Abstract
With the goal of establishing efficacious peptide‐based immunotherapy for patients with bone and soft tissue sarcomas, we previously identified the cytotoxic T lymphocyte‐defined osteosarcoma antigenic gene Papillomavirus binding factor. The present study was designed to determine the status of HLA class I expression in osteosarcoma and other bone and soft tissue sarcomas. Seventy‐four formalin‐fixed paraffin‐embedded specimens of various bone and soft tissue sarcomas, including 33 osteosarcomas, were stained with the anti‐HLA class I monoclonal antibody EMR8‐5, which we recently generated. The expression of HLA class I was lost or downregulated in 46 of these specimens (62%). With respect to osteosarcoma, loss or downregulation of HLA class I expression was seen in 13 (52%) of 25 primary tumors and seven (88%) of eight metastatic tumors. In six of 11 HLA class I‐negative osteosarcoma specimens, the expression of β‐2 microglobulin was also lost. Subsequently the prognostic significance of HLA class I expression was analyzed in 21 patients with osteosarcoma who had completed multidrug neoadjuvant chemotherapy and undergone adequate surgery. Patients with osteosarcoma highly expressing HLA class I showed significantly better overall and event‐free survival than those with HLA class I‐negative osteosarcoma. In contrast, such prognostic significance of HLA class I expression was not found in 15 patients with malignant fibrous histiocytoma of soft tissue. These findings suggest that the class I‐restricted cytotoxic T lymphocyte pathway plays a major role in immune surveillance of patients with osteosarcoma. (Cancer Sci 2006; 97: 1374–1380)
Bone and soft tissue sarcomas are malignant neoplasms of mesenchymal origin. Despite the relatively low prevalence of these tumors, the aggressive clinical behavior of especially high‐grade sarcomas gives rise to considerable concerns about their management.( 1 , 2 ) As exemplified by osteosarcoma, which is the most common primary sarcoma of bone, 30–40% of cases are still refractory to current multimodality treatments including definitive surgery and neoadjuvant chemotherapy.( 1 , 3 , 4 ) This emphasizes the need for alternative treatments including immunotherapy.
Immunotherapeutic trials for bone and soft tissue sarcomas were initially conducted in patients with osteosarcoma during the 1970s.( 5 , 6 ) As a result of several technological advances in tumor immunology following these studies, immunotherapeutic trials again started recently for various bone and soft tissue sarcomas in a more modern fashion.( 7 , 8 , 9 , 10 ) In these studies, dendritic cells( 7 ) and antigenic peptides( 8 , 10 ) have been used as vaccines. Nevertheless, only a few tumors showed decreases in their tumor burden in response to these vaccines,( 7 , 8 ) suggesting the presence of escape mechanisms from immune surveillance in patients with bone and soft tissue sarcomas.
It is known that tumor cells can lose HLA class I molecules from the cell surface, thereby escaping from recognition of tumor antigens by CD8+ T cells.( 11 , 12 , 13 ) Such loss or downregulation of HLA class I molecules has been demonstrated in malignant melanoma( 14 , 15 , 16 , 17 ) and a variety of carcinomas, including those arising from the breast,( 18 , 19 , 20 , 21 , 22 ) lung,( 23 , 24 ) stomach,( 25 ) colon,( 26 , 27 , 28 ) bladder( 29 ) and ovary.( 30 ) In contrast, few studies have so far addressed HLA class I expression in bone and soft tissue sarcoma tissues.( 31 , 32 )
We have recently generated a monoclonal antibody (mAb) against HLA class I molecules that can be used for formalin‐fixed, paraffin‐embedded specimens (Torigoe T, Asanuma H, Shimozawa K, Nakazawa E, Tamura Y, Hirohashi Y, Kitamura H, Honma I, Tsuruma T, Tsukamoto T, Hirata K, Hasegawa T and Sato N, submitted for publication, 2006). Using this mAb, we examined the expression profiles of HLA class I molecules immunohistochemically in various bone and soft tissue sarcomas, and analyzed the prognostic significance of HLA class I expression, focusing on osteosarcoma.
Materials and Methods
The present study was approved under institutional guidelines for the use of human subjects in research and the patients’ specimens were analyzed after having obtained informed written consent from the patients or their families.
Patients and samples. Formalin‐fixed paraffin‐embedded sections were obtained from 74 bone and soft‐tissue sarcomas consisting of 66 primary tumors (biopsy specimens) and eight metastatic tumors (surgical specimens). The primary tumors included 32 cases of bone sarcoma (25 osteosarcomas, five chondrosarcomas and two malignant fibrous histiocytomas) and 34 cases of soft tissue sarcoma (15 malignant fibrous histiocytomas, five liposarcomas, three rhabdomyosarcomas and two cases each of extraskeletal Ewing sarcoma, clear cell sarcoma, synovial sarcoma, leiomyosarcoma and alveolar soft‐part sarcoma as well as one malignant peripheral nerve sheath tumor). All metastatic tumors were lung metastases of osteosarcoma.
Antibodies. The mAb against HLA class I molecules, EMR8‐5, was generated previously in our laboratory. This mAb reacts with extracellular domains of HLA‐A*2402, A*0101, A*1101, A*0201, A*0207, B*0702, B*0801, B*1501, B*3501, B*4001, B*4002, B*4006, B*4403, Cw*0102, Cw*0801, Cw*1202 and Cw*1502 and can be used for immunostaining of formalin‐fixed paraffin‐embedded sections (Torigoe T, Asanuma H, Shimozawa K, Nakazawa E, Tamura Y, Hirohashi Y, Kitamura H, Honma I, Tsuruma T, Tsukamoto T, Hirata K, Hasegawa T and Sato N, submitted for publication, 2006). A polyclonal antibody for β‐2 microglobulin was purchased from Polysciences (Warrington, PA, USA). mAb for CD4 and CD8 were purchased from Dako (Glostrup, Denmark).
Immunohistochemistry. Formalin‐fixed paraffin‐embedded sections of biopsy specimens were deparaffinized and then boiled for 20 min in a microwave oven for antigen retrieval. The sections were blocked with 1% non‐fat dry milk and stained with a streptavidin–biotin complex (Nichirei, Tokyo, Japan) as described previously.( 33 ) The sections were then stained with hematoxylin. Positive reactivity of the EMR8‐5 and anti‐β‐2 microglobulin antibodies was confirmed by staining of vascular endothelial cells and lymphocytes in sections of tumor specimens. Sections of a normal testis obtained from an autopsy specimen were used as an external negative control for immunostaining.
The reactivity of EMR8‐5 was determined by staining of the plasma membranes of tumor cells. The reactivity of the anti‐β‐2 microglobulin antibody was determined by staining of the cytoplasm and plasma membranes of tumor cells. The expression status of HLA class I and β‐2 microglobulin was graded semiquantitatively according to the modified classification described by Al‐Batran et al.:( 17 ) negative (positive cells < 5%), low (= 5% positive cells = 50%), and high (positive cells > 50%) (Fig. 1). Diffuse expression and heterogeneous expression were regarded as high grade and low grade, respectively. Focal expression was graded as low or negative according to the percentage of positive cells.
Figure 1.
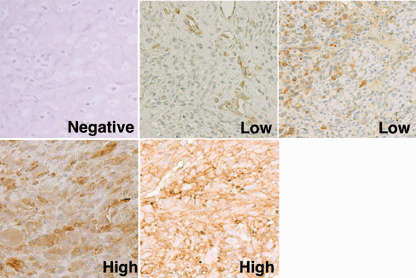
Immunohistochemical grading of tumor specimens. Representative sections of osteosarcoma specimens stained with the anti‐HLA class I monoclonal antibody EMR8‐5 are shown (original magnification, ×200). ‘Negative’ indicates that less than 5% of tumor cells were stained positively. ‘Low’ indicates a positive tumor cell number from 5 to 50%. ‘High’ indicates a positive tumor cell number of over 50%. As seen typically in the low‐grade section, endothelial cells were stained positively with EMR8‐5, which was used as an internal positive control. The same immunohistochemical grading was used for sections stained with the antibody against anti‐β‐2 microglobulin.
Infiltration of T cells into tumor tissues detected by the anti‐CD4 mAb and anti‐CD8 mAb was evaluated by semiquantitative scoring on a scale of + (scattered or mild infiltration), ++ (moderate infiltration) and +++ (diffuse infiltration), as described by Al‐Batran et al.( 17 ) (Fig. 2). The relationship between HLA class I expression on tumor cells and T‐cell infiltration was analyzed using Fisher's exact probability test. A probability of less than 0.05 was considered statistically significant.
Figure 2.
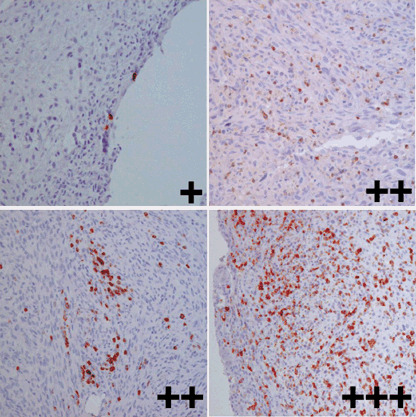
Grading of T‐cell infiltration. Representative sections of osteosarcoma specimens stained with an anti‐CD8 monoclonal antibody are shown (original magnification, ×200). Infiltration of T cells into tumor tissues was evaluated by semiquantitative scoring on a scale of + (scattered or mild infiltration), ++ (moderate infiltration) and +++ (diffuse infiltration).
Reverse transcription–polymerase chain reaction. Expression of HLA class I and β‐2 microglobulin mRNA was determined in five fresh‐frozen biopsy specimens of osteosarcoma by reverse transcription–polymerase chain reaction (RT‐PCR). An osteosarcoma cell line, U2OS, was used as a positive control. Total RNA was extracted from specimens using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and reverse transcribed with SuperScriptII RNase H− Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. PCR was carried out with KOD dash DNA polymerase (Toyobo, Tokyo, Japan). For detection of HLA class I, the forward primer 5′‐CGCCGTGGATAGAGCAGG‐3′ and the reverse primer 5′‐GCGATGTAATCCTTGCCG‐3′ were used. They were complementary to the conserved sequences in exon 2 and exon 3 of all HLA class I mRNA, respectively.( 34 ) The mixture was denatured at 98°C for 2 min, followed by 30 cycles at 98°C for 15 s, 60°C for 2 s, and 74°C for 1 min. For detection of β‐2 microglobulin, the forward primer 5′‐GAAAACGGGAAAGTCCCTCT‐3′ and the reverse primer 5′‐AGATCCAGCCCTGGACTAGC‐3′ were used. The mixture was denatured at 98°C for 2 min, followed by 30 cycles at 98°C for 15 s, 57°C for 2 s, and 74°C for 1 min. Primers for the glyceraldehyde‐3‐phosphate dehydrogenase (G3PDH) housekeeping gene were used as an internal control. Reaction products were analyzed by electrophoresis in 1.0% agarose gels with ethidium bromide.
Survivorship analysis. Survivorship analysis was carried out for 21 patients with osteosarcoma who had completed the protocols consisting of pre‐ and postoperative adjuvant chemotherapy and adequate surgical tumor resection.( 35 ) The remaining four patients were excluded because of discontinuation of the chemotherapy protocols in three, and an inadequate surgical margin in one. Two patients with lung metastasis at biopsy were included. These 21 patients were followed up for an average of 47 months (range from 9 to 125 months). Survivorship was also analyzed in 15 patients (11 men and four women) with malignant fibrous histiocytoma of soft tissue. They were 52.4 years old on average (range from 27 to 77 years) and followed up for an average of 53.1 months (range from 18 to 120 months). Survival was estimated using Kaplan–Meier plots. Prognostic significance of the expression status of HLA class I in overall and event‐free survival of patients with osteosarcoma was determined by univariate analysis using the log‐rank test.( 36 ) A probability of less than 0.05 was considered statistically significant.
Results
Expression of HLA class I in bone and soft tissue sarcomas. To determine the expression profiles of HLA class I molecules in bone and soft tissue sarcomas, we carried out immunohistochemical staining of 74 paraffin‐embedded tumor specimens with the anti‐HLA class I mAb EMR8‐5. Table 1 summarizes the results. Of the 74 sarcoma specimens, 28 (38%) were graded as having high expression of HLA class I molecules. Twenty‐two specimens (30%) were graded as having low expression and the remaining 24 specimens (32%) were negative for HLA class I expression. Collectively the expression of HLA class I was lost (negative‐grade expression) or downregulated (low‐grade expression) in 62% of bone and soft tissue sarcomas.
Table 1.
Expression status of HLA class I molecules in bone and soft tissue sarcoma biopsy specimens
| Specimen | n | HLA class I status | ||
|---|---|---|---|---|
| Negative | Low | High | ||
| Bone tumors | ||||
| Osteosarcoma | ||||
| Primary lesion | 25 | 5 | 8 | 12 |
| Lung metastatic lesion | 8 | 6 | 1 | 1 |
| Chondrosarcoma | 5 | 3 | 1 | 1 |
| Bone malignant fibrous histiocytoma | 2 | 1 | 0 | 1 |
| Soft tissue tumors | ||||
| Malignant fibrous histiocytoma | 15 | 2 | 6 | 7 |
| Liposarcoma | 5 | 2 | 2 | 1 |
| Rhabdomyosarcoma | 3 | 1 | 1 | 1 |
| Extraskeletal Ewing sarcoma | 2 | 0 | 1 | 1 |
| Clear cell sarcoma | 2 | 0 | 1 | 1 |
| Synovial sarcoma | 2 | 2 | 0 | 0 |
| Leiomyosarcoma | 2 | 1 | 0 | 1 |
| Alveolar soft‐part sarcoma | 2 | 1 | 0 | 1 |
| Malignant peripheral nerve sheath tumor | 1 | 0 | 1 | 0 |
| Total | 74 | 24 | 22 | 28 |
The distribution of HLA class I‐positive cells was graded as negative (positive cells < 5%), low (5% < positive cells ≤ 50%) or high (positive cells > 50%).
With respect to osteosarcoma, loss or downregulation of HLA class I expression was seen in 13 (52%) of the 25 primary tumors and seven (88%) of the eight metastatic tumors. Of these osteosarcoma specimens, both primary and lung metastatic tumor specimens were obtained from four patients (cases 5, 11, 12 and 17 in Table 2). As shown representatively in Fig. 3, there were no remarkable changes in the expression of HLA class I between the primary tumors and the metastatic tumors.
Table 2.
The grade of HLA class I expression and the prognosis in 21 patients with osteosarcoma
| Patient | Age (years) | Location | Surgical staging system | HLA class I status | Event‐free survival (months) | Overall survival (months) | Prognosis | T‐cell infiltration | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Original lesion | Lung metastatic lesion | CD8+ | CD4+ | |||||||
| 1 | 18 | Left 2nd rib | IIB | Low | – | 106 | 106 | CDF | + | – |
| 2 | 33 | Pelvis | IIB | Negative | – | 8 | 13 | DOD | + | – |
| 3 | 20 | Right distal femur | IIB | High | – | 72 | 72 | CDF | ++ | – |
| 4 | 15 | Left distal femur | IIB | High | – | 106 | 106 | CDF | ++ | ++ |
| 5 | 15 | Left distal femur | IIB | High | High | 20 | 33 | DOD | + | ++ |
| 6 | 15 | Left proximal fibula | IIB | Low | – | 13 | 74 | DOD | + | – |
| 7 | 13 | Left proximal humerus | IIB | Low | – | 7 | 9 | DOD | + | – |
| 8 | 7 | Left distal femur | IIB | High | – | 85 | 85 | CDF | + | – |
| 9 | 10 | Left proximal tibia | IIB | Low | – | 72 | 72 | CDF | + | – |
| 10 | 13 | Right distal femur | IIB | High | – | 6 | 11 | DOD | + | – |
| 11 | 27 | Right proximal tibia | IIB | Negative | Negative | 8 | 18 | DOD | + | – |
| 12 | 18 | Right distal femur | IIIB | Low | Low | 0 | 16 | DOD | + | – |
| 13 | 20 | Right distal humerus | IIB | High | – | 67 | 67 | CDF | + | – |
| 14 | 69 | Left distal femur | IIB | High | – | 38 | 38 | CDF | ++ | – |
| 15 | 15 | Left distal femur | IIB | High | – | 32 | 32 | CDF | + | – |
| 16 | 46 | Pelvis | IIB | Low | – | 40 | 40 | CDF | + | – |
| 17 | 40 | Right distal femur | IIB | Negative | Low | 12 | 20 | DOD | + | – |
| 18 | 19 | Right proximal tibia | IIB | Low | – | 35 | 35 | CDF | + | – |
| 19 | 15 | Left distal femur | IIB | High | – | 26 | 26 | CDF | +++ | ++ |
| 20 | 48 | Left distal femur | IIB | Negative | – | 17 | 18 | NED | ++ | ++ |
| 21 | 15 | Right distal femur | IIIB | Negative | – | 0 | 17 | NED | ND | ND |
CDF, continuous disease free; DOD, dead of disease; ND, not determined; NED, no evidence of disease.
Figure 3.
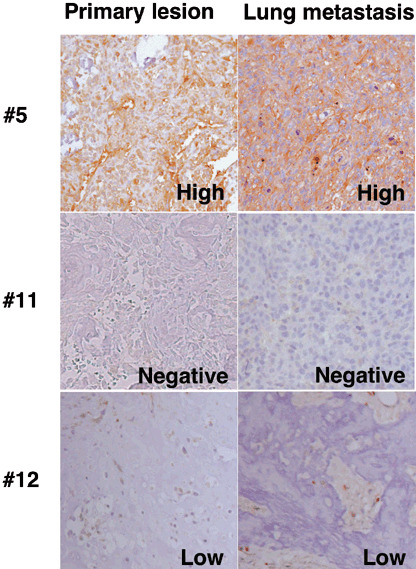
Expression of HLA class I in primary and metastatic osteosarcoma. Sections of the primary tumor and the lung metastatic tumor obtained from the same patients were stained with our anti‐HLA class I monoclonal antibody (original magnification, ×200). The numbers in the figure correspond to patient numbers in Table 2.
Expression of HLA class I mRNA was examined in five fresh‐frozen biopsy specimens of osteosarcoma, including three specimens where HLA class I was defined as negative by immunostaining (cases 11, 17 and 20). As shown in Table 3 and Fig. 4, HLA class I mRNA was similarly detectable in all cases, irrespective of the immunohistochemical status of HLA class I expression.
Table 3.
Comparative expression of HLA class I and β‐2 microglobulin in osteosarcomas
| Patient | HLA class I | β‐2 microglobulin | ||
|---|---|---|---|---|
| Protein | mRNA | Protein | mRNA | |
| 2 | Negative | ND | High | ND |
| 11 | Negative | + | High | + |
| 17 | Negative | + | High | + |
| 20 | Negative | + | High | + |
| 21 | Negative | ND | Negative | ND |
| 12 | Low | + | Low | + |
| 15 | High | + | High | + |
ND, not determined.
Figure 4.
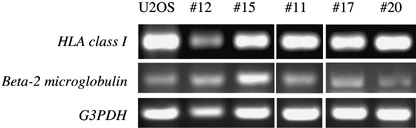
Expression of HLA class I and β‐2 microglobulin mRNA in osteosarcoma. The expression of HLA class I and β‐2 microglobulin mRNA was examined in five fresh frozen osteosarcoma specimens. U20S is an osteosarcoma cell line used as a positive control. Immunohistochemical status of HLA class I expression was low in patient 12, high in patient 15 and negative in patients 11, 17 and 20. G3PDH, glyceraldehyde‐3‐phosphate dehydrogenase.
Expression of β‐2 microglobulin in osteosarcomas. To determine the role of β‐2 microglobulin in the loss of HLA class I expression, we subsequently stained specimens with an anti‐β‐2 microglobulin antibody focusing on HLA class I‐negative osteosarcomas (Table 3; Fig. 5). β‐2 microglobulin was expressed highly in four of five osteosarcoma specimens that were negative for HLA class I as defined by immunostaining. The remaining osteosarcoma specimen was negative for both HLA class I and β‐2 microglobulin. The expression status of β‐2 microglobulin mRNA was consistent with the results of immunostaining (Fig. 4).
Figure 5.
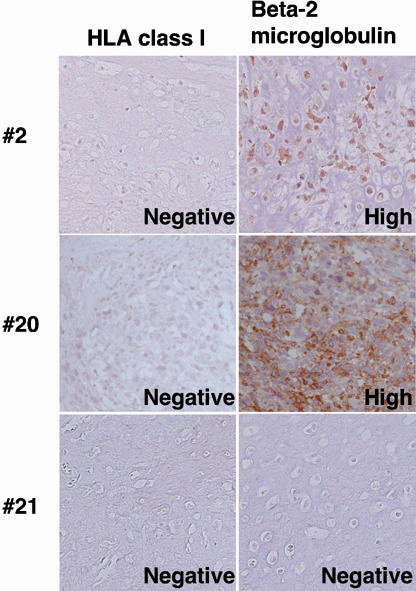
Expression of β‐2 microglobulin in HLA class I‐negative osteosarcoma. Sections of HLA class I‐negative primary osteosarcoma were stained with the anti‐β‐2 microglobulin antibody (original magnification, ×200). In patients 2 and 20, the anti‐β‐2 microglobulin antibody reacted highly with their specimens. In contrast, in patient 21 the reactivity was graded as negative for both the anti‐HLA class I monoclonal antibody and the anti‐β‐2 microglobulin antibody.
Prognostic significance of HLA class I expression in osteosarcoma and malignant fibrous histiocytoma. We then analyzed the prognostic significance of HLA class I expression in 21 patients with osteosarcoma (Table 2). Osteosarcoma was chosen as a prototype of high‐grade sarcomas and was treated uniformly with definitive surgical excision and multidrug adjuvant chemotherapy. As shown in Fig. 6, patients who had osteosarcoma with high expression of HLA class I showed better overall survival than those with low expression of HLA class I or those with negative expression. There was a significant difference in the overall survival between patients with osteosarcoma highly expressing HLA class I and those with negative expression of HLA class I (P = 0.032). Similarly, osteosarcomas with high expression of HLA class I had a significantly better prognosis for event‐free survival than those with negative expression (P = 0.0014) (Fig. 7).
Figure 6.
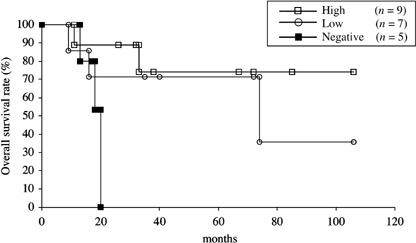
Overall survival of 21 patients with osteosarcoma stratified by HLA class I status. Overall survival was estimated using Kaplan–Meier plots. The date that the histological diagnosis was made was used as time 0. There was a significant difference between patients with osteosarcoma highly expressing HLA class I and those with negative expression of HLA class I (P = 0.032).
Figure 7.
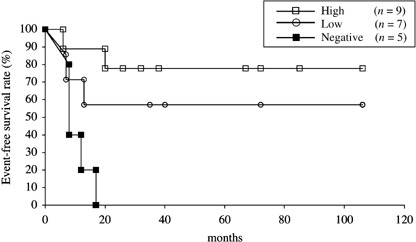
Event‐free survival of 21 patients with osteosarcoma stratified by HLA class I status. Event‐free survival was estimated using Kaplan–Meier plots. The date that the histological diagnosis was made was used as time 0. Osteosarcomas with high expression of HLA class I had a significantly better prognosis than those with negative expression (P = 0.0014).
For comparison, we also analyzed the prognostic significance of HLA class I expression in 15 patients with malignant fibrous histiocytoma of soft tissue (Fig. 8). Although statistical analysis was not carried out due to the small sample size, there was no apparent relationship between HLA class I expression status and overall survival of these patients.
Figure 8.
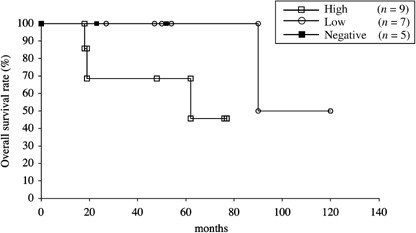
Overall survival of 15 patients with malignant fibrous histiocytoma of soft tissue stratified by HLA class I status. Overall survival was estimated using Kaplan–Meier plots. The date that the histological diagnosis was made was used as time 0.
Relationship between HLA class I expression and T‐cell infiltration in osteosarcoma tissues. To determine the relationship between HLA class I expression and T‐cell infiltration, we stained 20 osteosarcoma specimens with anti‐CD4 and anti‐CD8 mAb (Table 2). Infiltration of CD4+ T cells was seen in four osteosarcoma specimens. In contrast, CD8+ T cells were found in all 20 osteosarcoma specimens to various extents (Table 2). Moderate (++) or diffuse (+++) infiltration of CD8+ T cells was detected in five specimens. Of these, HLA class I was highly expressed in four specimens, even though statistical analysis failed to reveal a significant relationship (P = 0.12).
Discussion
The present study was designed to determine the status of HLA class I expression in osteosarcoma and other bone and soft tissue sarcomas with a view toward future immunotherapeutic trials using peptide vaccines derived from papillomavirus binding factor, which we defined as an osteosarcoma antigen.( 37 ) The anti‐HLA class I mAb used in the present study, EMR8‐5, recognizes the common epitope of HLA‐A, B and C molecules in formalin‐fixed, paraffin‐embedded tissue sections. EMR8‐5 appears suitable for immunohistochemical analysis of osteosarcoma specimens as they often exhibit osteoblastic features that make production of cryostat sections problematic.
There are two other monoclonal anti‐HLA class I antibodies, HC10 and HCA2, that have been well characterized and can be used for the immunostaining of formalin‐fixed tissue specimens. However, HC10 barely reacts with HLA‐A allele proteins, and HCA2 reacts to some HLA‐A allele proteins, but not to most HLA‐B or C allele proteins.( 38 , 39 )
We found that the expression of HLA class I was lost or downregulated in 47 (63%) of 74 formalin‐fixed paraffin‐embedded specimens of various bone and soft tissue sarcomas. Previously, Mechtersheimer et al. examined 82 frozen specimens of various sarcomas using the anti‐HLA class I mAb W6/32.( 31 ) They reported loss or downregulation of HLA class I expression in 28 specimens (34%). With respect to osteosarcoma, Mechtersheimer et al. evaluated four specimens and none of them showed downregulation of HLA class I expression.( 31 ) In the present study, the frequency of loss or downregulation of HLA class I expression was 88% (7/8) in the metastatic tumors, which was higher than in the primary tumors (52%, 13/25). In addition, patients with osteosarcoma exhibiting high expression of HLA class I showed significantly better overall and event‐free survival than did those with HLA class I‐negative osteosarcoma. In contrast, such prognostic significance of HLA class I expression was not found in 15 patients with malignant fibrous histiocytoma of soft tissue. These findings suggest that the class I‐restricted cytotoxic T lymphocyte pathway plays a major role in immune surveillance of patients with osteosarcoma who undergo multidrug chemotherapy protocols. At the same time, they imply that the loss or downregulation of HLA class I expression may become a substantial obstacle in immunotherapeutic approaches for patients with osteosarcoma.
Although the sample size was very small, the expression of HLA class I was negative in two of two synovial sarcoma specimens in the present study, and was downregulated in four of five specimens in the study by Mechtersheimer et al.( 31 ) The propensity of synovial sarcoma cells to lose HLA class I may have also served as an obstacle for immunotherapeutic trials such as the one we undertook using an SYT–SSX fusion gene‐derived peptide vaccine.( 10 )
Apart from sarcomas, the loss or downregulation of HLA class I molecules occurred in 33% of head and neck cancers, 54% of breast cancers, 31% of lung cancers, 32% of renal cancers, 43% of colon cancers, 55% of cervix cancers, 70% of prostate cancers and 51% of melanomas.( 11 ) Such loss or downregulation of HLA class I has been associated with disease progression of malignant melanoma( 14 ) and ovary carcinoma,( 30 ) and with poor prognosis in colorectal cancer.( 28 ) In contrast, some studies of malignant melanoma have shown a lack of prognostic significance of HLA class I expression.( 15 , 16 ) It should also be noted that total loss of HLA class I has been proposed as an indicator of good prognosis in breast cancer( 21 ) and non‐small cell lung cancer.( 24 )
There are several mechanisms proposed to explain abnormal HLA class I phenotypes: (i) structural alterations of the genes that encode HLA class I antigen subunits; (ii) impaired transcriptional activity of these genes; (iii) deregulation of antigen‐processing machinery components responsible for functional HLA class I expression such as β‐2 microglobulin and transporters associated with antigen processing (TAP‐1 and TAP‐2);( 40 ) and (iv) degradation of HLA class I proteins by proteasomes. We analyzed these possibilities by examining the expression of HLA class I mRNA and also the expression of β‐2 microglobulin protein and mRNA (Table 3). HLA class I mRNA was detectable in all five osteosarcoma tissues examined, including three tissues with negative cell surface expression of HLA class I proteins. β‐2 microglobulin protein was negative in one of five osteosarcoma tissues with negative cell surface expression of HLA class I proteins. In this particular case, deregulation of β‐2 microglobulin is likely attributed to the loss of HLA class I expression. The remaining cases may be explained by the possibility that β‐2 microglobulin has non‐functional mutations, as has been reported in malignant melanoma.( 41 ) However, we found no mutations in the entire open reading frame of β‐2 microglobulin mRNA in five osteosarcoma tissues examined, including three HLA class I negative osteosarcomas (Tsukahara T, unpublished observation, 2006). Other possibilities, such as deregulation of TAP and the involvement of proteasomes, remain to be elucidated by further experiments including western blotting.
The higher frequency of loss of HLA class I expression seen in metastatic osteosarcomas (88%) than in primary osteosarcomas (52%) can be explained by the following mechanisms: (i) osteosarcoma cells with loss of HLA class I expression metastasized selectively; (ii) the expression of HLA class I was lost during the process of metastasis or at the metastatic sites; and (iii) the group of osteosarcoma cases with loss of HLA class I expression showed higher frequency of metastasis. In this regard, the expression status of HLA class I did not alter or decrease in the metastatic lesion compared with that in the primary lesion in four patients. Therefore we assume that the regulatory mechanism of HLA class I expression does not differ between the primary lesion and the metastatic lesion. Primary osteosarcomas with loss of HLA class I have a propensity to metastasize, which eventually leads to poor survival of patients, as seen in the present study.
Al‐Batran et al. reported a significant correlation between the expression of HLA class I and the infiltration of T cells in stage IV melanoma tissues.( 17 ) With respect to osteosarcoma, Trieb et al. examined the expression of HLA‐DR and infiltration of T cells.( 42 ) Although T cells infiltrated 33 of 35 tumor specimens( 42 ) there was no signification correlation between T‐cell infiltration and HLA‐DR expression in osteosarcoma. In the present study, moderate or diffuse infiltration of CD8+ T cells was found mostly in osteosarcoma tissues with high HLA class I expression. However, the relationship between HLA class I expression and infiltration of CD8+ T cells was not statistically significant.
In conclusion, we showed for the first time that the status of HLA class I expression affected the overall survival and event‐free survival of patients with osteosarcoma. Because of the small sample size of the present analysis, larger studies need to be conducted to verify the significance of HLA class I molecules for prognosis and the application of T cell‐based immunotherapy for patients with bone and soft tissue sarcomas.
Acknowledgments
This work was supported by a Grant‐in‐Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 16209013 to N. Sato) and a postdoctoral fellowship of the Japan Society for the Promotion of Science (grant no. 02568 to T. Tsukahara).
References
- 1. Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med 1999; 341: 342–52. [DOI] [PubMed] [Google Scholar]
- 2. Singer S, Demetri GD, Baldini EH, Fletcher CD. Management of soft‐tissue sarcomas: an overview and update. Lancet Oncol 2000; 1: 75–85. [DOI] [PubMed] [Google Scholar]
- 3. Meyers PA, Schwartz CL, Krailo M et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high‐dose methotrexate. J Clin Oncol 2005; 23: 2004–11. [DOI] [PubMed] [Google Scholar]
- 4. Ferrari S, Smeland S, Mercuri M et al. Neoadjuvant chemotherapy with high‐dose ifosfamide, high‐dose methotrexate, cisplatin, and doxorubicin for patients with localized osteosarcoma of the extremity: a joint study by the Italian and Scandinavian Sarcoma Groups. J Clin Oncol 2005; 23: 8845–52. [DOI] [PubMed] [Google Scholar]
- 5. Marcove RC. A clinical trial of autogenous vaccines in the treatment of osteogenic sarcoma. Beitr Pathol 1974; 153: 65–72. [DOI] [PubMed] [Google Scholar]
- 6. Neff JR, Enneking WF. Adoptive immunotherapy in primary osteosarcoma. An interim report. J Bone Joint Surg Am 1975; 57: 145–8. [PubMed] [Google Scholar]
- 7. Geiger J, Hutchinson R, Hohenkirk L, McKenna E, Chang A, Mule J. Treatment of solid tumours in children with tumour‐lysate‐pulsed dendritic cells. Lancet 2000; 356: 1163–5. [DOI] [PubMed] [Google Scholar]
- 8. Dagher R, Long LM, Read EJ et al. Pilot trial of tumor‐specific peptide vaccination and continuous infusion interleukin‐2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: an inter‐institute NIH study. Med Pediatr Oncol 2002; 38: 158–64. [DOI] [PubMed] [Google Scholar]
- 9. Dillman R, Barth N, Selvan S et al. Phase I/II trial of autologous tumor cell line‐derived vaccines for recurrent or metastatic sarcomas. Cancer Biother Radiopharm 2004; 19: 581–8. [DOI] [PubMed] [Google Scholar]
- 10. Kawaguchi S, Wada T, Ida K et al. Phase I vaccination trial of SYT‐SSX junction peptide in patients with disseminated synovial sarcoma. J Transl Med 2005; 3: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T‐cell recognition: molecular mechanisms and functional significance. Adv Immunol 2000; 74: 181–273. [DOI] [PubMed] [Google Scholar]
- 12. Algarra I, Garcia‐Lora A, Cabrera T, Ruiz‐Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother 2004; 53: 904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bubenik J. MHC class I down‐regulation: tumour escape from immune surveillance? Int J Oncol 2004; 25: 487–91. [PubMed] [Google Scholar]
- 14. Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down‐regulation of HLA class I antigen‐processing molecules in malignant melanoma: association with disease progression. Am J Pathol 1999; 154: 745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kamarashev J, Ferrone S, Seifert B et al. TAP1 down‐regulation in primary melanoma lesions: an independent marker of poor prognosis. Int J Cancer 2001; 95: 23–8. [DOI] [PubMed] [Google Scholar]
- 16. Hofbauer GF, Burkhart A, Schuler G, Dummer R, Burg G, Nestle FO. High frequency of melanoma‐associated antigen or HLA class I loss does not correlate with survival in primary melanoma. J Immunother 2004; 27: 73–8. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Batran SE, Rafiyan MR, Atmaca A et al. Intratumoral T‐cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res 2005; 65: 3937–41. [DOI] [PubMed] [Google Scholar]
- 18. Cabrera T, Angustias Fernandez M, Sierra A et al. High frequency of altered HLA class I phenotypes in invasive breast carcinomas. Hum Immunol 1996; 50: 127–34. [DOI] [PubMed] [Google Scholar]
- 19. Redondo M, Garcia J, Villar E et al. Major histocompatibility complex status in breast carcinogenesis and relationship to apoptosis. Hum Pathol 2003; 34: 1283–9. [DOI] [PubMed] [Google Scholar]
- 20. Gobbi G, Mirandola P, Micheloni C et al. Expression of HLA class I antigen and proteasome subunits LMP‐2 and LMP‐10 in primary vs metastatic breast carcinoma lesions. Int J Oncol 2004; 25: 1625–9. [PubMed] [Google Scholar]
- 21. Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer 2005; 117: 248–55. [DOI] [PubMed] [Google Scholar]
- 22. Kageshita T, Ishihara T, Campoli M, Ferrone S. Selective monomorphic and polymorphic HLA class I antigenic determinant loss in surgically removed melanoma lesions. Tissue Antigens 2005; 65: 419–28. [DOI] [PubMed] [Google Scholar]
- 23. Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC. Loss of antigen‐presenting molecules (MHC class I and TAP‐1) in lung cancer. Br J Cancer 1996; 73: 148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramnath N, Tan D, Li Q et al. Is downregulation of MHC class I antigen expression in human non‐small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother 2005; 55: 891–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ferron A, Perez‐Ayala M, Concha A et al. MHC class I and II antigens on gastric carcinomas and autologous mucosa. J Immunogenet 1989; 16: 413–23. [DOI] [PubMed] [Google Scholar]
- 26. Gutierrez J, Lopez‐Nevot MA, Cabrera T et al. Class I and II HLA antigen distribution in normal mucosa, adenoma and colon carcinoma: relation with malignancy and invasiveness. Exp Clin Immunogenet 1987; 4: 144–52. [PubMed] [Google Scholar]
- 27. Cabrera T, Collado A, Fernandez MA et al. High frequency of altered HLA class I phenotypes in invasive colorectal carcinomas. Tissue Antigens 1998; 52: 114–23. [DOI] [PubMed] [Google Scholar]
- 28. Watson NF, Ramage JM, Madjd Z et al. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int J Cancer 2006; 118: 6–10. [DOI] [PubMed] [Google Scholar]
- 29. Romero JM, Jimenez P, Cabrera T et al. Coordinated downregulation of the antigen presentation machinery and HLA class I/β2‐microglobulin complex is responsible for HLA‐ABC loss in bladder cancer. Int J Cancer 2005; 113: 605–10. [DOI] [PubMed] [Google Scholar]
- 30. Vitale M, Pelusi G, Taroni B et al. HLA class I antigen down‐regulation in primary ovary carcinoma lesions: association with disease stage. Clin Cancer Res 2005; 11: 67–72. [PubMed] [Google Scholar]
- 31. Mechtersheimer G, Staudter M, Majdic O, Dorken B, Moldenhauer G, Moller P. Expression of HLA‐A,B,C, β2‐microglobulin (β2m), HLA‐DR‐DP‐DQ and of HLA‐D‐associated invariant chain (Ii) in soft‐tissue tumors. Int J Cancer 1990; 46: 813–23. [DOI] [PubMed] [Google Scholar]
- 32. Fernandez JE, Concha A, Aranega A, Ruiz‐Cabello F, Cabrera T, Garrido F. HLA class I and II expression in rhabdomyosarcomas. Immunobiology 1991; 182: 440–8. [DOI] [PubMed] [Google Scholar]
- 33. Kaya M, Wada T, Akatsuka T et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res 2000; 6: 572–7. [PubMed] [Google Scholar]
- 34. Liu K, Kao KJ. Measurement of relative quantities of different HLA‐A and ‐B mRNAs in cells by reverse transcription–polymerase chain reaction and denaturing gradient gel electrophoresis. J Immunol Meth 1997; 203: 67–75. [DOI] [PubMed] [Google Scholar]
- 35. Wada T, Isu K, Takeda N, Usui M, Ishii S, Yamawaki S. A preliminary report of neoadjuvant chemotherapy NSH‐7 study in osteosarcoma: preoperative salvage chemotherapy based on clinical tumor response and the use of granulocyte colony‐stimulating factor. Oncology 1996; 53: 221–7. [DOI] [PubMed] [Google Scholar]
- 36. Kawaguchi S, Wada T, Nagoya S et al. Extraskeletal myxoid chondrosarcoma: a multi‐institutional study of 42 cases in Japan. Cancer 2003; 97: 1285–92. [DOI] [PubMed] [Google Scholar]
- 37. Tsukahara T, Nabeta Y, Kawaguchi S et al. Identification of human autologous cytotoxic T‐lymphocyte‐defined osteosarcoma gene that encodes a transcriptional regulator, papillomavirus binding factor. Cancer Res 2004; 64: 5442–8. [DOI] [PubMed] [Google Scholar]
- 38. Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA‐B locus heavy chains permit biochemical characterization of certain HLA‐C locus products. J Immunol 1986; 137: 2299–306. [PubMed] [Google Scholar]
- 39. Stam NJ, Vroom TM, Peters PJ, Pastoors EB, Ploegh HL. HLA‐A‐ and HLA‐B‐specific monoclonal antibodies reactive with free heavy chains in western blots, in formalin‐fixed, paraffin‐embedded tissue sections and in cryo‐immuno‐electron microscopy. Int Immunol 1990; 2: 113–25. [DOI] [PubMed] [Google Scholar]
- 40. Chang CC, Campoli M, Ferrone S. HLA class I defects in malignant lesions: what have we learned? Keio J Med 2003; 52: 220–9. [DOI] [PubMed] [Google Scholar]
- 41. Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. β2‐Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J Clin Invest 1998; 101: 2720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trieb K, Lechleitner T, Lang S, Windhager R, Kotz R, Dirnhofer S. Evaluation of HLA‐DR expression and T‐lymphocyte infiltration in osteosarcoma. Pathol Res Pract 1998; 194: 679–84. [DOI] [PubMed] [Google Scholar]


