Abstract
Gypican‐3 (GPC3) has been recognized as an oncofetal protein in hepatic neoplasms and yolk sac tumors. To characterize a distinct subgroup of gastric carcinoma (GC) expressing GPC3 (GPC3‐GC), primary and metastatic GC tissues were evaluated by immunohistochemistry with special focus on their related entities: hepatoid, clear‐cell, and α‐fetoprotein‐producing GC. GPC3‐GC was defined as focal GPC3‐GC when 10–49% of neoplastic cells were positive, and as diffuse GPC3‐GC when more than 50% of cells were positive. Among 926 GC cases, 101 (11%) were GPC3‐GC, of which 45 were diffuse and 56 were focal GPC3‐GC. Specific histological patterns, such as the hepatoid and clear‐cell patterns, were frequently observed in diffuse GPC3‐GC (38 and 49%, respectively) and in focal GPC3‐GC (4 and 25%, respectively), whereas these patterns were extremely rare in GPC3‐negative GC. Immunoreactive α‐fetoprotein was only identified in GPC3‐GC (38% of diffuse and 14% of focal GPC3‐GC). Both diffuse and focal GPC3‐GC showed nodal metastasis more frequently (67 and 55%, respectively) than GPC3‐negative GC (34%), and the diffuse GPC3‐GC had significantly more T2–4 and M1 stage cases. GPC3 immunostaining was present in 57 out of 61 nodal metastases (93%) and in all four liver metastases examined. Importantly, diffuse GPC3 expression was observed in the liver metastasis, even if the primary tumor was focal GPC3‐GC. GPC3‐GC is a distinctive group of GC, which unifies hepatoid, clear‐cell, and α‐fetoprotein‐producing GC. GPC3 is expected to be a target of forthcoming immunotherapy for a patient bearing this specific type of GC. (Cancer Sci 2009; 100: 626–632)
Abbreviations:
- AFP
α‐fetoprotein
- GC
gastric carcinoma
- GPC3
glypican 3
- GPC3‐GC
glypican 3‐expressing gastric carcinoma
- HCC
hepatocellular carcinoma
- muc
mucinous adenocarcinoma
- pap
papillary adenocarcinoma
- por1
solid‐type poorly differentiated adenocarcinoma
- por2
non‐solid‐type poorly differentiated adenocarcinoma
- sig
signet ring cell adenocarcinoma
- TMA
tissue microarray
- tub1
well‐differentiated tubular adenocarcinoma
- tub2
moderately differentiated tubular adenocarcinoma
- ZHX2
zinc fingers and homeoboxes 2
Gypican‐3 is a cell‐surface heparan sulfate proteoglycan that is linked to the extracytoplasmic cell‐surface membrane by a glycosylphosphatidylinositol anchor.( 1 ) Heparan sulfate proteoglycans are known to interact with growth factors through heparan sulfate chains and thereby serve as coreceptors for heparin‐binding growth factors. Recently, GPC3 has been recognized as an oncofetal protein in the fetal liver and neoplasms of hepatocellular lineage, that is, hepatoblastoma and HCC.( 2 ) Similar to α‐fetoprotein, GPC3 is expressed in yolk sac tumors of the testis as well.( 3 , 4 ) Immunohistochemistry for GPC3 labels much more neoplastic cells than that of AFP in testicular yolk sac tumors, although GPC3 is occasionally expressed in other types of germ cell tumors. The parallel expression pattern of GPC3 and AFP has been further demonstrated in our comparative study of GPC3, AFP, hepatocyte antigen, and protein‐induced vitamin K absence or antagonist II in AFP‐producing GC.( 5 )
Gastric carcinoma is the fourth most common malignancy worldwide, with approximately 870 000 new cases occurring yearly, and mortality due to GC is the second highest following lung carcinoma.( 6 ) To overcome this carcinoma, it is important to identify its distinct subgroups with characteristic marker phenotypes. AFP‐producing GC is such an example, and is characterized by an elevated serum AFP level in cancer‐bearing patients. AFP‐producing GC takes a particularly aggressive clinical course and has a poor survival rate with marked vascular invasion and high rates of liver metastasis.( 7 , 8 , 9 ) These facts have led to the proposal of AFP‐producing GC as an entity in itself, but the absence of effective therapy against this type of GC prevented further application of the entity in clinical medicine. This is largely due to the fact that AFP is primarily a secretary protein, and thus it is difficult to target the cells that produce it with a specific antibody. However, in our previous study, which concerned 10 cases of AFP‐producing GC, the immunoreactivity for GPC3 was stronger and more sensitive than that for AFP.( 5 ) These results imply that GPC3 may serve as a cell‐surface marker of AFP‐producing GC and raises the possibility that GPC3‐GC constitute a distinct subset of GC.
In the present study, we have extended our observation to clarify the pathological features of GPC3‐GC with special focus on its related entities of GC subgroups, such as hepatoid GC, clear‐cell GC, and AFP‐producing GC. There is increasing evidence that GPC3 is a sensitive serum marker( 10 , 11 , 12 ) and an effective immunotherapeutic target for the treatment of GPC3‐expressing cancers such as HCC and malignant melanoma.( 13 , 14 ) Therefore, it is necessary to characterize this subgroup of GC for these clinical applications.
Materials and Methods
All of the specimens used in the present study were retrieved from the archives of the Department of Pathology, Tokyo University Hospital from 1990 to 2007. In total, 926 GC cases were evaluated. All samples were fixed in 10% formalin, embedded in paraffin, cut into 4 µm‐thick sections, and stained with hematoxylin–eosin for routine pathological examination. Tumor staging was carried out according to the tumor–node–metastasis stage system.( 15 ) T and N factors were determined by histological evaluation. In the present study, the GC cases consisted of 479 early (T1) and 447 advanced (T2–4) cases. Histological subtypes were classified according to the criteria of the Japanese Research Society for Gastric Cancer,( 16 ) consisting of pap, tub1, tub2, por1, por2, and sig. Carcinoma with marked extracellular mucin was classified as muc. They were also classified according to the Lauren classification system: intestinal and diffuse type.( 17 ) The degree of lymphatic and venous invasion was also assessed according to the criteria of the Japanese Research Society for Gastric Cancer as follows: 0 for no invasion, 1 for minimal invasion, 2 for moderate invasion, and 3 for marked invasion.
Specific histological patterns, which have also been reported in AFP‐producing GC, were evaluated, such as hepatoid, clear‐cell, and fetal gut‐like patterns in GPC3‐positive and GPC3‐negative GC.( 18 , 19 ) In the criteria of the Japanese Research Society for Gastric Cancer, hepatoid pattern is included in por1, whereas fetal gut‐like pattern is included in tub1 or tub2. Clear‐cell pattern is variously classified, such as por1 when showing sheet‐like proliferation, pap when showing papillary growth, and tub1 or tub2 when showing tubular structures. Then, the distribution of GPC3‐positive cells was compared to these specific histological patterns.
Construction of TMA and full section study. TMA was used for the first screening in the immunohistochemical analysis, which contained 24 GC cases on each slide. Duplicate 2‐mm cores were obtained for each carcinoma from formalin‐fixed, paraffin‐embedded tissue blocks. When any positive cells were identified in the carcinoma tissues of the TMA, full sections of the representative lesions of the carcinoma were prepared for further study. For GPC3‐GC cases with nodal involvement or distant metastases (N1‐3 or M1 grade), the metastatic lesions were also examined for GPC3 expression by immunohistochemistry.
Immunohistochemistry. Monoclonal antibodies for GPC3 were generated in our laboratory as reported previously.( 2 ) For the immunohistochemical staining, primary antibodies against GPC3 (monoclonal, GPC‐C02, 1:2500) and AFP (rabbit polyclonal, 1:2000; Dako, Copenhagen, Denmark) were used. Four micrometer‐thick sections from the TMA samples and from each block of the tumor tissue were stained using an automated stainer (Ventana Benchmark; Ventana Medical Systems, Tucson, AZ, USA) with both positive and negative controls. The positive control sections were carcinoma tissues of HCC, which had been demonstrated as positive for GPC3 and AFP.
Scoring of immunohistochemical staining. For the TMA evaluation, cases with no positively stained cells were identified as negative for GPC3, and cases with any positive cells were further assessed using full tissue sections. Membranous and cytoplasmic immunoreactivity for GPC3 was semiquantitatively evaluated in a representative full section and scored using the percentage of tumor cells with positive staining as follows: negative (<10%), focal (10–49%), and diffuse (more than 50%). AFP‐producing GC was defined when AFP production was confirmed by immunohistochemistry in more than 1% tumor cells.
Statistical analysis. Correlations between clinicopathological parameters and the expression of GPC3 or AFP were analyzed by the χ2‐test or Fisher's exact test. A P‐value of less than 0.05 was considered statistically significant.
Results
Immunohistochemical analysis of GPC3 expression in GC. Immunohistochemistry of GPC3 was first applied to the TMA sections, which included 926 GC cases. The expression of GPC3 was not observed in non‐neoplastic gastric mucosa except in some vascular endothelial cells. GPC3 expression in the carcinomas was observed in the cell membrane and the cytoplasm of the tumor cells (Fig. 1). Positive membranous staining was observed at the luminal border of neoplastic cells in the glandular structures (Fig. 1a) and at the circumference of individual cells in the trabecular structures (Fig. 1b). There were 185 cases from the GC‐TMA that showed positive cells in either of the two tissue sections. These cases were further evaluated in detail with one or two full sections of the tumor. Eighty‐four out of 185 cases were considered to be negative because they showed positive immunoreactivity for GPC3 in less than 10% of the tumor population. Thus, 101 out of 926 cases (11%) were positive for GPC3, 45 cases (5% of the total GC cases) exhibited diffuse immunoreactivity (GPC3‐GC, diffuse type), whereas the other 56 (6%) showed focal immunoreactivity (GPC3‐GC, focal type).
Figure 1.
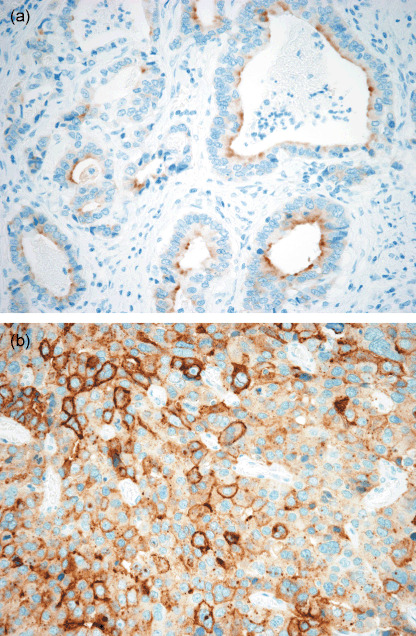
Glypican 3 expression in gastric carcinoma. (a) Positive staining at the luminal border of glandular structures of tubular type gastric carcinoma (×400). (b) Diffuse membranous and cytoplasmic staining of glypican 3 in the trabecular structure of poorly differentiated solid type gastric carcinoma (×400).
Histological patterns and distribution of GPC3‐positive cells in GPC3‐GC. To clarify how GPC3‐GC was histologically classified in routine surgical pathology of GC, the histological subtypes were determined by the predominance of common histological patterns according to the criteria of the Japanese Research Society for Gastric Cancer. Tub2 (45%) was the most common histological subtype, followed by por1 (21%), pap (12%), and tub1 (12%) in the GPC3‐GC (Fig. 2). Sig (2%) was far less common in the GPC3‐GC cases, although it was third in the GPC3‐negative GC cases (172 of 825 cases, 21%). In the Lauren classification system, the frequency of GPC3 positivity was significantly higher in intestinal‐type GC (69 out of 541, 12.8%) than in diffuse‐type GC (32 out of 385, 8.3%, P = 0.033).
Figure 2.
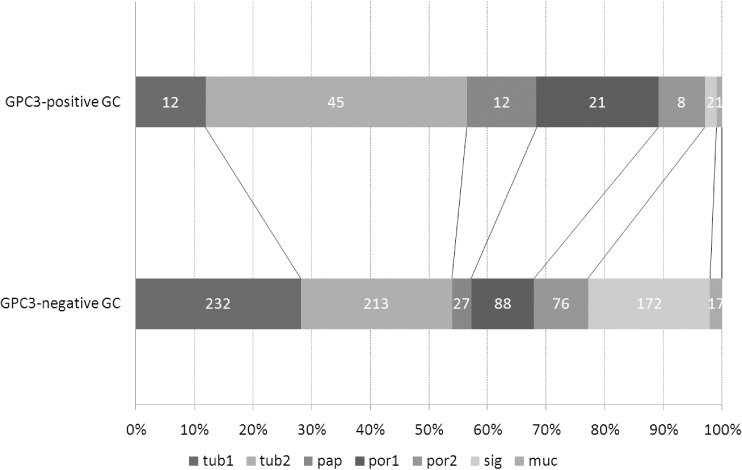
Histological subtypes, determined by the predominant common pattern, in gastric carcinoma (GC) with and without expression of glypican 3 (GPC3). Histological subtype was determined in GPC3‐expressing GC and GPC3‐negative GC according to the definition of the Japanese Research Society for Gastric Cancer. muc, mucinous type; pap, papillary type; por1, solid poorly differentiated type; por2, non‐solid poorly differentiated type; sig, signet ring cell type; tub1, well‐differentiated tubular type; tub2, moderately differentiated tubular type. These proportions were significantly different between the two groups (P < 0.0001, χ2‐test). Note that the proportion of papillary type is significantly higher in GPC3‐expressing GC.
Next, common and specific histological patterns were evaluated in GPC3‐GC (Table 1). Papillary and tubular patterns were characteristic of GPC3‐GC, which were observed in 54 and 85% of samples, respectively. As for the specific histological patterns, the hepatoid pattern was mainly composed of cuboidal or polygonal cells with abundant eosinophilic cytoplasm. The tumor cells tended to be arranged in solid nests or in a trabecular fashion and separated by narrow fibrous stroma (Fig. 3a). Nineteen out of 101 cases of GPC3‐GC (19%) had a hepatoid component in at least 10% of the tumor population. The clear‐cell pattern consisted of tumor cells with clear cytoplasm showing moderate to severe cytological atypia, similar to ovarian clear‐cell adenocarcinoma or yolk sac tumor (Fig. 3b). Tubules and papillary structures were present in varying proportions, but papillary structures dominated in most cases. Some of them also showed a focal solid area composed of sheets of polygonal clear cells (Fig. 3c). The clear‐cell pattern was observed in 36 cases of the GPC3‐GC (36%). Hepatoid and clear‐cell components coexisted in 13 GPC3‐GC cases (13%). Fetal gut‐like pattern was a particular form of clear‐cell pattern. They showed glandular structures with primitive appearance resembling the developing gut epithelium in early gestation, consisting of regular columnar cells with clear cytoplasm and an oval nucleus showing mild atypia (Fig. 3d). This pattern was relatively rare (4%) in the GPC3‐GC cases. These specific patterns, hepatoid, clear‐cell, and fetal gut‐like, were extremely rare in GPC3‐negative GC, being present in one case (0.1%), seven cases (0.8%), and none, respectively.
Table 1.
Histological patterns and α‐fetoprotein (AFP) immunoreactivity in glypican 3‐expressing gastric carcinomas
| Findings | Total(n = 101) | Diffuse type(n = 45) | Focal type(n = 56) | Statistical difference |
|---|---|---|---|---|
| Common | ||||
| Papillary | 55 (54%) | 27 (60%) | 28 (50%) | ns |
| Tubular | 86 (85%) | 36 (80%) | 50 (89%) | ns |
| Solid | 30 (30%) | 19 (42%) | 11 (20%) | 0.0135 |
| Scirrhous | 23 (23%) | 8 (18%) | 15 (27%) | ns |
| Specific | ||||
| Hepatoid | 19 (19%) | 17 (38%) | 2 (4%) | <0.0001 |
| Clear cell | 36 (36%) | 22 (49%) | 14 (25%) | 0.0127 |
| Fetal gut‐like | 4 (7%) | 3 (7%) | 1 (2%) | ns |
| Other findings | ||||
| Intraluminal eosinophilic material | 68 (67%) | 31 (69%) | 37 (66%) | ns |
| Hyaline globule | 25 (25%) | 13 (29%) | 12 (21%) | ns |
| AFP positivity | ||||
| Total | 25 (25%) | 17 (38%) | 8 (14%) | 0.0065 |
| T1 (n = 33) | 4 (12%) | 3 (30%) | 1 (4%) | ns |
| T2–4 (n = 68) | 21 (31%) | 14 (40%) | 7 (21%) | ns |
ns, not significant.
Figure 3.
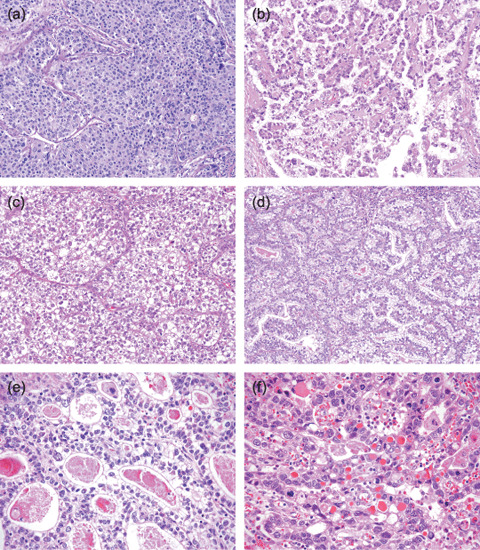
Histological features of glypican 3 (GPC3)‐expressing gastric carcinoma (GC). (a–d) Characteristic histological patterns in glypican 3 (GPC3)‐expressing gastric carcinoma (GC). (a) Hepatoid pattern showing sheet‐like proliferation of polygonal tumor cells with abundant eosinophilic cytoplasm (hematoxylin–eosin [HE], ×200). (b) Clear‐cell pattern showing papillary proliferation bearing a close resemblance to ovarian clear‐cell adenocarcinoma (HE, ×200). (c) Solid area of clear‐cell pattern composed of a sheet of polygonal clear cells (HE, ×200). (d) Fetal gut‐like appearance of clear‐cell pattern showing a tubulopapillary structure lined by clear columnar cells with relatively mild cytological atypia (HE, ×200). (e) Eosinophilic material in the luminal structure and (f) intracytoplasmic hyaline globules in GPC3‐expressing GC (HE, ×400). These materials often showed positive staining for GPC3.
The distribution of GPC3‐positive cells corresponded to these specific patterns, and the positive cells were scarce in the regions showing non‐hepatoid solid, scirrhous, signet ring cell, or mucinous patterns. Therefore, when histological features were compared between diffuse and focal types of GPC3‐GC (Table 1), the hepatoid pattern was more frequently observed in the diffuse type (38%) than in the focal type (4%, P < 0.0001). The clear‐cell pattern was common in both diffuse (49%) and focal (25%) types. Otherwise, GPC3‐positve cells did not show a specific tendency toward particular spatial distributions, and were present in both superficial and deep areas of GC.
Other notable findings were intraluminal eosinophilic material and hyaline globules. Intraluminal eosinophilic material was occasionally identified in the neoplastic glandular structures, which were often stained positively for GPC3 (Fig. 3e). It is likely that this material contains the secretory form of GPC3.( 20 ) Various amounts of intracytoplasmic hyaline globules were identified in 25 out of 101 cases of GPC3‐GC (25%) (Fig. 3f). They were periodic acid–Schiff positive and diastase resistant. Some of them were also immunohistochemically positive for GPC3. The frequencies of these structures were not different between diffuse and focal types of GPC3‐GC.
Production of AFP in GPC3‐GC. To clarify the relationship between AFP production and GPC3 expression in GC, immunohistochemistry of AFP was similarly applied to both TMA and full sections of GC with reference to clinical information about serum AFP level. AFP production was detected in 25 out of 926 GC by immunohistochemical staining and these GC were defined as AFP‐producing GC. Preoperative serum AFP levels were elevated (>10 ng/mL) in all of these patients with available data (n = 16). All AFP‐producing GC showed positive immunostaining for GPC3, diffusely in 17 cases and focally in eight cases (Table 1). Out of 25 AFP‐producing GC, 15 had a hepatoid component, 17 had a clear‐cell component, and 10 had both of them. Twenty‐two out of 25 cases of AFP‐producing GC showed stronger and more extensive staining for GPC3 compared with AFP immunoreactivity (Fig. 4), and AFP‐positive cells were consistently observed in the GPC3‐positive areas of these cases. In the other three cases, there were positive cells for both GPC3 and AFP in a limited region, but most of the carcinoma cells showed both immunoreactivities in the same cell.
Figure 4.
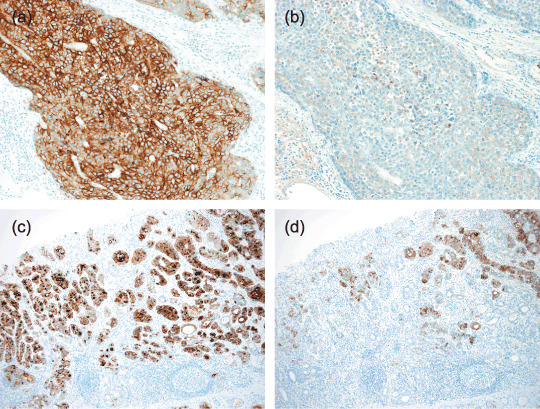
Immunohistochemistry of α‐fetoprotein (AFP) in glypican 3 (GPC3)‐expressing gastric carcinomas (GC). Parallel immunostaining of (a,c) GPC3 and (b,d) AFP demonstrates both immunoreactive cells in the (a,b) hepatoid pattern and (c,d) clear‐cell tubular pattern of GPC3‐expressing GC. The numbers of cells immunoreactive for AFP were generally less than those of GPC3‐immunoreactive cells (×200).
α‐Fetoprotein immunostaining was observed in 38% of diffuse‐type GPC3‐GC and 14% of focal‐type GPC3‐GC. The frequency of positivity was lower in the T1‐stage of GPC3‐GC (12%) than in T2–4 stages (31%). However, this was due to the lowest positive rate (4%) being in the T1 stage of the focal type of GPC3‐GC.
Clinicopathological features of GPC3‐GC. The clinicopathological characteristics of the GC cases were analyzed with regard to GPC3 expression (Table 2). In diffuse‐type GPC3‐GC, the frequency was significantly higher in the male patients, T2–4 grade tumors, and those cases positive for lymph node metastasis (N1–N3), compared with the GPC3‐negative GC. Similarly, lymph node metastasis was more frequent in focal‐type GPC3‐GC than in GPC3‐negative GC. Six out of 45 diffuse‐type cases (13%) and two out of 56 focal‐type cases (4%) had distant metastases at the onset of disease (M1 tumors), whereas only nine out of 825 (1%) cases showed distant metastases in the GPC3‐negative GC. Metastatic sites were in the liver (n = 5) and bone (n = 1) in diffuse‐type GPC3‐GC, and in the liver (n = 2) in focal‐type GPC3‐GC. On the other hand, the metastatic sites were in the liver (n = 8) and intestine (n = 1) in GPC3‐negative GC.
Table 2.
Clinicopathological features of glypican 3 (GPC3)‐expressing gastric carcinoma (GC)
| Clinicopathological features | GPC3‐expressing GC | GPC3‐negative GC n = 825 (89%) | Statistical difference | ||
|---|---|---|---|---|---|
| Diffuse | Focal | ||||
| n = 45 (5%) | n = 56 (6%) | GPC3‐diffuse vs GPC3‐negative | GPC3‐focal vs GPC3‐negative | ||
| Age (years), (mean ± SD) | 65.9 ± 11.8 | 66.9 ± 9.8 | 64.5 ± 11.1 | ns | ns |
| Sex (M/F) | 40/5 | 46/10 | 604/214 | 0.024 | ns |
| Tumor size (mm), (mean ± SD) | 57.3 ± 37.7 | 50.2 ± 31.4 | 47.2 ± 35.3 | 0.051 | ns |
| Tumor site (U/M/L) † | 18/16/10 | 11/24/21 | 173/346/295 | 0.0236 | ns |
| TNM factors | |||||
| T1/T2–4 | 10/35 | 23/33 | 446/379 | <0.0001 | ns |
| M0/M1 | 39/6 | 54/2 | 816/9 | <0.0001 | ns |
| N0/N1‐3 | 15/30 | 25/31 | 548/277 | <0.0001 | 0.0009 |
| ly0/ly1‐3 | 20/25 | 23/33 | 529/296 | 0.0077 | 0.0006 |
| v0/v1‐3 | 8/37 | 20/36 | 501/324 | <0.0001 | 0.0002 |
Tumor sites were classified into proximal (U), middle (M), and distal (L) third of the stomach. Twelve cases were excluded that were remnant cancers or spreading throughout the stomach.
ns, not significant.
Immunostaining for GPC3 in metastasis. The expression of GPC3 in the metastatic lesions of GPC3‐positive GC was evaluated (Table 3). Out of 61 GPC3‐positive GC cases with nodal involvement, 57 (93%) cases also showed immunoreactivity for GPC3 in metastatic tumors. In 30 cases of diffuse‐type GPC3‐GC, the metastasis exhibited diffuse staining for GPC3 in 21 cases, focal staining in eight cases, and was negative in one case. In 31 cases of focal‐type GPC3‐GC, the metastasis showed diffuse staining in nine cases, focal staining in 19 cases, and was negative in three cases.
Table 3.
Glypican 3 expression in the metastatic tumors of glypican 3‐expressing gastric carcinoma
| Glypican 3 expression | Lymph node metastasis | Liver metastasis | ||
|---|---|---|---|---|
| Primary diffuse | Primary focal | Primary diffuse | Primary focal | |
| 30 | 31 | 2 | 2 | |
| Diffuse | 21 | 9 | 2 | 2 |
| Focal | 8 | 19 | 0 | 0 |
| Negative | 1 | 3 | 0 | 0 |
Liver metastases were surgically resected in four cases, two each in diffuse and focal types of GPC3‐GC, in which GPC3 expression was further evaluated. All of the metastatic tumors showed diffuse expression of GPC3.
Discussion
Glypican 3 was expressed in 11% of GC in the present study, of which 5% were diffuse‐type GPC3‐GC and 6% were focal‐type GPC3‐GC. Diffuse‐type GPC3‐GC showed significantly higher T‐grade than GPC3‐negative GC, whereas there was no significant difference between focal‐type GPC3‐GC and GPC3‐negative GC in T‐grade. This result indicated that the proportion of GPC3‐positive cells increased with tumor progression, which was possibly due to their growth advantage because GPC3 was shown to stimulate the growth of HCC cells in vitro and in vivo by activating canonical Wnt signaling.( 21 ) This result disagrees with that of Zhu et al. who suggested that GPC3 may act as a tumor suppressor in GC because of the decrease in GPC3‐mRNA in GC tissues,( 22 ) but is compatible with that from our previous study.( 5 ) Their results differed in detecting the GPC3‐expressing GC subgroup presumably due to the smaller number of cases examined rather than the methods used, as immunohistochemical expression was well correlated with that of transcripts in HCC and HCC cell lines.( 23 ) A recent study by Baumhoer et al. demonstrated that 10 of 70 (14%) GC cases, 9 of 45 (20%) intestinal‐type GC, and 1 of 25 (4%) diffuse‐type GC were positive for GPC3 in immunohistochemical analysis.( 24 ) Their result is consistent with our findings on the frequency of GPC3‐GC and its histological features showing predominantly tubular and papillary patterns that correspond to intestinal type, although they used a commercially available antibody for GPC3 (clone 1G12; Biomosaics, Burlington, VT, USA). We have confirmed almost the same staining properties of both antibodies in a total of 20 GC cases, 10 cases each of GPC3‐positive and GPC3‐negative GC (data not shown).
The rat homolog of human GPC3, OCI‐5, is expressed during development of the fetal gut and is involved in intestinal morphogenesis.( 25 , 26 ) The OCI‐5 transcript is maximally expressed in the embryonic gut at day 16 of development and gradually decreases from day 19, becoming undetectable by day 24 postparturition. The expression profile of GPC3 in the human fetal gut is less obvious. However, according to our preliminary studies on fetal tissues, hepatocyte and renal tubular epithelium cells display diffuse immunoreactivity throughout the gestational period, and the gastrointestinal immature epithelium also shows a small amount of GPC3 in early gestational life (T. Ushiku, unpublished data, 2008). In the present study, four cases of GPC3‐GC showed a morphology that closely simulated primitive or fetal‐type gastrointestinal epithelium. Therefore, GPC3‐expression in GC implicates oncofetal protein expression in epithelial cells in general, but some may represent fetal differentiation to the gastrointestinal epithelium.
Specific histological patterns, such as the hepatoid and clear‐cell patterns, were most often present in diffuse‐type GPC3‐GC, although the clear‐cell pattern was also relatively frequent in focal‐type GPC3‐GC. Immunoreactive AFP was identified in 38% of diffuse‐type GPC3‐GC and also in 14% of focal‐type GPC3‐GC. The intimate relationships among the histological patterns and AFP production in GC have been well recognized: hepatoid GC, clear‐cell GC, and AFP‐producing GC are known as particular subsets of GC that overlap one another and account for 0.7–2.2, 4.8–7.5, and 2.7–5.4% of total GC, respectively.( 7 , 8 , 27 , 28 ) Considering the reported frequencies and the observation in the present study of GPC3‐GC, we propose that the relationships of these entities are as shown in Figure 5. GPC3‐GC, both diffuse and focal, contain nearly all of the cases of AFP‐producing GC, and most of the cases of hepatoid GC and clear‐cell GC. In our results, the positive rates of GPC3 were 100, 95, and 84% in AFP‐producing, hepatoid, and clear‐cell GC, respectively.
Figure 5.
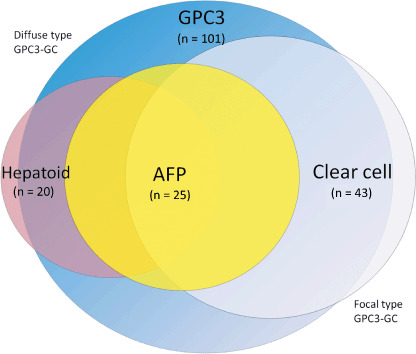
Schematic presentation of the relationships of glypican 3 (GPC3)‐expressing gastric carcinoma (GC) with α‐fetoprotein (AFP)‐producing, hepatoid, and clear‐cell GC. GPC3‐expressing GC, both diffuse and focal types, shows the highest frequency, and includes most of the cases of AFP‐producing, hepatoid, and clear‐cell GC. These three entities overlap to a considerable degree.
Comparative immunohistochemistry of GPC3 and AFP in the present study confirmed that AFP‐producing GC invariably expressed GPC3. Simultaneous expression of GPC3 and AFP is also observed in tumors such as HCC, hepatoblastoma, yolk sac tumor, and some ovarian clear‐cell carcinomas.( 2 , 3 , 29 , 30 , 31 ) Therefore, there may be a common mechanism that regulates the expression of both proteins. Recent studies have disclosed that the Zhx2 gene, the mouse orthologue to human ZHX2, acts as a repressor of both AFP and Gpc3 in the adult mouse liver.( 32 , 33 ) The ZHX2 gene is often silenced in human HCC in association with ZHX2 promoter hypermethylation, and ZHX2 mRNA expression was decreased in HCC cases with a high serum AFP level.( 34 ) These facts imply that silenced ZHX2 mRNA can reactivate the expression of AFP in HCC. In the present study, the proportion of AFP expression in GPC3‐GC was significantly lower in T1 cases (4 of 33, 12%) than in T2–4 cases (21 of 68, 31%) (P < 0.05), suggesting that AFP expression might be induced within GPC3‐positive cancer cells. It is of further interest to investigate the relationship and sequence of promoter methylation, ZHX2 repression, and the expression of GPC3 and AFP.
α‐Fetoprotein‐producing GC is considered an aggressive type of GC and is likely to recur and metastasize.( 7 , 8 , 9 ) In the present study, both diffuse and focal types of GPC3‐GC showed lymph node metastasis more frequently than GPC3‐negative GC. The proportions of cases at the T2–4 and M1 stages were significantly higher in diffuse‐type GPC3‐GC. It is expected that effective multimodal therapy will be developed against these advanced diseases. GPC3 is an ideal tumor antigen candidate for immunotherapy because it is expressed in a set of cancers but not in normal tissues, except for placenta and fetal liver. There is increasing evidence that GPC3 is an effective immunotherapeutic target in the treatment of GPC3‐expressing cancers such as HCC and malignant melanoma.( 13 , 14 ) Nishimura et al. developed GPC3 peptide vaccine‐based immunotherapy and started a phase I clinical trial for patients with advanced HCC.( 35 ) Another approach is monoclonal antibody therapy, such as anti‐CD20 antibody for B‐cell lymphoma or anti‐HER2 antibody for breast cancer. Nakano et al. generated anti‐GPC3 antibodies with a high level of antibody‐dependent cellular cytotoxicity against HCC and started a phase I clinical trial.( 36 ) The membranous expression pattern of GPC3 in GPC3‐GC is suitable for such an antibody therapy. It is worth noting that GPC3 immunostaining was present in 57 out of 61 nodal metastases (93%) and in all four liver metastases examined (100%). Importantly, GPC3 expression was observed in more than 50% of neoplastic cells of metastatic tumors of the liver, even if the primary tumor was focal‐type GPC3‐GC. The staining pattern change, from focal in the primary tumor to diffuse in metastasis, was also observed in 9 out of 31 cases with lymph node metastasis. These facts indicate that GPC3‐expressing components predominate in the metastatic sites, and further suggest that GPC3 should be the target molecule for immunotherapy for patients with metastasis in GPC3‐GC irrespective of whether the distribution of GPC3 immunoreactivity in the primary tumor is focal or diffuse, when such therapy is developed for practical use.
There are several additional facts that should be noted with regard to the application of GPC3 in the management of GC patients. GPC3 can be used as a serum marker to follow the recurrence and metastasis of GPC3‐GC( 10 , 11 , 12 ) as the extra cellular domain of GPC3 is shed into the circulation. On the other hand, caution is necessary in the application of GPC3 immunohistochemistry to metastatic tumors of the liver, especially when the patient has a history of GC.( 37 , 38 )
In conclusion, GPC3‐GC is a distinctive subset of GC, a unifying entity of hepatoid, clear‐cell, and AFP‐producing GC. GPC3 is not a surrogate marker, but is expected to be a target for forthcoming immunotherapy for patients with GPC3‐GC.
Acknowledgments
This work was supported by Grants‐in‐Aid for Young Scientists (B) 19790251 and the program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation.
References
- 1. Filmus J. Glypicans in growth control and cancer. Glycobiology 2001; 11: 19R–23R. [DOI] [PubMed] [Google Scholar]
- 2. Yamauchi N, Watanabe A, Hishinuma M et al . The glypican 3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol 2005; 18: 1591–8. [DOI] [PubMed] [Google Scholar]
- 3. Ota S, Hishinuma M, Yamauchi N et al . Oncofetal protein glypican‐3 in testicular germ‐cell tumor. Virchows Arch 2006; 449: 308–14. [DOI] [PubMed] [Google Scholar]
- 4. Zynger DL, Dimov ND, Luan C, Teh BT, Yang XJ. Glypican 3: a novel marker in testicular germ cell tumors. Am J Surg Pathol 2006; 30: 1570–5. [DOI] [PubMed] [Google Scholar]
- 5. Hishinuma M, Ohashi KI, Yamauchi N et al . Hepatocellular oncofetal protein, glypican 3 is a sensitive marker for alpha‐fetoprotein‐producing gastric carcinoma. Histopathology 2006; 49: 479–86. [DOI] [PubMed] [Google Scholar]
- 6. Ohgaki H, Matsukura N. Stomach cancer. In: Stewart BW, Kleihues P, eds. World Cancer Report. Lyon: IARC Press, 2003: 194–7. [Google Scholar]
- 7. Kono K, Amemiya H, Sekikawa T et al . Clinicopathologic features of gastric cancers producing alpha‐fetoprotein. Dig Surg 2002; 19: 359–65. [DOI] [PubMed] [Google Scholar]
- 8. Chang YC, Nagasue N, Abe S, Taniura H, Kumar DD, Nakamura T. Comparison between the clinicopathologic features of AFP‐positive and AFP‐negative gastric cancers. Am J Gastroenterol 1992; 87: 321–5. [PubMed] [Google Scholar]
- 9. Nagai E, Ueyama T, Yao T, Tsuneyoshi M. Hepatoid adenocarcinoma of the stomach. A clinicopathologic and immunohistochemical analysis. Cancer 1993; 72: 1827–35. [DOI] [PubMed] [Google Scholar]
- 10. Hippo Y, Watanabe K, Watanabe A et al . Identification of soluble NH2‐terminal fragment of glypican‐3 as a serological marker for early‐stage hepatocellular carcinoma. Cancer Res 2004; 64: 2418–23. [DOI] [PubMed] [Google Scholar]
- 11. Nakatsura T, Yoshitake Y, Senju S et al . Glypican‐3, overexpressed specifically in human hepatocellular carcinoma, is a novel tumor marker. Biochem Biophys Res Commun 2003; 306: 16–25. [DOI] [PubMed] [Google Scholar]
- 12. Capurro M, Wanless IR, Sherman M et al . Glypican‐3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 2003; 125: 89–97. [DOI] [PubMed] [Google Scholar]
- 13. Motomura Y, Senju S, Nakatsura T et al . Embryonic stem cell‐derived dendritic cells expressing glypican‐3, a recently identified oncofetal antigen, induce protective immunity against highly metastatic mouse melanoma, B16‐F10. Cancer Res 2006; 66: 2414–22. [DOI] [PubMed] [Google Scholar]
- 14. Komori H, Nakatsura T, Senju S et al . Identification of HLA‐A2‐ or HLA‐A24‐restricted CTL epitopes possibly useful for glypican‐3‐specific immunotherapy of hepatocellular carcinoma. Clin Cancer Res 2006; 12: 2689–97. [DOI] [PubMed] [Google Scholar]
- 15. Sobin LH, Wittekind C, eds. TNM Classification of Malignant Tumors, 6th edn. New York: John Wiley & Sons, 2002. [Google Scholar]
- 16. Japanese Gastric Cancer Association . Japanese Classification of Gastric Carcinoma, 2nd English edn. Gastric Cancer 1998; 1: 10–24. [DOI] [PubMed] [Google Scholar]
- 17. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 18. Kodama T, Kameya T, Hirota T et al . Production of alpha‐fetoprotein, normal serum proteins, and human chorionic gonadotropin in stomach cancer: histologic and immunohistochemical analyses of 35 cases. Cancer 1981; 48: 1647–55. [DOI] [PubMed] [Google Scholar]
- 19. Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K. α‐Fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn 1993; 43: 654–61. [DOI] [PubMed] [Google Scholar]
- 20. Filmus J, Shi W, Wong ZM, Wong MJ. Identification of a new membrane‐bound heparan sulphate proteoglycan. Biochem J 1995; 311: 561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican‐3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 2005; 65: 6245–54. [DOI] [PubMed] [Google Scholar]
- 22. Zhu Z, Friess H, Kleeff J et al . Glypican‐3 expression is markedly decreased in human gastric cancer but not in esophageal cancer. Am J Surg 2002; 184: 78–83. [DOI] [PubMed] [Google Scholar]
- 23. Midorikawa Y, Ishikawa S, Iwanari H et al . Glypican‐3, overexpressed in hepatocellular carcinoma, modulates FGF2 and BMP‐7 signaling. Int J Cancer 2003; 103: 455–65. [DOI] [PubMed] [Google Scholar]
- 24. Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4387 tissue samples. Am J Clin Pathol 2008; 129: 899–906. [DOI] [PubMed] [Google Scholar]
- 25. Li M, Choo B, Wong ZM, Filmus J, Buick RN. Expression of OCI‐5/glypican 3 during intestinal morphogenesis: regulation by cell shape in intestinal epithelial cells. Exp Cell Res 1997; 235: 3–12. [DOI] [PubMed] [Google Scholar]
- 26. Filmus J, Church JG, Buick RN. Isolation of a cDNA corresponding to a developmentally regulated transcript in rat intestine. Mol Cell Biol 1988; 8: 4243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ghotli ZA, Serra S, Chetty R. Clear cell (glycogen rich) gastric adenocarcinoma: a distinct tubulo‐papillary variant with a predilection for the cardia/gastro‐oesophageal region. Pathology 2007; 39: 466–9. [DOI] [PubMed] [Google Scholar]
- 28. Gao YB, Zhang DF, Jin XL, Xiao JC. Preliminary study on the clinical and pathological relevance of gastric hepatoid adenocarcinoma. J Dig Dis 2007; 8: 23–8. [DOI] [PubMed] [Google Scholar]
- 29. Zynger DL, Gupta A, Luan C, Chou PM, Yang GY, Yang XJ. Expression of glypican 3 in hepatoblastoma: an immunohistochemical study of 65 cases. Hum Pathol 2008; 39: 224–30. [DOI] [PubMed] [Google Scholar]
- 30. Esheba GE, Pate LL, Longacre TA. Oncofetal protein Glypican‐3 distinguishes yolk Sac tumor from clear cell carcinoma of the ovary. Am J Surg Pathol 2008; 32: 600–7. [DOI] [PubMed] [Google Scholar]
- 31. Stadlmann S, Gueth U, Baumhoer D, Moch H, Terracciano L, Singer G. Glypican‐3 expression in primary and recurrent ovarian carcinomas. Int J Gynecol Pathol 2007; 26: 341–4. [DOI] [PubMed] [Google Scholar]
- 32. Morford LA, Davis C, Jin L, Dobierzewska A, Peterson ML, Spear BT. The oncofetal gene glypican 3 is regulated in the postnatal liver by zinc fingers and homeoboxes 2 and in the regenerating liver by alpha‐fetoprotein regulator 2. Hepatology 2007; 46: 1541–7. [DOI] [PubMed] [Google Scholar]
- 33. Perincheri S, Dingle RW, Peterson ML, Spear BT. Hereditary persistence of alpha‐fetoprotein and H19 expression in liver of BALB/cJ mice is due to a retrovirus insertion in the Zhx2 gene. Proc Natl Acad Sci USA 2005; 102: 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lv Z, Zhang M, Bi J, Xu F, Hu S, Wen J. Promoter hypermethylation of a novel gene, ZHX2, in hepatocellular carcinoma. Am J Clin Pathol 2006; 125: 740–6. [DOI] [PubMed] [Google Scholar]
- 35. Nishimura Y, Nakatsura T, Senju S. [Usefulness of a novel oncofetal antigen, glypican‐3, for diagnosis and immunotherapy of hepatocellular carcinoma]. Nihon Rinsho Meneki Gakkai Kaishi 2008; 31: 383–91. [DOI] [PubMed] [Google Scholar]
- 36. Nakano K, Orita T, Nezu J et al . Anti‐glypican 3 antibodies cause ADCC against human hepatocellular carcinoma cells. Biochem Biophys Res Commun 2009; 378: 279–84. [DOI] [PubMed] [Google Scholar]
- 37. Ligato S, Mandich D, Cartun RW. Utility of glypican‐3 in differentiating hepatocellular carcinoma from other primary and metastatic lesions in FNA of the liver: an immunocytochemical study. Mod Pathol 2008; 21: 626–31. [DOI] [PubMed] [Google Scholar]
- 38. Kandil D, Leiman G, Allegretta M et al . Glypican‐3 immunocytochemistry in liver fine‐needle aspirates: a novel stain to assist in the differentiation of benign and malignant liver lesions. Cancer 2007; 111: 316–22. [DOI] [PubMed] [Google Scholar]


