Abstract
Ret finger protein (RFP) is a nuclear protein with transcriptional repressive activity that is highly expressed in a variety of human and rodent tumor cell lines. We examined the expression of RFP in human endometrial cancer and assessed its clinical significance. Formalin‐fixed, paraffin‐embedded sections from endometrial cancer tissues were immunostained with the RFP antibody, and the staining intensity was evaluated. The clinicopathological factors examined were age, International Federation of Gynecology and Obstetrics stage, tumor grade, myometrial invasion, and pelvic lymph node metastasis. Overall survival (OS) and progression‐free survival (PFS) were evaluated using the Kaplan–Meier method, and multivariate analysis was performed using the Cox proportional hazard analysis. Of the 119 cancer tissues, 57 (47.9%) cases were positive for RFP immunoreactivity. RFP expression was not associated with any of the clinicopathological parameters examined. However, positive RFP expression significantly predicted poorer OS and PFS compared with negative expression (OS, P = 0.0011; PFS, P < 0.0001). In the multivariate analyses, positive RFP expression was an independent prognostic factor for survival in this study. RFP knockdown significantly impaired cancer cell migration and invasion in vitro with concomitant decreases of integrins β1 and α2. Positive RFP expression is a predictive marker for an unfavorable clinical outcome in patients with endometrial cancer. (Cancer Sci 2009; 100: 1895–1901)
Endometrial cancer is the most common gynecologic malignancy in the United States.( 1 ) In Japan, its incidence has dramatically increased over the past decade. Patients with deep myometrial invasion, poorly differentiated tumors, serous or clear cell histology, or extension of their disease to other organs or lymph nodes within the pelvis are at higher risk for disease recurrence.( 2 ) Several molecular markers have been reported to predict disease outcome of prognosis of endometrial cancer,( 3 ) although few have been translated into widespread clinical use. Additional prognostic indicators will improve care in that patients at a higher risk of relapse or death from their disease may be identified early enough to benefit from aggressive treatment.
Ret finger protein (RFP) was originally identified as a fusion protein with RET receptor tyrosine kinase.( 4 ) RFP contains a tripartite motif consisting of a RING finger, a B‐box zinc finger, and a coiled‐coil domain. RFP exhibits transcriptional repressive activity, and is associated with and colocalizes with Enhancer of Polycomb 1, Mi‐2β, and histone deactylase 1 (HDAC1) in the nucleus.( 5 , 6 ) RFP mRNA is highly expressed in a variety of human and rodent tumor cell lines. In addition, RFP protein is detectable in male germ cells and is present at a low level in peripheral and central neurons, hepatocytes, and adrenal chromaffin cells.( 5 , 7 ) However, in cancer patients, the exact function of RFP remains unclear.
The fact that RFP is strongly expressed in a variety of human cancer lines suggests that it may be involved in tumor development and/or progression. If this is indeed the case, RFP, in addition to being a prognostic indicator, may represent a novel molecular target for chemotherapeutics. In the present study, we performed an immunohistochemical analysis of RFP expression in endometrial cancer tissues to determine whether RFP expression correlated with clinicopathological factors or clinical outcomes in endometrial cancer patients.
Materials and Methods
Patients and tissue samples. A total of 119 samples were obtained from patients with endometrial endometrioid adenocarcinoma who underwent surgical treatment at Nagoya University Hospital between 1992 and 2007. All patients provided informed consent. The age of the patients ranged from 28 to 86 years with a median age of 57 years. None of these patients had undergone neo‐adjuvant chemotherapy. All patients underwent a total abdominal or radical hysterectomy plus bilateral salpingo‐oophorectomy. At the time of laparotomy, peritoneal fluid samples were obtained for cytologic testing. Systemic pelvic lymphadenectomy was performed in 92 (77.3%) patients. All patients were staged according to the 1988 International Federation of Gynecology and Obstetrics (FigO) criteria: 72 were stage I, 16 were stage II, 24 were stage III, and seven were stage IV. Histologic grade was assigned according to the World Health Organization classification: 50 were G1, 51 were G2, and 18 were G3. All tissue samples were fixed in 10% formalin, embedded in paraffin and routinely stained with hematoxylin–eosin for histological examination. In this study, all patients with FigO stage IC and higher received postoperative chemotherapy with six cycles of either cisplatin/doxorubicin/cyclophosphamide or cisplatin/etoposide from 1992 to 1999 and paclitaxel/carboplatin after 2000. Patients who received postoperative radiation therapy or any preoperative treatment were excluded from this study as the number of patients in this subset was too few to analyze. Patients with tumor recurrences were treated with chemotherapy, local radiation therapy, or surgical resection, when possible.
Antibodies. The rabbit polyclonal RFP antibody has been described previously.( 7 ) Other antibodies used in this study included integrin β1 (ITGB1) monoclonal antibody, integrin α2 (ITGA2) monoclonal antibody, integrin αV (ITGAV) monoclonal antibody, integrin α5 (ITGA5) monoclonal antibody, integrin β3 (ITGB3) monoclonal antibody (BD Biosciences, San Jose, CA, USA) and β‐actin monoclonal antibody (Sigma, St. Louis, MO, USA). BHA2.1 monoclonal antibody that specifically recognizes the α2β1 integrin complex was purchased from Chemicon (Temecula, CA, USA).
Immunohistochemistry. Immunihistochemical analysis was performed as described previously.( 8 ) Formalin‐fixed, paraffin‐embedded sections from endometrial cancer patients were deparaffinized and rehydrated. Slides were rinsed thoroughly with Protein Blocking Agent (UltraTech HRP Streptavidin–Biotin Detection System; Beckman Coulter, Fullerton, CA, USA) and endogenous peroxidase was blocked in 3% hydrogen peroxide. Antigen retrieval was performed by autoclaving at 121°C for 10 min in 0.1 mol/L citrate buffer (pH 7.0) with 0.5% NP‐40 (Sigma‐Aldrich, St. Louis, MO, USA). The sections were incubated with rabbit polyclonal RFP antibody (1 µg/mL) for 60 min and followed by incubation with the secondary biotinylated goat polyvalent antibody (Beckman Coulter) for 10 min. The samples were incubated with peroxidase‐conjugated streptavidin for 10 min and the reaction products were visualized using 3,3′‐diaminobenzidine tetrahydrochloride and H2O2. Counterstaining was performed using hematoxylin. When greater than 10% of the tumor cells were stained with the antibody regardless of staining intensity, the specimen was categorized as positive for RFP expression. Each specimen was scored twice by two independent observers who were blinded to the clinical parameters and other prognostic factors.
Cell culture. The human endometrial adenocarcinoma cell line HEC50, the human cervical adenocarcinoma cell line HeLa, and the human breast adenocarcinoma cell line MDA‐MB‐231 were maintained in RPMI with 10% fetal bovine serum (FBS) and penicillin‐streptomycin. These cells were incubated at 37°C in a humidified atmosphere of 5% CO2.
RNA interference. RFP, ITGB1, ITGA2 and control siRNA were purchased from Qiagen (Valencia, CA, USA). The siRNAs were transfected into cell lines using Lipofectoamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The target sequences were RFP, 5′‐GGUACAUCGACUUCCU CUATT‐3′; ITGB1, 5′‐GAGGATATTACTCAGATCCAA‐3′; and ITGA2, 5′‐CCCGAGCACATCATTTATATA‐3′.
Western blot analysis. Cells were washed twice with ice cold PBS and treated with lysis buffer (50 mM Tris‐HCl [pH 7.4], 120 mM NaCl, 5 mM MgCl2, 0.8% Nonidet P‐40, 10% glycerol, 1 mM DTT, and 1 mM PMSF) containing Complete Protease Inhibitor Mixture (Roche, Basel, Switzerland). The lysates were briefly sonicated. Western blotting was performed as previously described.( 5 )
Northern blot analysis. Total RNAs from HEC50, MDA‐MB‐231, and HeLa cells were isolated using Trizol (Invitrogen) and separated on 1% agarose–formamide gels with formaldehyde and transferred onto Hybond‐XL nylon membranes (Amersham Biosciences, Milwaukee, WI, USA). Human ITGA2, ITGB1, RFP, andβ‐actin cDNA fragments were labeled with [32P] dCTP (Perkin Elmer, Waltham, MA, USA) using the High Prime DNA‐labeling system (Roche Diagnostics, Basel, Switzerland) and used as probes for northern hybridization at 65°C for 20 h. Signals were detected on Fuji X‐ray films (Fuji, Tokyo, Japan) after exposure for an appropriate time.
Quantitative RT‐PCR. Pre‐designed primers and probes for RFP, ITGB1, and ITGA2 cDNAs were purchased from Nippon EGT (Toyama, Japan). All of the real‐time quantitative RT‐PCR reactions were performed using the TaqMan PCR Core Reagents Kit and the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Amplification of 18S rRNA was used as the normalization control.
Cell proliferation assay. The cell proliferation assay was performed as described previously.( 9 ) Cells were seeded in triplicate in 96‐well plates at a density of 2000 cells in a volume of 200 µl of RPMI‐1640 containing 10% FCS, and cultured for 1–5 days. Cell viability was assayed by a modified tetrazolium salt 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide assay using a Cell Titer 96 Aqueous One Solution Cell Proliferation Assay kit (Promega, Madison, WI, USA) according to the manufacturer's instructions. Absorbance was measured at 492 nm using a microplate reader (Multiskan Bichromatic; Vienna, VA, USA).
In vitro cell migration assay. Tumor cell migration was evaluated using 24‐well transwell chambers (Corning, Corning, NY, USA) containing filters (6.5 mm in diameter) with an 8.0‐µm pore size. HEC50, MDA‐MB‐231, and HeLa cells were suspended at a final concentration of 2.5 × 104/mL in serum‐free medium in the upper chamber, and 800 µL of 1% FBS medium was added to the lower wells. To inhibit α2β1 integrin, 10 µg/mL BHA2.1 (Chemicon) was added to the upper wells. After 8‐h incubation, the remaining tumor cells on the upper surface of filters were removed by wiping with cotton swabs, and the cells which had invaded though to the lower surface were visualized with May–Grunward Giemsa. The number of cells was counted under a microscope at a magnification of ×100 or ×200. Data were obtained from three independent experiments performed in triplicate.
In vitro invasion assay. Tumor cell invasion was evaluated using 24‐well Matrigel invasion chambers (24 wells; BD Biocoat Matrigel invasion chamber; BD Biosciences). The cells were plated in the same manner as in the migration assay and incubated for 16 h.
Statistical analysis. The association between negative versus positive RFP expression and clinicopathological parameters was evaluated using the χ2‐test. The RFP expression was used for further analysis regarding overall survival (OS) and progression‐free survival (PFS). The univariate survival analysis was based on the Kaplan–Meier method. Comparison between the survival curves was analyzed using the log‐rank test. The OS was defined as the time between the date of surgery and the last date of follow‐up or the date of death owing to endometrial cancer. The PFS was defined as the time interval between the date of surgery and the date of progression/recurrence or date of last follow‐up. The prognostic significance of positive RFP expression concerning other pathological variables was assessed using multivariate Cox's proportional hazard's analysis. Stat View software version 5.0 (SAS Institution, Cary, NC, USA) was used for all statistical analyses and a P‐value of <0.05 was considered significant. Statistical analyses for in vitro migration and invasion assays were performed using the unpaired Student's t‐test. Differences were considered significant at P < 0.05.
Results
Immunohistochemical detection of RFP in endometrial cancer tissues. When greater than 10% of the tumor cells were stained with the RFP antibody, it was categorized as positive (Fig. 1). RFP expression was almost undetectable in the tumor stroma and normal endometrial glands (Fig. 1g). The clinical characteristics of the patients are summarized in Supplementary Table S1. Of the 119 endometrial cancer specimens examined in this study, 57 (47.9%) cases were positive for RFP immunoreactivity. No significant association was found between RFP immunoreactivity and any of the clinicopathological parameters tested: age, FigO stage, tumor grade, myometrium invasion, and pelvic lymph node metastasis (Table 1); however, the percentage of RFP‐positive cases was greater in the patients with pelvic lymph node metastasis (69%) than in those without metastasis (45%) (P = 0.073).
Figure 1.
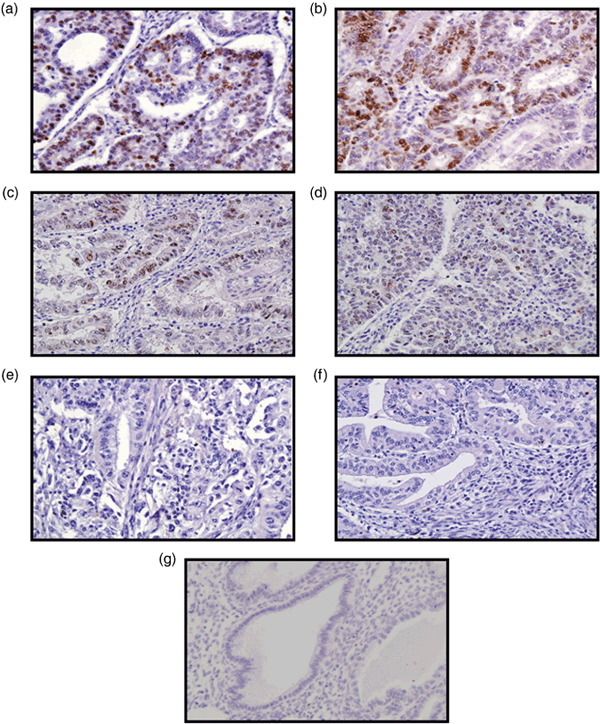
Immunohistochemical analysis of Ret finger protein (RFP) in endometrial cancer. (a,b) Strongly positive, (c) moderately positive, (d) weakly positive, and (e) negative cases. (f) No primary antibody. (g) Normal endometrium. When greater than 10% of the cancer cells were stained with the RFP antibody regardless of staining intensity, the specimen was categorized as positive for RFP expression.
Table 1.
Association between Ret finger protein (RET) expression and clinicopathologic factors in patients with endometrial cancer
| Variables | No. of patients | RFP negative | Expression positive | P‐values |
|---|---|---|---|---|
| Age (years) | ||||
| <60 | 70 | 39 | 31 | 0.346 |
| ≥60 | 49 | 23 | 26 | |
| FigO stage | ||||
| I | 71 | 39 | 32 | 0.453 |
| II–IV | 48 | 23 | 25 | |
| Grade | ||||
| 1 | 50 | 29 | 21 | 0.273 |
| 2,3 | 69 | 33 | 36 | |
| Myometrial invasion | ||||
| <1/2 | 74 | 41 | 33 | 0.355 |
| ≥1/2 | 45 | 21 | 24 | |
| Pelvic lymph node metastasis | ||||
| No | 103 | 57 | 46 | 0.073 |
| Yes | 16 | 5 | 11 | |
FigO, International Federation of Gynecology and Obstetrics.
Correlation of RFP expression with survival of endometrial cancer patients. The median OS of the patients was 55 months (range, 1–160). At the end of the follow‐up period, 104 (87.4%) patients were alive, 15 (12.6%) patients died from their disease, and nine (7.6%) patients were alive with disease. None of the patients were lost to follow‐up. During the follow‐up period, the total number of cases in which death and progression/recurrence were observed was 24 (20%). The 5‐year OS rates of patients who were negative (n = 62) and positive (n = 57) for RFP expression were 96.7 and 72.5%, respectively (Supplementary Table S2). In the univariate analyses, FigO stage, myometrial invasion, pelvic lymph node metastasis, and positive RFP expression were significant predictors of poor OS (Supplementary Table S2). For PFS, FigO stage, tumor grade, myometrial invasion, pelvic lymph node metastasis, and RFP expression were significant factors (Supplementary Table S2).
Figure 2 shows the OS and PFS curves with respect to RFP expression. Both OS and PFS in patients with positive RFP expression were significantly lower than those in patients with negative RFP expression (OS, P = 0.0011; PFS, P < 0.0001). We further divided all cases into two groups according to FigO stage (stage I and stage II–IV). As expected, there was a significant difference in OS between RFP‐negative and ‐positive groups (stage I, P = 0.0232; stage II–IV, P = 0.0194, respectively) (Fig. 2). Additionally, there was a significant difference in PFS between the two groups (stage I, P = 0.0045; stage II–IV, P = 0.0005, respectively) (Fig. 2). Similarly, all cases were stratified according to tumor grade (grade I and grade II/III). The expression of RFP was a significant prognostic factor in the grade II/III group. (OS; P = 0.001, PFS; P < 0.0001, respectively) (Supplementary Fig. S1).
Figure 2.
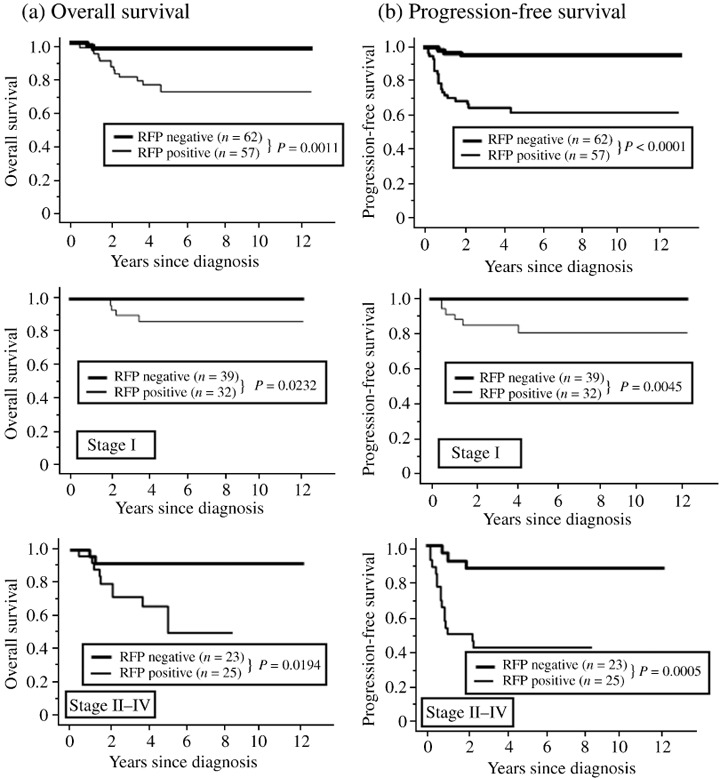
The Kaplan–Meier survival curves of 119 patients with endometrial cancer stratified by International Federation of Gynecology and Obstetrics (FigO) stage with respect to Ret finger protein (RFP) expression. (a) Overall survival: all cases (upper panel), stage I cases (middle panel), stage II–IV cases (lower panel). (b) Progression‐free survival: all cases (upper panel), stage I cases (middle panel), stage II–IV cases (lower panel).
Furthermore, we asked whether OS and PFS were affected by the surgical procedures (total hysterectomy vs radical hysterectomy; systemic pelvic lymphadenectomy [–]vs [+]). Interestingly, both the OS and PFS of RFP‐positive patients, but not of RFP‐negative patients, were significantly improved by radical hysterectomy or pelvic lymphadenectomy (Supplementary Figs S2 and S3). These findings suggest that RFP expression profiles in endometrial cancer patients may provide useful information for making a choice of the surgical procedures.
Multivariate analysis of prognostic variables in endometrial cancer patients. In the multivariate OS analyses, age, FigO stage, tumor grade, myometrium invasion, pelvic lymph node metastasis, and RFP expression were entered into the Cox proportional hazard analysis. The relative risk (RR) for positive RFP expression was 6.767 (95% confidence interval, 1.494–30.641; P = 0.013). Furthermore, in the multivariate analysis for PFS including the same factors, the expression of RFP retained its significance as an independent prognostic factor of poor PFS. The relative risk (RR) for positive RFP expression was 9.361 (95% confidence interval, 2.761–31.742; P = 0.0003).
Relationship between RFP expression and cancer cell migration. To investigate the mechanism by which RFP expression associates with poor prognosis, we evaluated the effects of knockdown of RFP expression on cancer cell migration and proliferation. RFP siRNA was transfected into HEC50, MDA‐MB‐231, and HeLa cells. RFP knockdown resulted in a significant impairment of cell migration in vitro (Fig. 3a,b). In the cell proliferation assay, RFP siRNA transfection significantly decreased proliferation of HeLa cells, but not of the other two cell lines (Fig. 3c).
Figure 3.
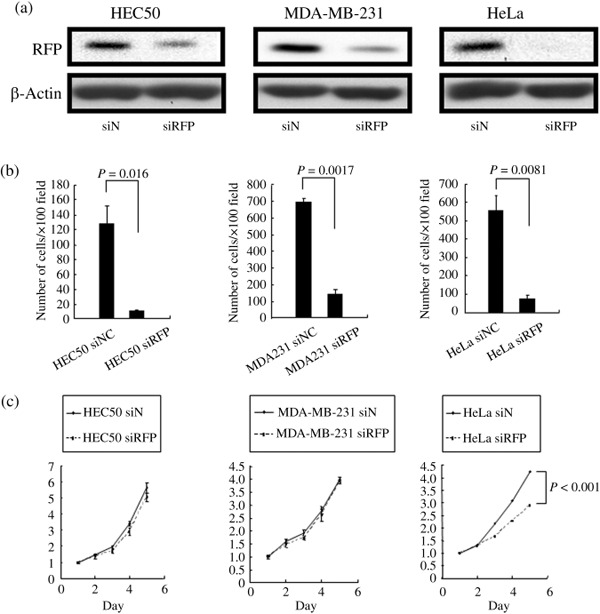
The effects of knockdown of Ret finger protein (RFP) expression on cancer cell migration and proliferation. (a) Control siRNA and RFP siRNA were transfected into HEC50, MDA‐MB‐231, and HeLa cells 72 h before the assays. Total cell lysates from each cell line were subjected to western blotting with RFP or β‐actin antibody. (b) In vitro migration assay. Transfection of RFP siRNA showed a significant impairment of cell migration in three cell lines. (c) Cell proliferation assay. Transfection of RFP siRNA significantly decreased proliferation of HeLa cells, but not of the other two cell lines.
Decrease of integrins β1 and α2 expression by RFP siRNA transfection. To determine which cellular components were affected by RFP siRNA transfection, expression of integrins, matrix metalloproteinases (MMPs), and E‐cadherin, which were involved in cell migration and invasion, were analyzed by western blotting. The expression levels of E‐cadherin, MMP2, MMP7, and MT‐MMP‐1 were not significantly changed by RFP knockdown (data not shown). In contrast, integrin β1 (ITGB1) and integrin α2 (ITGA2) expression were significantly decreased by RFP siRNA transfection in the three cell lines (Fig. 4a). Changes in the expression levels of the other integrins (ITGAV, ITGA5, and ITGB3) were variable depending on the cell lines.
Figure 4.
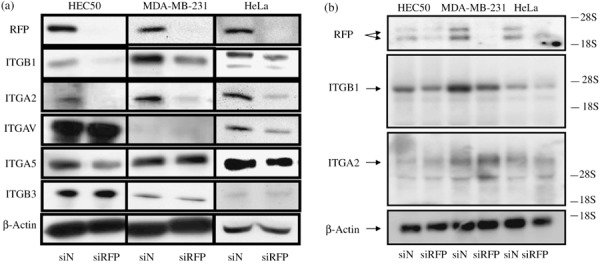
Decrease of integrin β1 (ITGB1) and integrin α2 (ITGA2) protein expression by transfection of RFP siRNA. (a) Total lysates from HEC50, MDA‐MB‐231, and HeLa cells were subjected to western blotting with RFP or integrin antibodies (indicated at left side). (b) Expression levels of ITGB1 and ITGA2 mRNA were analyzed by northern blotting. The ITGB1, but not ITGA2, mRNA level was significantly changed by RFP knockdown.
Since RFP is involved in gene transcription,( 5 ) we next investigated whether RFP affected ITGB1 and ITGA2 mRNA levels. In northern blotting, expression of ITGB1 mRNA, but not of ITGA2 mRNA, was significantly decreased by RFP knockdown (Fig. 4b), suggesting the possibility that expression of ITGB1 and ITGA2 proteins is regulated by different mechanisms.
Roles of ITGB1 and ITBA2 in cancer cell migration and invasion. Finally, to confirm the importance of ITGB1, ITGA2, and RFP in cancer cell migration and invasion, siRNA for ITGB1, ITGA2 or RFP was transfected into cancer cells. Knockdown of ITGB1, ITGA2, and RFP expression was confirmed by quantitative RT‐PCR (Supplementary Fig. S4). As shown in Figure 5(a,b), knockdown of these gene expressions markedly decreased cancer cell migration and invasion. Moreover, cancer cell migration and invasion were impaired by the addition of α2β1 integrin antibody (BHA2.1) (Fig. 5c,d).
Figure 5.
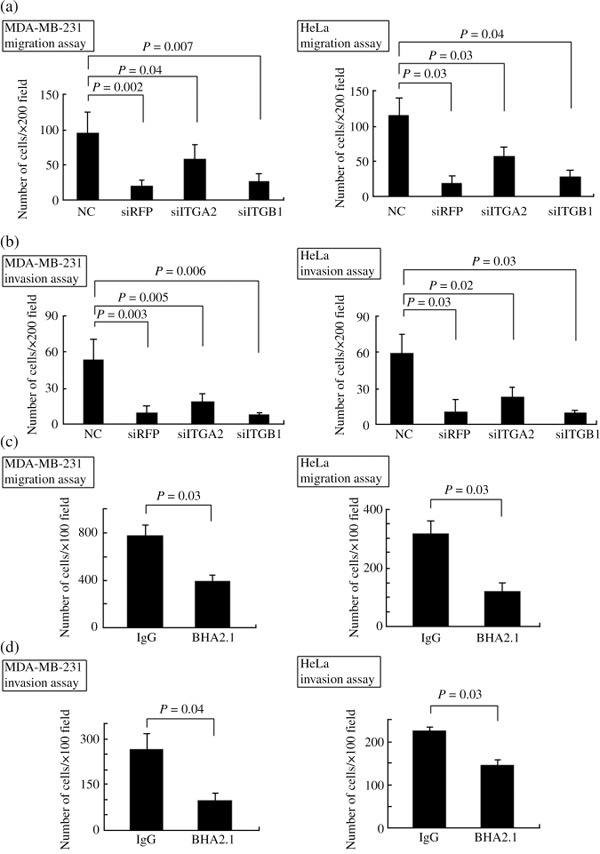
Roles of integrin β1 (ITGB1), integrin α2 (ITGA2), and Ret finger protein (RFP) in cancer cell migration and invasion. siRNA for ITGB1, ITGA2, or RFP was transfected into cancer cells. (a,b) Knockdown of ITGB1, ITGA2, or RFP expression markedly impaired cancer cell migration and invasion. (c,d) Cancer cell migration and invasion were inhibited by addition of α2β1 integrin antibody (BHA2.1). (Left panel) MDA‐MB‐231 cells, (right panel) HeLa cells.
Discussion
In the present study, we evaluated the expression of RFP in endometrial cancer using 119 surgical specimens and found that RFP expression in cancer cells was correlated with poor clinical outcome. This is the first report to demonstrate the detailed clinicopathological and prognostic impact of RFP expression in human endometrial cancer.
To elucidate the mechanism by which RFP expression contributed to the clinical outcome of endometrial cancer patients, we investigated the effect of RFP knockdown on cell proliferation and migration using three cancer cell lines. RFP knockdown affected the proliferation of HeLa cells but not of the other two cell lines (HE50 and MDA‐MB‐231 cells). This finding implies that RFP may regulate the expression of different genes involved in cell proliferation between three cell lines. In contrast, cell migration of these three cell lines was markedly impaired by RFP knockdown. In addition, among the proteins involved in cell migration (E‐cadherin, MMPs, and integrins), we found that ITGB1 and ITGA2 expression significantly decreased in RFP knockdown cells, suggesting the importance of these integrins in progression of endometrial cancer.
Tumor invasion and metastasis are complex, multistep processes requiring the dynamic interaction of tumor cells with the host environment.( 10 ) Integrins are known to be crucial regulators of tumor cell interactions with the microenvironment. There are an increasing number of reports in the literature implicating ITGB1 in tumorigenesis, both in vitro and in vivo.( 11 , 12 , 13 ) ITGB1 has been studied extensively in the biology of solid tumors, and its expression has been shown to correlate with poor prognosis in cancers of the lung,( 14 ) pancreas,( 15 ) and cutaneous melanoma.( 16 ) ITGB1 has also been implicated in the progression and metastasis of human breast cancer.( 17 , 18 ) Moreover, increased expression of the α2β1 integrin heterodimer has been observed in melanoma and gastric cancer, and this integrin species is thought to be a predictive marker for metastatic disease.( 19 , 20 , 21 ) Our findings also suggest that the α2β1 integrin heterodimer may play a crucial role in the progression of endometrial cancer.
At present, the transcriptional mechanism that regulates ITGB1 and ITGA2 expression in cancer cells remains elusive. As RFP is known to be a transcriptional regulator,( 5 , 6 ) we postulated that RFP knockdown down‐regulated the expression of ITGB1 and ITGA2 mRNA. Northern blot analysis revealed that RFP knockdown significantly decreased the ITGB1, but not ITGA2, mRNA level. This finding suggested that ITGB1 expression is regulated at a transcriptional level whereas ITGA2 could be regulated at a post‐transcriptional level including the degradation pathway. Alternatively, heterodimer formation of ITGA2 and ITGB1 may stabilize the ITGA2 protein. Further investigation is necessary to elucidate the mechanisms of down‐regulation of ITGB1 and ITGA2 proteins by RFP knockdown.
We have recently demonstrated that RFP complexes with HDAC1 and transcription factor NF‐Y. We found that this protein complex confers the resistance to anticancer drugs by decreasing the expression of thioredoxin binding protein‐2 (TBP‐2).( 22 ) TBP‐2 inhibits thioredoxin,( 23 , 24 ) a scavenger of ROS, and sensitizes cells to oxidative stress and cisplatin.( 25 , 26 ) Interestingly, HDAC inhibitors sensitize cancer cells to oxidative stress by inhibition of the HDAC1/RFP/NF‐Y complex, which results in re‐expression of TBP‐2.( 22 ) Thus, it is also possible that RFP expression is associated with the poor clinical outcome of endometrial cancer by regulating cancer cell resistance to oxidative stress.
In conclusion, we demonstrated here that RFP expression correlated with poor prognosis in endometrial cancer patients. RFP was an independent prognostic factor for PFS and OS. In addition, consistent with the fact that RFP regulates cancer cell migration, RFP‐positive cases in patients with pelvic lymph node metastasis (69%) were greater than those in patients without metastasis (45%). Therefore, RFP is not only a potential prognostic indicator in endometrial cancer but may also represent a molecular target against which to develop novel chemotherapeutic agents.
Disclosure Statement
No potential conflicts of interest are disclosed.
Supporting information
Fig. S1. The Kaplan–Meier survival curves of Ret finger protein (RFP) expression stratified by grade. (a,b) Overall survival: grade I and grade II/III; (c,d) progression‐free survival: grade I and grade II/III.
Fig. S2. The Kaplan–Meier survival curves of 119 patients with endometrial cancer with respect to surgical procedure. Left panel, overall survival; right panel, progression‐free survival. (a,b) The Kaplan–Meier survival curves of radical hysterectomy versus total hysterectomy in all cases. (c,d) The Kaplan–Meier survival curves of radical hysterectomy versus total hysterectomy in Ret finger protein (RFP)‐negative patients. (e,f) The Kaplan–Meier survival curves of radical hysterectomy versus total hysterectomy in RFP‐positive patients.
Fig. S3. The Kaplan–Meier survival curves of 119 patients with endometrial cancer with respect to systemic pelvic lymphadenectomy. Left panel, overall survival; right panel, progression‐free survival. (a,b) The Kaplan–Meier survival curves of lymphadenectomy (+) versus lymphadenectomy (–) in all cases. (c,d) The Kaplan–Meier survival curves of lymphadenectomy (+) versus lymphadenectomy (–) in Ret finger protein (RFP)‐negative patients. (e,f) The Kaplan–Meier survival curves of lymphadenectomy (+) versus lymphadenectomy (–) in RFP‐positive patients.
Fig. S4. Quantitative real‐time PCR (qRT‐PCR) analysis for evaluating the effect of siRNA. SiRNAs for Ret finger protein (RFP), integrin α2 (ITGA2), or integrin β1 (ITGB1) were transfected into HeLa (a), MDA‐MB‐231 (b), or HEC50 (c) cells. Pre‐designed primers for each cDNA were purchased from Nippon EGT and used for qRT‐PCR. Values represent mean ± SD of three independent experiments.
Table S1. Clinicopathologic characteristics of endometrial cancer patients
Table S2. Univariate analysis of various clinicopathologic parameters in relation to survival of patients with endometrial cancer
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgments
This work was supported by Grants‐in‐Aid for Global Center of Excellence (GCOE) Research, Scientific Research (A), and Scientific Research on the Priority Area ‘Cancer’ from the Ministry of Education, Culture, Sports, Science and Technology of Japan to M.T.
References
- 1. Jemal A, Thomas A, Murray T et al . Cancer statistics, 2002. CA Cancer J Clin 2002; 52: 23–47. [DOI] [PubMed] [Google Scholar]
- 2. Morrow CP, Bundy BN, Kurman RJ et al . Relationship between surgical‐pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol 1991; 40: 55–65. [DOI] [PubMed] [Google Scholar]
- 3. Inoue M. Current molecular aspects of the carcinogenesis of the uterine endometrium. Int J Gynecol Cancer 2001; 11: 334–9. [DOI] [PubMed] [Google Scholar]
- 4. Takahashi M, Inaguma Y, Hiai H et al . Developmentally regulated expression of a human ‘finger’‐containing gene encoded by the 5′ half of the ret transforming gene. Mol Cell Biol 1988; 8: 1853–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimono Y, Murakami H, Kawai K et al . Mi‐2 beta associates with BRG1 and RET finger protein at the distinct regions with transcriptional activating and repressing abilities. J Biol Chem 2003; 278: 51638–45. [DOI] [PubMed] [Google Scholar]
- 6. Shimono Y, Murakami H, Hasegawa Y et al . RET finger protein is a transcriptional repressor and interacts with enhancer of polycomb that has dual transcriptional functions. J Biol Chem 2000; 275: 39411–9. [DOI] [PubMed] [Google Scholar]
- 7. Tezel G, Nagasaka T, Iwahashi N et al . Different nuclear/cytoplasmic distributions of RET finger protein in different cell types. Pathol Int 1999; 49: 881–6. [DOI] [PubMed] [Google Scholar]
- 8. Hasegawa M, Moritani S, Murakumo Y et al . CD109 expression in basal‐like breast carcinoma. Pathol Int 2008; 58: 288–94. [DOI] [PubMed] [Google Scholar]
- 9. Kitamura T, Asai N, Enomoto A et al . Regulation of VEGF‐mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol 2008; 10: 329–37. [DOI] [PubMed] [Google Scholar]
- 10. Ramsay AG, Marshall JF, Hart IR. Integrin trafficking and its role in cancer metastasis. Cancer Metastasis Rev 2007; 26: 567–78. [DOI] [PubMed] [Google Scholar]
- 11. Wang F, Hansen RK, Radisky D et al . Phenotypic reversion or death of cancer cells by altering signaling pathways in three‐dimensional contexts. J Natl Cancer Inst 2002; 94: 1494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weaver VM, Petersen OW, Wang F et al . Reversion of the malignant phenotype of human breast cells in three‐dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol 1997; 137: 231–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zutter MM, Santoro SA, Staatz WD et al . Re‐expression of the alpha 2 beta 1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci USA 1955; 92: 7411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oshita F, Kameda Y, Hamanaka N et al . High expression of integrin beta1 and p53 is a greater poor prognostic factor than clinical stage in small‐cell lung cancer. Am J Clin Oncol 2004; 27: 215–9. [DOI] [PubMed] [Google Scholar]
- 15. Böttger TC, Maschek H, Lobo M et al . Prognostic value of immunohistochemical expression of beta‐1 integrin in pancreatic carcinoma. Oncology 1999; 56: 308–13. [DOI] [PubMed] [Google Scholar]
- 16. Nikkola J, Vihinen P, Vlaykova T et al . Integrin chains beta1 and alphav as prognostic factors in human metastatic melanoma. Melanoma Res 2004; 14: 29–37. [DOI] [PubMed] [Google Scholar]
- 17. Jonjiæ N, Lucin K, Krstulja M et al . Expression of beta‐1 integrins on tumor cells of invasive ductal breast carcinoma. Pathol Res Pract 1993; 189: 979–84. [DOI] [PubMed] [Google Scholar]
- 18. Gui GP, Wells CA, Browne PD et al . Integrin expression in primary breast cancer and its relation to axillary nodal status. Surgery 1995; 117: 102–8. [DOI] [PubMed] [Google Scholar]
- 19. Vuoristo M, Vihinen P, Vlaykova T et al . Increased gene expression levels of collagen receptor integrins are associated with decreased survival parameters in patients with advanced melanoma. Melanoma Res 2007; 17: 215–23. [DOI] [PubMed] [Google Scholar]
- 20. Matsuoka T, Yashiro M, Nishimura S et al . Increased expression of alpha2beta1‐integrin in the peritoneal dissemination of human gastric carcinoma. Int J Mol Med 2000; 5: 21–5. [DOI] [PubMed] [Google Scholar]
- 21. Kawamura T, Endo Y, Yonemura Y et al . Significance of integrin alpha2/beta1 in peritoneal dissemination of a human gastric cancer xenograft model. Int J Oncol 2001; 18: 809–15. [PubMed] [Google Scholar]
- 22. Kato T, Shimono Y, Hasegawa M et al . Characterization of the HDAC1 complex that regulates cancer cell sensitivity to oxidative stress. Cancer Res 2009; 69: 3597–604. [DOI] [PubMed] [Google Scholar]
- 23. Nishiyama A, Matsui M, Iwata S et al . Identification of thioredoxin‐binding protein‐2/vitamin D (3) up‐regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem 1999; 274: 21645–50. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, De Keulenaer GW, Lee RT. Vitamin D (3)‐up‐regulated protein‐1 is a stress‐responsive gene that regulates cardiomyocyte viability through interaction with thioredoxin. J Biol Chem 2002; 277: 26496–500. [DOI] [PubMed] [Google Scholar]
- 25. Baker AF, Koh MY, Williams RR et al . Identification of thioredoxin‐interacting protein 1 as a hypoxia‐inducible factor 1alpha‐induced gene in pancreatic cancer. Pancreas 2008; 36: 178–86. [DOI] [PubMed] [Google Scholar]
- 26. Junn E, Han SH, Im JY et al . Vitamin D3 up‐regulated protein 1 mediates oxidative stress via suppressing the thioredoxin function. J Immunol 2000; 164: 6287–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. The Kaplan–Meier survival curves of Ret finger protein (RFP) expression stratified by grade. (a,b) Overall survival: grade I and grade II/III; (c,d) progression‐free survival: grade I and grade II/III.
Fig. S2. The Kaplan–Meier survival curves of 119 patients with endometrial cancer with respect to surgical procedure. Left panel, overall survival; right panel, progression‐free survival. (a,b) The Kaplan–Meier survival curves of radical hysterectomy versus total hysterectomy in all cases. (c,d) The Kaplan–Meier survival curves of radical hysterectomy versus total hysterectomy in Ret finger protein (RFP)‐negative patients. (e,f) The Kaplan–Meier survival curves of radical hysterectomy versus total hysterectomy in RFP‐positive patients.
Fig. S3. The Kaplan–Meier survival curves of 119 patients with endometrial cancer with respect to systemic pelvic lymphadenectomy. Left panel, overall survival; right panel, progression‐free survival. (a,b) The Kaplan–Meier survival curves of lymphadenectomy (+) versus lymphadenectomy (–) in all cases. (c,d) The Kaplan–Meier survival curves of lymphadenectomy (+) versus lymphadenectomy (–) in Ret finger protein (RFP)‐negative patients. (e,f) The Kaplan–Meier survival curves of lymphadenectomy (+) versus lymphadenectomy (–) in RFP‐positive patients.
Fig. S4. Quantitative real‐time PCR (qRT‐PCR) analysis for evaluating the effect of siRNA. SiRNAs for Ret finger protein (RFP), integrin α2 (ITGA2), or integrin β1 (ITGB1) were transfected into HeLa (a), MDA‐MB‐231 (b), or HEC50 (c) cells. Pre‐designed primers for each cDNA were purchased from Nippon EGT and used for qRT‐PCR. Values represent mean ± SD of three independent experiments.
Table S1. Clinicopathologic characteristics of endometrial cancer patients
Table S2. Univariate analysis of various clinicopathologic parameters in relation to survival of patients with endometrial cancer
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


