||
| Treatment | ||||||||
| Surgery alone | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Surgery combined with either radiation, chemotherapy, or both | 4.95 (4.36–5.63) | 2.84 (2.49–3.25) | 4.95 (4.36–5.63) | 2.87 (2.51–3.28) | ||||
| No surgery (radiation alone, chemo alone, or combined radiation + chemotherapy) | 10.27 (9.07–11.63) | 4.04 (3.52–4.63) | 10.27 (9.07–11.63) | 4.121 (3.59–4.73) | ||||
| Other treatments and unknown | 9.31 (7.96–10.88) | 4.48 (3.79–5.28) | 9.31 (7.96–10.88) | 4.501 (3.82–5.31) | ||||
In the case of ‘unemployment’, high area‐based SES means a low percentage of male unemployment in each municipality. In the case of ‘education’, high area‐based SES means a high percentage of college or graduate school graduates in each municipality. In model 1 we controlled for age; in model 2 we controlled for age plus biological factors (cancer stage and histology); in model 3 we controlled for all of the variables in model 2, plus treatment type. CI, confidence interval.
Table 6.
Effects of area‐based socioeconomic status (SES) on corpus cancer cumulative 5‐year survival estimated by Cox proportional hazards regression models
| Independent variable | Unemployment Univariate Hazard ratio (95%CI) | Model 1 Hazard ratio (95%CI) | Model 2 Hazard ratio (95%CI) | Model 3 Hazard ratio (95%CI) | Education Univariate Hazard ratio (95%CI) | Model 1 Hazard ratio (95%CI) | Model 2 Hazard ratio (95%CI) | Model 3 Hazard ratio (95%CI) |
|---|---|---|---|---|---|---|---|---|
| Area‐based SES † | ||||||||
| High | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Middle | 1.27 (1.08–1.49) | 1.26 (1.08–1.49) | 1.10 (0.94–1.30) | 1.10 (0.94–1.30) | 1.26 (1.09–1.46) | 1.30 (1.13–1.51) | 1.15 (0.99–1.33) | 1.17 (1.01–1.36) |
| Low | 1.99 (1.69–2.35) | 1.72 (1.46–2.03) | 1.54 (1.31–1.82) | 1.56 (1.32–1.84) | 1.43 (1.21–1.69) | 1.36 (1.15–1.61) | 1.17 (0.99–1.39) | 1.17 (0.99–1.39) |
| Age under 40 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 40–49 | 1.18 (0.76–1.82) | 1.14 (0.74–1.76) | 1.09 (0.71–1.68) | 1.10 (0.72–1.71) | 1.18 (0.76–1.82) | 1.17 (0.76–1.81) | 1.11 (0.72–1.72) | 1.14 (0.74–1.75) |
| 50–59 | 1.46 (0.97–2.19) | 1.37 (0.91–2.07) | 1.25 (0.83–1.89) | 1.26 (0.84–1.90) | 1.46 (0.97–2.19) | 1.44 (0.95–2.16) | 1.28 (0.85–1.92) | 1.29 (0.86–1.95) |
| 60–69 | 2.73 (1.81–4.10) | 2.50 (1.66–3.77) | 2.14 (1.42–3.22) | 2.12 (1.41–3.20) | 2.73 (1.81–4.10) | 2.70 (1.79–4.05) | 2.26 (1.50–3.41) | 2.26 (1.50–3.40) |
| 70–79 | 4.20 (2.77–6.38) | 3.71 (2.44–5.65) | 3.27 (2.15–4.99) | 2.95 (1.93–4.48) | 4.20 (2.77–6.38) | 4.12 (2.71–6.27) | 3.59 (2.36–5.47) | 3.24 (2.13–4.93) |
| 80+ | 8.07 (5.09–12.81) | 7.38 (4.65–11.72) | 6.61 (4.26–10.51) | 4.88 (3.05–7.81) | 8.07 (5.09–12.81) | 8.13 (5.13–12.91) | 6.86 (4.32–10.91) | 5.07 (3.17–8.11) |
| Cancer stage | ||||||||
| Localized | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Regional | 4.50 (3.85–5.27) | 4.51 (3.85–5.38) | 3.93 (3.33–4.64) | 4.50 (3.85–5.27) | 4.51 (3.85–5.28) | 3.98 (3.37–4.70) | ||
| Distant | 12.78 (10.71–15.26) | 11.84 (9.91–14.27) | 9.44 (7.82–11.39) | 12.78 (10.71–15.26) | 11.82 (9.88–14.14) | 9.50 (7.86–11.47) | ||
| Unknown | 3.50 (2.83–4.32) | 3.12 (2.52–3.86) | 2.43 (1.93–3.06) | 3.50 (2.83–4.32) | 3.16 (2.55–3.91) | 2.45 (1.94–3.09) | ||
| Treatment | ||||||||
| Surgery alone | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Surgery combined with either radiation, chemotherapy, or both | 2.31 (1.94–2.74) | 1.41 (1.17–1.70) | 2.31 (1.94–2.74) | 1.36 (1.13–1.64) | ||||
| No surgery (radiation alone, chemotherapy alone, or combined radiation + chemotherapy) | 8.33 (6.64–10.47) | 2.74 (2.15–3.50) | 8.33 (6.64–10.47) | 2.721 (2.13–3.48) | ||||
| Other treatments and unknown | 5.15 (4.04–6.56) | 2.35 (1.80–3.07) | 5.15 (4.04–6.56) | 2.321 (1.78–3.03) | ||||
In the case of ‘unemployment’, high area‐based SES means a low percentage of male unemployment in each municipality. In the case of ‘education’, high area‐based SES means a high percentage of college or graduate school graduates in each municipality. In model 1 we controlled for age; in model 2 we controlled for age plus biological factors (cancer stage); in model 3 we controlled for all of the variables in model 2, plus treatment type. CI, confidence interval.
Discussion
In this population‐based analysis of a metropolitan representative sample in Japan, we have shown substantial socioeconomic disparities in survival following cervical and corpus cancer, which remained statistically significant even after controlling for age, cancer stage, histology and treatment. We have also shown differences in the distribution of cancer stage, histology and treatment by SES in cervical cancer, as well as differences in the distribution of cancer stage and treatment following corpus cancer.
Many studies have indicated a significant association between low SES and poorer cancer survival in Western countries,( 3 ) including the well‐established socioeconomic disparities in survival among breast cancer patients in the USA.( 20 ) The association between low SES and poorer survival among cervical/corpus cancer patients has also been examined in previous studies outside of Japan, using different measures of SES, for example, education,( 5 , 7 , 21 ) occupation,( 8 , 9 , 10 , 11 ) housing tenure,( 11 ) income,( 21 ) poverty,( 5 ) and composite measure.( 4 , 12 , 13 ) In these studies, stage of cancer at diagnosis has been found to be the most important explanatory factor in the association between cancer survival and SES.( 2 , 4 , 13 , 22 ) We additionally controlled for type of treatment, although we did not have information on the quality of treatment. Choice of treatment depends on histological type, cancer stage, age and the health status of patients, among other things. Importantly, even after controlling for these prognostic factors, disparities in survival by SES still remained. Several explanations can be offered for our findings.
First, our use of survival as an end‐point reflects mortality from all causes of death, which might have overestimated SES differences. According to a population‐based study by Auvinen et al., the difference between all‐cause mortality and cancer‐specific mortality following cervical and corpus cancer was about 5%, but the impact on the estimated magnitude of SES differences was relatively small.( 11 ) In order to address this issue, analyses of cancer‐cause specific survival are needed.
Another data issue is that we excluded ‘part unspecified’ uterus cancer cases (ICD, 10th revision, code C55) which may have affected survival differences by SES. However, reanalysis of cervical cancer including 929 cases with ICD, 10th revision, code C55 also indicated residual SES differences after adjustment for prognostic factors. The reanalyzed results of the fully adjusted regression models were almost identical to the results shown in Table 5. Hazard ratios for patients from middle and high unemployment areas compared to patients from low unemployment areas were 1.09 (95% confidence interval [CI] 1.02–1.18) and 1.30 (95%CI 1.21–1.40), respectively. Hazard ratios for middle and low education SES were 1.05 (95%CI 0.98–1.12) and 1.16 (95%CI 1.08–1.25).
The vital status of several patients (out of the 4708 cervical cancer and 812 corpus cancer patients who originally resided in Osaka City from 1975–1992) were not ascertained by the registers in Osaka City office 5 years after the diagnosis, but via matching to the cancer death certificate file. This may have overestimated cancer survival. However, if this source of potential misclassification was corrected, the survival differences by SES could become larger because such cases occurred only among patients from the middle and low SES strata.
A second issue in interpreting our findings is that other explanatory factors may have been related to both patient and tumor characteristics.( 6 , 11 , 23 , 24 ) Complications of treatment and psychosocial factors might be important as explanatory factors related to patient survival. Low SES patients tend to suffer from more comorbidity.( 25 , 26 , 27 ) Differences in susceptibility to complications might be closely related to general health status, including nutrition status or lifestyle factors such as smoking, drinking and exercise. Health care seeking behavior prior to diagnosis as well as compliance with treatment after diagnosis also vary between SES groups.( 28 ) In turn, these factors depend on knowledge and awareness at the individual level, as well as social networks and social ties at the interpersonal level.( 29 ) Tumor characteristics similarly vary across SES,( 30 ) including histology( 21 ) and exposure to different risk factors.( 31 , 32 ) Exposure to risk factors, such as infection with the papillomavirus, fertility history, cigarette smoking and diet for cervical cancer,( 33 , 34 , 35 ) as well as obesity, age at menopause, lower parity, smoking, use of estrogen replacement therapy and oral contraceptive use for corpus cancer( 36 , 37 , 38 , 39 , 40 ) vary across SES groups, and may in turn contribute to differences in tumor characteristics.
In the present study, we used area‐based SES measurements as a proxy for individual‐level SES because we lacked information on the latter. Accordingly, our findings need to be interpreted with caution. For example, we were unable to determine which SES groups were at increased risk of lower survival within low SES areas. Conversely, our findings have suggested the existence of substantial disparities in survival following cervical and corpus cancer. Our study suggest that socioeconomic data at the ecological level, which are available from routine government sources, can serve as effective tools for assessing and monitoring cancer survival inequalities.
In conclusion, our study has demonstrated, for the first time in Japan, SES differences in survival following cervical and corpus cancer. We also indicated differences in the distribution of prognostic factors by SES. The Japan Ministry of Health, Labor and Welfare recommends cervical cancer screening to be carried out every other year for females aged over 20 years and increased health education efforts targeting the prevention of cervical and corpus cancer. Only 20% of Japanese women have received cervical cancer screening within the past year.( 41 ) Our findings point to the need for appropriate interventions including health education, screening, and improvement of access to care and treatment to ameliorate the survival difference across SES groups.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad and a Grant‐in‐Aid for Cancer Research (14‐2) from the Ministry of Health, Labour and Welfare of Japan. We would like to thank all who work in the Osaka Cancer Registry.
References
- 1. Lawson HW, Henson R, Bobo JK, Kaeser MK. Implementing recommendations for the early detection of breast and cervical cancer among low‐income women. MMWR Recomm Rep 2000; 49: 37–55. [PubMed] [Google Scholar]
- 2. Morgan MA, Behbakht K, Benjamin I, Berlin M, King SA, Rubin SC. Racial differences in survival from gynecologic cancer. Obstet Gynecol 1996; 88: 914–8. [DOI] [PubMed] [Google Scholar]
- 3. Kogevinas M, Porta M. Socioeconomic differences in cancer survival: a review of the evidence. In: Kogevinas M, Pearce N, Susser M, Boffetta P, eds. Social Inequalities and Cancer. Lyon: IARC Scientific Publications, 1997; 177–206. [PubMed] [Google Scholar]
- 4. Lamont DW, Symonds RP, Brodie MM, Nwabineli NJ, Gillis CR. Age, socio‐economic status and survival from cancer of cervix in the West of Scotland 1980–87. Br J Cancer 1993; 67: 351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh GK, Miller BA, Hankey BF, Edwards BK. Persistent area socioeconomic disparities in U.S. incidence of cervical cancer, mortality, stage, and survival, 1975–2000. Cancer 2004; 101: 1051–7. [DOI] [PubMed] [Google Scholar]
- 6. Auvinen A, Karjalainen S. Possible explanations for social class differences in cancer patient survival. In: Kogevinas M, Pearce N, Susser M, Boffetta P, eds. Social Inequalities and Cancer. Lyon: IARC Scientific Publications, 1997; 377–97. [PubMed] [Google Scholar]
- 7. Greenwald HP, Polissar NL, Dayal HH. Race, socioeconomic status and survival in three female cancers. Ethn Health 1996; 1: 65–75. [DOI] [PubMed] [Google Scholar]
- 8. Milner PC, Watts M. Effect of socioeconomic status on survival from cervical cancer in Sheffield. J Epidemiol Community Health 1987; 41: 200–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murphy M, Goldblatt P, Thornton‐Jones H, Silcocks P. Survival among women with cancer of the uterine cervix: influence of marital status and social class. J Epidemiol Community Health 1990; 44: 293–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vagero D, Persson G. Cancer survival and social class in Sweden. J Epidemiol Community Health 1987; 41: 204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Auvinen A, Karjalainen S, Pukkala E. Social class and cancer patient survival in Finland. Am J Epidemiol 1995; 142: 1089–102. [DOI] [PubMed] [Google Scholar]
- 12. Coleman MP, Babb P, Sloggett A, Quinn M, De Stavola B. Socioeconomic inequalities in cancer survival in England and Wales. Cancer 2001; 91: 208–16. [DOI] [PubMed] [Google Scholar]
- 13. Sankaranarayanan R, Nair MK, Jayaprakash PG et al. Cervical cancer in Kerala: a hospital registry‐based study on survival and prognostic factors. Br J Cancer 1995; 72: 1039–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ministry of Health, Labour and Welfare. Vital Statistics. Tokyo: Statistics and Information Department Minister's Secretariat, 2002. (In Japanese). [Google Scholar]
- 15. Ajiki W, Tsukuma H, Oshima A. Cancer incidence and incidence rates in Japan in 1999: estimates based on data from 11 population‐based cancer registries. Jpn J Clin Oncol 2004; 34: 352–6. [DOI] [PubMed] [Google Scholar]
- 16. Ioka A, Tsukuma H, Ajiki W, Oshima A. Trends in uterine cancer incidence in Japan 1975–98. Jpn J Clin Oncol 2003; 33: 645–6. [DOI] [PubMed] [Google Scholar]
- 17. Ioka A, Ajiki W, Tsukuma H, Oshima A. [Survival of gynecological cancer patients in Osaka, Japan]. Nippon Rinsho 2004; 62 (Suppl. 10): 49–54. (In Japanese). [PubMed] [Google Scholar]
- 18. Parkin DM, Plummer MR. Comparability and quality of data. In: Parkin DM, Whelan SL, Ferlay J, Teppo DB, Thomas DB, eds. Cancer Incidence in Five Continents vol 3. Lyon: IARC Scientific Publications, 2002;57–73. [Google Scholar]
- 19. IACR, IARC, ENCR . International rules for multiple primary cancer. Internal report No. 2004/02. Lyon: IARC, 2004. [Google Scholar]
- 20. Cohart EM. Socioeconomic distribution of cancer of the female sex organs in New Haven. Cancer 1995; 8: 34–41. [DOI] [PubMed] [Google Scholar]
- 21. Steinhorn SC, Myers MH, Hankey BF, Pelham VF. Factors associated with survival differences between black women and white women with cancer of the uterine corpus. Am J Epidemiol 1986; 124: 85–93. [DOI] [PubMed] [Google Scholar]
- 22. Inthasorn P, Carter J, Valmadre S, Beale P, Russell P, Dalrymple C. Analysis of clinicopathologic factors in malignant mixed Mullerian tumors of the uterine corpus. Int J Gynecol Cancer 2002; 12: 348–53. [DOI] [PubMed] [Google Scholar]
- 23. Kogevinas M, Marmot MG, Fox AJ, Goldblatt PO. Socioeconomic differences in cancer survival. J Epidemiol Community Health 1991; 45: 216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leon DA, Wilkinson RG. Inequalities in prognosis: socioeconomic differences in cancer and heart disease survival. In: Fox J, ed. Health Inequalities in European Countries. Aldershot: Gower, 1989; 280–300. [Google Scholar]
- 25. Lynch JW, Kaplan GA, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all‐cause mortality, cardiovascular mortality, and acute myocardial infarction? Am J Epidemiol 1996; 144: 934–42. [DOI] [PubMed] [Google Scholar]
- 26. Lynch JW, Kaplan GA, Shema SJ. Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. N Engl J Med 1997; 337: 1889–95. [DOI] [PubMed] [Google Scholar]
- 27. Kennedy BP, Kawachi I, Glass R, Prothrow‐Stith D. Income distribution, socioeconomic status, and self rated health in the United States: multilevel analysis. BMJ 1998; 317: 917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richardson JL, Langholz B, Bernstein L, Burciaga C, Danley K, Ross RK. Stage and delay in breast cancer diagnosis by race, socioeconomic status, age and year. Br J Cancer 1992; 65: 922–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Waxler‐Morrison N, Hislop TG, Mears B, Kan L. Effects of social relationships on survival for women with breast cancer: a prospective study. Soc Sci Med 1991; 33: 177–83. [DOI] [PubMed] [Google Scholar]
- 30. Chirikos TN, Horner RD. Economic status and survivorship in digestive system cancers. Cancer 1985; 56: 210–7. [DOI] [PubMed] [Google Scholar]
- 31. Mendall MA, Goggin PM, Molineaux N et al. Childhood living conditions and Helicobacter pylori seropositivity in adult life. Lancet 1992; 339: 896–7. [DOI] [PubMed] [Google Scholar]
- 32. Boffetta P, Kogevinas M, Simonato L, Wilbourn J, Saracci R. Current perspectives on occupational cancer risks. Int J Occup Environ Health 1995; 1: 315–25. [DOI] [PubMed] [Google Scholar]
- 33. Stuver S, Adami HO. Cervical cancer. In: Adami HO, Hunter DJ, Trichoponlos D, eds. Textbook of Cancer Epidemiology. Oxford: Oxford University Press, 2002; 340–58. [Google Scholar]
- 34. Munoz N, Bosch FX. Epidemiology of cervical cancer. IARC Sci Publ 1989; 9–39. [PubMed]
- 35. Winkelstein W Jr. Smoking and cervical cancer – current status: a review. Am J Epidemiol 1990; 131: 945–57. [DOI] [PubMed] [Google Scholar]
- 36. Weiderpass E, Adami HO, Baron JA et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst 1999; 91: 1131–7. [DOI] [PubMed] [Google Scholar]
- 37. Persson P, Adami HO. Endometrial cancer. In: Adami HO, Hunter DJ, Trichoponlos D, eds. Textbook of Cancer Epidemiology. Oxford: Oxford University Press, 2002; 359–62. [Google Scholar]
- 38. Cooper GS, Sandler DP. Age at natural menopause and mortality. Ann Epidemiol 1998; 8: 229–35. [DOI] [PubMed] [Google Scholar]
- 39. Brinton LA, Barrett RJ, Berman ML, Mortel R, Twiggs LB, Wilbanks GD. Cigarette smoking and the risk of endometrial cancer. Am J Epidemiol 1993; 137: 281–91. [DOI] [PubMed] [Google Scholar]
- 40. Stanford JL, Brinton LA, Berman ML et al. Oral contraceptives and endometrial cancer: do other risk factors modify the association? Int J Cancer 1993; 54: 243–8. [DOI] [PubMed] [Google Scholar]
- 41. Ministry of Health, Labour and Welfare . Comprehensive Survey of Living Conditions of the People on Health and Welfare. Tokyo: Statistics and Information Department Minister's Secretariat, 2001. (In Japanese). [Google Scholar]


