Abstract
(Cancer Sci 2010; 101: 815–819)
Recently, it was observed that nestin is preferentially expressed in basal/myoepithelial cells of the mammary gland, and that this intermediate filament may be used as a myoepithelial marker. However, the clinical and prognostic implications of nestin as a marker for breast cancer are still unclear. We examined mastectomy specimens from 150 breast cancers and matching, adjacent non‐cancerous tissues using immunohistochemistry and western blotting. Overall, triple‐negative breast cancers – that is, breast cancers that do not express estrogen receptors (ER), progesterone receptors (PR), or human epidermal growth factor receptor 2 (HER2/neu) – had higher expression rates for nestin than the other breast cancers (57.14%vs 9.30%; P < 0.001). In triple‐negative breast cancers, significantly increased nestin expression rates were observed in patients with lymph node metastasis compared with those without node metastasis (25.00%vs 76.92%; P = 0.032). A similar phenomenon was observed for invasive ductal carcinomas compared with ductal carcinoma in situ (16.67%vs 73.33%; P = 0.046). Nestin expression was also found to be significantly related to ER, PR, and P53 expression (P < 0.05). Nestin expression was associated with both shorter breast cancer‐specific survival and poor relapse‐free survival in the lymph node‐positive group (P = 0.028 and P = 0.012 respectively). After Cox regression was carried out, nestin was not shown to be an independent prognostic factor for breast cancer. These findings substantiate the possibility of using nestin as a marker for triple‐negative breast cancer. Triple‐negative breast cancer progression is associated with nestin; however, the underlying mechanisms of this relationship require further investigation.
Breast carcinoma is the most common malignancy in women and is the second leading cause of cancer death in women.( 1 ) Over the last 30 years, deaths from breast cancer have approximately tripled in Japan, which historically has had a low incidence of breast cancer.( 2 ) According to the World Health Organization, more than 1.2 million people will be diagnosed with breast cancer each year worldwide.( 2 )
Breast cancer is a heterogeneous disease embracing several different phenotypes with consistently different biological characteristics.( 3 ) Hormonal therapies (tamoxifen, anti‐estrogens) and adjuvant chemotherapy (trastuzumab [Herceptin]) have benefited millions of patients with breast cancer.( 4 , 5 ) Their success, however, is limited to a subset of patients whose tumors express estrogen receptors (ER), progesterone receptors (PR), human epidermal growth factor receptor 2 (HER2/neu), tumor protein 53 (P53), and so on.( 6 ) Therefore, the status of these proteins has prognostic ramifications for breast cancer.
Nestin, a primeval protein marker for neural stem cells, is also expressed in follicle stem cells and their immediate, differentiated progeny.( 7 ) It has recently received attention as a marker for newly formed endothelial cells. In their study, Teranishi et al. concluded that nestin is an angiogenesis marker for proliferating endothelial cells in colorectal cancer tissue.( 8 ) Recently, studies based on Western patients demonstrated that nestin is preferentially expressed in basal/myoepithelial cells of the mammary gland, and that this intermediate filament may be used as a myoepithelial marker.( 9 , 10 ) However, the clinical and prognostic implications of nestin as a marker for breast cancer are still unclear.
Currently, there is no reported study addressing nestin expression in Asian breast cancer patients. Therefore, we examined 150 mastectomy specimens obtained from patients with breast cancers to investigate the expression of nestin in relation to clinicopathological features, immunohistochemical markers, and amplification of key genes in breast cancer, and to determine the prognostic impact of nestin expression.
Materials and Methods
Patients and tissue specimens. For this study, we selected 150 patients who had histologically confirmed breast cancer and underwent radical operations in the Surgical Oncology Department of the First Affiliated Hospital of the China Medical University between January 2000 and December 2003. The inclusion criteria were as follows: (i) curative operations were carried out; (ii) resected specimens were pathologically examined; (iii) more than 10 lymph nodes were pathologically examined after operation; and (iv) a complete medical record was available.
Thin slices of tumor tissue of all cases received in our histopathology unit were fixed in 4% formaldehyde solution (pH 7.0) for periods not exceeding 24 h. The tissues were processed routinely for paraffin embedding, and 4 μm‐thick sections were cut and placed on glass slides coated with 3‐aminopropyl triethoxysilane for immunohistochemistry. Tissue samples were stained with hematoxylin–eosin to determine the histological type and grade of tumors.
Antibodies. Monoclonal mouse anti‐human nestin and monoclonal mouse anti‐human P53 (prediluted) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal mouse anti‐human ER (prediluted) was from Dako (Shanghai, China). Monoclonal mouse anti‐human PR (dilution 1:100), monoclonal mouse anti‐human HER2/neu (dilution 1:100; Dako).
Immunohistochemical analysis. Nuclear staining for ER, PR, and P53 was graded 1+ if <10% of the cells were stained, 2+ if 10–50% of the cells were stained, and 3+ if >50% of the cells were stained. Grades 2+ and 3+ were considered positive, whereas absence of staining and 1+ staining were considered negative. Similar standards were used for staining intensity in HER‐2/neu; only grade 3+ (high intensity) was considered positive. Nestin expression was classified semiquantitatively according to the following criteria: 0 if <1% of neoplastic cells discretely expressed nestin in their cytoplasm; 1+ if ≥1 and <10% of morphologically unequivocal neoplastic cells discretely expressed nestin in their cytoplasm; and 2+ if ≥10% of morphologically unequivocal neoplastic cells discretely expressed nestin in their cytoplasm. Samples scored as 1+ or 2+ were considered positive.
Statistical analysis. All data were analyzed with SPSS statistics software (Version 13.0, Chicago, IL, USA). Relationships between tumor markers and other parameters were studied using χ2‐test, Fisher’s extract test, or independent t‐tests. Linear regression was used to study the correlation among ER, PR, HER‐2/neu, P53, and nestin protein expression. Regression (r) values were regarded as indicating no correlation (0.0–0.2); a low degree of correlation (0.2–0.4); a moderate degree of correlation (0.4–0.6); a marked degree of correlation (0.6–0.8); or a high correlation (0.8–1.0). Disease‐specific survival was analyzed using the Kaplan–Meier method. The log‐rank test was used to analyze survival differences. Multivariate analysis was carried out using the Cox proportional hazards model selected in forward stepwise. A P‐value of less than 0.05 was considered statistically significant.
Results
Patient characteristics. Nineteen cases had ductal carcinoma in situ (DCIS), and 131 cases had invasive ductal carcinomas (IDC). The mean age of 150 patients was 51.04 years (range, 27–80 years). There were 96 patients without lymph node metastasis, 36 with pN1, 13 with pN2, and five with pN3 metastasis. Positive expression of nestin, ER, PR, HER2/neu, and P53 was found in 24, 65, 73, 30, and 55 cases respectively. After analysis, the mean age of patients was similar among nestin+, nestin−, ER+, ER−, PR+, PR−, HER2/neu+, HER2/neu−, P53+, and P53− cases (43.40, 52.57, 49.48, 48.88, 50.49, 51.29, and 49.91 years, respectively; P > 0.05).
Correlations between nestin expression and clinicopathological features. Immunohistochemical examination showed that nestin was located in the cytoplasm and strongly expressed in the tumor cells of triple‐negative breast cancers (Fig. 1a,b). Moreover, myoepithelial cells displayed strong nestin expression in matching, adjacent non‐cancerous tissues, whereas epithelial cells displayed little or no staining (Fig. 1c). Nestin protein expression in paired breast tissues was measured using western blotting. Strong 177.439‐kD bands were detected in triple‐negative breast cancers, whereas weak or no bands were detected in other breast cancers or matching and adjacent non‐cancerous tissues (Fig. 1d).
Figure 1.
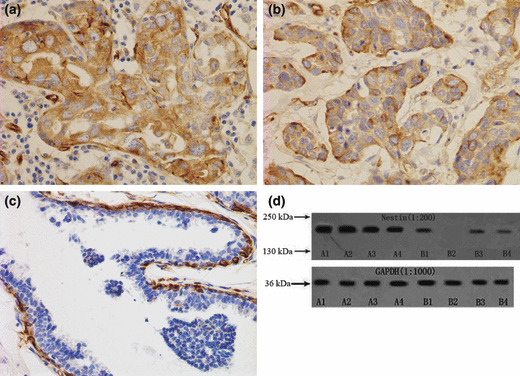
(a,b) Nestin located in the cytoplasm in triple‐negative breast cancers. (c) Nestin expressed in myoepithelial cells. (d) Nestin protein expression in paired breast tissues was measured by western blotting. Protein (50 μL) from each sample was evaluated with expression of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) as a loading control. Strong 177.439‐kD bands were detected in triple‐negative breast cancers (A1–4), whereas weaker or no bands were detected in other breast cancers (B1,2) or matching and adjacent non‐cancerous tissues (B3,4).
Overall, triple‐negative breast cancers had higher expression rates for nestin than the other cancers (57.14%vs 9.30%; P < 0.001) (Table 1). Cases with histological grade III tumors had a higher expression rate of nestin than the others (0 vs 3.22%vs 58.33% for grades I, II, and III respectively; P = 0.001). The nestin expression rate in DCIS was lower than in IDC, but was not significantly different (15.79%vs 16.03%; P > 0.05) (Table 1). Moreover, the expression rate of nestin in pT1 tumors was not significantly different from that in pT3 tumors (12.90 vs 12.77%vs 32.00% for pT1 vs pT2 vs pT3 respectively; P = 0.057), nor was it significantly different between patients with lymph node metastasis and those without (P = 0.209).
Table 1.
Correlations between nestin expression and clinicopathological features
| Variable | n | Nestin− | Nestin+ | P‐value |
|---|---|---|---|---|
| Age | ||||
| <35 years | 14 | 12 | 2 | 0.854 |
| >35 years | 136 | 114 | 22 | |
| Tumor size | ||||
| T1 | 31 | 27 | 4 | 0.057 |
| T2 | 94 | 82 | 12 | |
| T3 | 25 | 17 | 8 | |
| Histological grade | ||||
| I | 21 | 21 | 0 | 0.001 |
| II | 93 | 90 | 3 | |
| III | 36 | 15 | 21 | |
| Stage | ||||
| DCIS | 19 | 16 | 3 | 0.979 |
| IDC | 131 | 110 | 21 | |
| Lymphovascular invasion status | ||||
| Negative | 68 | 57 | 11 | 0.957 |
| Positive | 82 | 69 | 13 | |
| Metastatic nodes | ||||
| pN0 | 97 | 85 | 12 | 0.209 |
| pN1 | 35 | 30 | 5 | |
| pN2 | 13 | 9 | 4 | |
| pN3 | 5 | 2 | 3 | |
| Triple‐negative breast cancer | ||||
| Yes | 21 | 9 | 12 | 0.000 |
| No | 129 | 117 | 12 | |
DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma.
We also evaluated the relationship between nestin expression and clinicopathological features in triple‐negative breast cancers. Interestingly, significantly increased nestin expression rates were observed in patients with lymph node metastasis compared with those without node metastasis (25.00%vs 76.92%; P = 0.032). A similar phenomenon was observed in IDC compared with DCIS (16.67%vs 73.33%; P = 0.046). However, no similar phenomenon was observed in the 129 patients without triple‐negative breast cancers.
Nestin expression and immunohistochemical markers. After univariate analysis, the nestin expression rate was found to be significantly lower in ER+ and PR+ cases than in ER− or PR− cases (P = 0.015 and P = 0.001 respectively) (Table 2). However, P53+ cases had higher nestin expression rates compared with P53− cases (P = 0.006) (Table 2). We carried out logistic analysis on the above factors in order to the exclude the effects of confounding factors. After multivariate analysis, P53 expression was not found to be related to nestin expression (P = 0.081) (Table 3).
Table 2.
Correlations between nestin expression and immunohistochemical markers
| Variable | n | Nestin− | Nestin+ | P‐value |
|---|---|---|---|---|
| ER | ||||
| − | 85 | 66 | 19 | 0.015 |
| + | 65 | 60 | 5 | |
| PR | ||||
| − | 77 | 57 | 20 | 0.001 |
| + | 73 | 69 | 4 | |
| HER‐2/neu | ||||
| − | 120 | 99 | 21 | 0.316 |
| + | 30 | 27 | 3 | |
| P53 | ||||
| − | 95 | 87 | 8 | 0.006 |
| + | 55 | 39 | 16 | |
ER, estrogen receptor; HER2/neu, human epidermal growth factor receptor 2; P53, tumor protein 53; PR, progesterone receptor.
Table 3.
Multivariate analysis of the factors related to nestin expression
| Characteristic | Exp(B) | 95% CI for Exp(B) | P‐value |
|---|---|---|---|
| ER | 0.129 | 0.035–0.482 | 0.002 |
| PR | 0.218 | 0.055–0.858 | 0.029 |
| HER2/neu | 0.322 | 0.071–1.469 | 0.143 |
| P53 | 2.790 | 0.880–8.843 | 0.081 |
| Constant | 0.617 |
CI, confidence interval; ER, estrogen receptor; HER2/neu, human epidermal growth factor receptor 2; P53, tumor protein 53; PR, progesterone receptor.
Correlation among ER, PR, P53, HER‐2, and nestin expression in ductal breast carcinoma. We found a significant positive correlation between ER and PR protein expression (r = 0.211, P = 0.021) (Table 4). Somewhat positive correlations between nestin and ER and between nestin and PR were found (r = 0.365, P < 0.001; r = 0.298, P = 0.001 respectively) (Table 4). P53 expression was also significantly related to nestin expression (r = 0.291, P = 0.001) (Table 4). However, no correlation was found between nestin and HER‐2/neu expression (r = 0.029, P = 0.750) (Table 4).
Table 4.
Correlations among estrogen receptor (ER), progesterone receptor (PR), tumor protein 53 (P53), human epidermal growth factor receptor 2 (HER‐2), and nestin expression in ductal breast carcinoma
| Characteristic | r‐value | F‐value | P‐value |
|---|---|---|---|
| ER vs PR | 0.211 | 5.501 | 0.021 |
| ER vs HER‐2/neu | 0.05 | 0.296 | 0.588 |
| ER vs P53 | 0.087 | 0.899 | 0.345 |
| P53 vs HER‐2/neu | 0.038 | 0.172 | 0.679 |
| Nestin vs ER | 0.365 | 18.154 | 0.000 |
| Nestin vs PR | 0.298 | 11.526 | 0.001 |
| Nestin vs P53 | 0.291 | 10.896 | 0.001 |
| Nestin vs HER‐2/neu | 0.029 | 0.102 | 0.750 |
Survival outcome. Overall, 28 (18.67%) patients received no further treatment after surgery, 50 (33.33%) patients received chemotherapy and radiotherapy as well as endocrine therapy, 22 (14.67%) patients received chemotherapy and endocrine therapy, 42 (28.00%) patients received chemotherapy alone, and the remaining eight (5.33%) patients received endocrine therapy alone. Survival analysis revealed that nestin was associated with breast cancer‐specific survival in 150 cases (P = 0.005, log rank test, Fig. 2a). Subgroup analysis demonstrated that nestin expression was not associated with breast cancer‐specific survival in the lymph node‐negative group (P = 0.135, log rank test, Fig. 2b), whereas nestin expression was associated with shorter breast cancer‐specific survival in the lymph node‐positive group (P = 0.028, log rank test, Fig. 2c). Furthermore, we observed the relationship between nestin status and breast cancer relapse‐free survival. It was observed that nestin was associated with breast cancer relapse‐free survival (P = 0.001, log rank test). Subgroup analysis demonstrated that nestin expression was associated with breast cancer relapse‐free survival in the lymph node‐positive group (P = 0.012, log rank test), but not in the lymph node‐negative group (P = 0.260, log rank test). After performing a Cox regression, nestin was not shown to be an independent prognostic factor of breast cancer.
Figure 2.
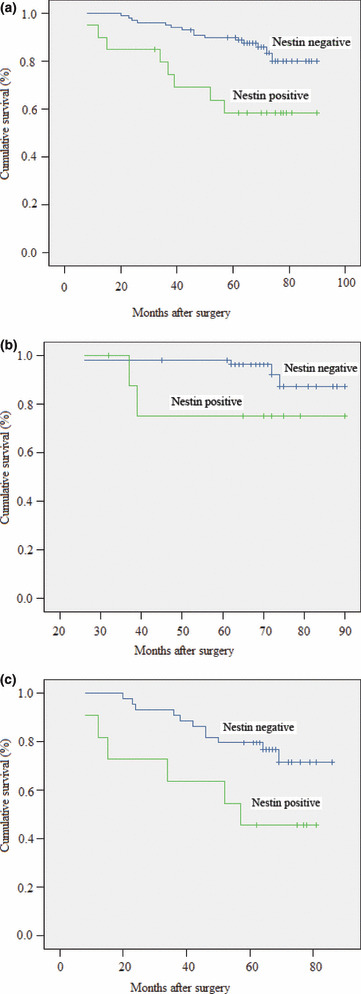
(a) Disease‐specific survival for patients with breast cancer according to nestin expression status (P = 0.005, log rank test). (b) Disease‐specific survival for patients with lymph node‐negative breast cancer according to nestin expression status (P = 0.135, log rank test). (c) Disease‐specific survival for patients with lymph node‐positive breast cancer according to nestin expression status (P = 0.028, log rank test).
Discussion
Breast cancer is a clinically heterogeneous disease. Histological type, grade, tumor size, lymph‐node involvement, and ER and HER2 receptor status all influence the prognosis and probability of response to systemic therapies, but do not fully capture the varied clinical course of breast cancer.( 11 , 12 , 13 ) Endocrine therapy and trastuzumab adjuvant treatment have benefited patients with ER+ cancers and HER2‐overexpressing cases.( 14 , 15 ) Therefore, the status of these proteins has prognostic ramifications in breast cancer. Consequently, much effort is focused on understanding the clinical significance of known markers, finding relationships between them and discovering new ones – all aimed at optimal utilization of the available therapies and development of novel therapies based on improved cancer models.
Nestin is a type VI intermediate filament protein. Structurally, nestin has the shortest head domain (N‐terminus) and the longest tail domain (C‐terminus) of all the intermediate filament proteins.( 16 , 17 ) Nestin is expressed by many types of cells during development, although its expression is usually transient and does not persist into adulthood.( 17 ) Nestin is an intermediate filament protein expressed in dividing cells during the early stages of development in the central nervous system (CNS), peripheral nervous system (PNS), myogenic, and other tissues.( 7 ) Nestin is utilized as a marker of proliferating and migrating cells; however, very little is known about its functions or regulation.
A study based on Western patients suggested that nestin is preferentially expressed in basal‐like breast carcinomas, predominantly expressed in triple‐negative breast cancers.( 9 ) Parry et al. reported that, although nestin expression was associated with basal‐like and triple‐negative phenotypes, high proliferation rates and p53 nuclear expression – all markers of poor prognosis – it was not associated with metastasis‐free survival or breast cancer‐specific survival.( 10 )
In our study, nestin was predominantly expressed in triple‐negative cancers, corroborating previous findings. Interestingly we observed that nestin expression was significantly correlated with lymph node metastasis in the triple‐negative cancers alone. Moreover, nestin expression was associated with poor prognoses in the lymph node‐positive group in our study – supporting the concept that nestin‐positive cancer cells may have higher lymphatic metastasis capability. There are some differences between our results and previous reported results. Parry et al. reported that nestin was not found to be associated with metastasis‐free survival or breast cancer‐specific survival in their study.( 10 ) There may be some reasons causing these differences, such as different sub‐groups and ethnic differences. After univariate analysis, nestin expression was significantly related to the expression of ER, PR, and P53 in our study. It demonstrated that nestin might play an important function in breast carcinoma development and metastasis. However, the specific role of nestin in breast cancer is unclear.
In summary, our findings show that nestin is consistently expressed in triple‐negative subgroups of breast cancer and correlates with clinicopathological and immunohistochemical characteristics. It demonstrated that nestin might be a new potential marker for breast cancer. However, the underlying mechanisms of nestin’s involvement are still unclear, so more and larger studies are warranted to investigate the prognostic impact of nestin expression in of breast carcinomas.
Acknowledgment
This work was supported in part by the gastric cancer laboratory of the Chinese Medical University.
References
- 1. Anim JT, John B, Abdulsathar SS et al. Relationship between the expression of various markers and prognostic factors in breast cancer. Acta Histochem 2005; 107: 87–93. [DOI] [PubMed] [Google Scholar]
- 2. Kasami M, Uematsu T, Honda M et al. Comparison of estrogen receptor, progesterone receptor and Her‐2 status in breast cancer pre‐ and post‐neoadjuvant chemotherapy. Breast 2008; 17: 523–7. [DOI] [PubMed] [Google Scholar]
- 3. Rody A, Diallo R, Poremba C et al. Estrogen receptor alpha and beta, progesterone receptor, pS2 and HER‐2/neu expression delineate different subgroups in ductal carcinoma in situ of the breast. Oncol Rep 2004; 12: 695–9. [PubMed] [Google Scholar]
- 4. Briest S, Stearns V. Tamoxifen metabolism and its effect on endocrine treatment of breast cancer. Clin Adv Hematol Oncol 2009; 7: 185–92. [PubMed] [Google Scholar]
- 5. Korkaya H, Wicha MS. HER‐2, notch, and breast cancer stem cells: targeting an axis of evil. Clin Cancer Res 2009; 15: 1845–7. [DOI] [PubMed] [Google Scholar]
- 6. Hussein MR, Abd‐Elwahed SR, Abdulwahed AR. Alterations of estrogen receptors, progesterone receptors and c‐erbB2 oncogene protein expression in ductal carcinomas of the breast. Cell Biol Int 2008; 32: 698–707. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman RM. The potential of nestin‐expressing hair follicle stem cells in regenerative medicine. Expert Opin Biol Ther 2007; 7: 289–91. [DOI] [PubMed] [Google Scholar]
- 8. Teranishi N, Naito Z, Ishiwata T et al. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol 2007; 3: 593–603. [PubMed] [Google Scholar]
- 9. Li H, Cherukuri P, Li N et al. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res 2007; 67: 501–10. [DOI] [PubMed] [Google Scholar]
- 10. Parry S, Savage K, Marchiò C, Reis‐Filho JS. Nestin is expressed in basal‐like and triple negative breast cancers. J Clin Pathol 2008; 61: 1045–50. [DOI] [PubMed] [Google Scholar]
- 11. Chen KT, Lee TW, Lo JM. In vivo examination of 188Re(I)‐tricarbonyl‐labeled trastuzumab to target HER2‐overexpressing breast cancer. Nucl Med Biol 2009; 36: 355–61. [DOI] [PubMed] [Google Scholar]
- 12. Jacquemier J, Charafe‐Jauffret E, Monville F et al. Association of GATA3, P53, Ki67 status and vascular peritumoral invasion are strongly prognostic in luminal breast cancer. Breast Cancer Res 2009; 11: R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vora HH, Patel NA, Rajvik KN et al. Cytokeratin and vimentin expression in breast cancer. Int J Biol Markers 2009; 24: 38–46. [DOI] [PubMed] [Google Scholar]
- 14. Ross JS, Slodkowska EA, Symmans WF et al. The HER‐2 receptor and breast cancer: ten years of targeted anti‐HER‐2 therapy and personalized medicine. Oncologist 2009; 14: 320–68. [DOI] [PubMed] [Google Scholar]
- 15. Ma CX, Sanchez CG, Ellis MJ. Predicting endocrine therapy responsiveness in breast cancer. Oncology 2009; 23: 133–42. [PubMed] [Google Scholar]
- 16. Wiese C, Rolletschek A, Kania G et al. Nestin expression – a property of multi‐lineage progenitor cells? Cell Mol Life Sci 2004; 61: 2510–22. [DOI] [PubMed] [Google Scholar]
- 17. Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci 1992; 103: 589–97. [DOI] [PubMed] [Google Scholar]


