Abstract
It has been reported that the 67‐kDa laminin receptor (67LR) is implicated in cancer metastasis. We recently showed that 37LRP, the 67LR precursor, is a hypoxia‐inducible factor 1 (HIF‐1) target gene exposed to hypoxia in gastric cancer. Here, we investigated the role of 67LR in hypoxic metastasis and invasion in gastric cancer. Immunohistochemical analysis, western blotting, and RT‐PCR assays revealed that 67LR was highly expressed in metastatic gastric cancers in vivo. Knockdown of the 67LR protein by RNA interference significantly decreased the adhesive, invasive, and in vivo metastatic abilities of the gastric cancer cell lines SGC7901 and MKN‐45. Western blot analysis showed that 67LR increased the expression of urokinase‐type plasminogen activator (uPA) and matrix metalloproteinase (MMP)‐9, and decreased tissue inhibitor of matrix metalloproteinase (TIMP)‐1 protein. We further showed that hypoxia induced 67LR expression in a time‐dependent manner and this induction was inhibited by HIF‐1 small‐interfering (si) RNA. Both ERK and JNK inhibitors significantly inhibited hypoxia‐induced expression of 67LR and the subsequent expression of uPA and MMP 9. SiRNA against 67LR or antibody against MMP9 and uPA significantly inhibited hypoxia‐induced in vitro invasive ability. Taken together, these results reveal that 67LR promotes the invasive and metastatic ability of the gastric cancer cells through increasing uPA and MMP 9 expression, with involvement of the ERK and JNK signal pathway in hypoxia‐induced 67 LR expressions and subsequent uPA and MMP9 expression. (Cancer Sci 2010)
Increasing evidence has demonstrated that hypoxia is an important micro‐environmental factor in prompting tumor metastasis.( 1 ) Hypoxia‐inducible factor 1 (HIF‐1) is key transcriptional regulator under hypoxia, and is involved in invasion and metastasis in cancer cells.( 2 , 3 ) The 67‐kDa laminin receptor (67LR) is a non‐integrin cell‐surface receptor with a high affinity for laminin. The 67LR is overexpressed on the surface of a variety of tumor cells and clinical data indicate it is involved in the progression of different tumor types.( 4 ) The strong correlation between 67LR overexpression and the metastatic potential of tumor cells suggests that 67LR plays an important role in the promoting the metastatic phenotype.( 5 , 6 )
We previously reported that 37LRP, the 67LR precursor, is an HIF‐1‐dependent hypoxia‐induced gene involved in gastric cancer multidrug resistance.( 7 ) However, the detailed mechanism of how 67LR contributes to hypoxia‐induced metastasis in gastric cancer remains unclear.
The aim of the present study was to investigate whether 67LR is related to hypoxia‐induced gastric cancer metastasis, and to determine the underlying mechanism of invasiveness and metastasis under hypoxic condition. We found that 67LR is overexpressed in metastatic gastric cancer, and correlates with HIF‐1. We also demonstrated that 67LR may prompt invasion and metastasis of gastric cancer in vitro and in vivo by regulating urokinase‐type plasminogen activator (uPA), matrix metalloproteinase (MMP)‐9, and tissue inhibitor of matrix metalloproteinase (TIMP)‐1 protein. Finally, we found that the ERK1/2 and c‐Jun NH2‐terminal kinase (JNK) signal pathway is involved in hypoxia‐induced 67LR expression.
Materials and Methods
Cell culture. Cell plates were precovered with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) at 5 μg/cm2. The human gastric adenocarcinoma cancer cell lines SGC7901, MKN‐45, MGC803, and AGS have been previously described.( 8 ) The XGC981 1‐L cell line was established in our laboratory. 9 All cells were maintained in Dulbecco’s modified Eagle medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 0.1 mg/mL streptomycin under normoxia at 37°C in a modular incubator chamber (Precision Scientific, Winchester, VA, USA), or under hypoxia with 5% CO2 and 1% O2 balanced with N2.
Tissue collection and immunochemistry. Primary site tissues from 62 nonmetastatic gastric cancers, 49 metastatic gastric cancers, and tissues of primary and metastatic sites in lymph node sites from 49 metastatic gastric cancers were obtained from patients who underwent surgery at the Department of General Surgery of Xijing Hospital. Signed informed consent was obtained from patients contributing surgical tissues dissected for the study. All gastric cancer cases were clinically and pathologically proven. Protocols used in the study were approved by the hospital’s Protection of Human Subjects Committee. Slides were dewaxed, rehydrated, incubated in 10% normal goat serum and 0.3% Triton X‐100 in phosphate‐buffered saline (PBS) for 1 h, and then incubated with polyclonal anti‐67LR Ab (1:1000; Abcam, Cambridge, UK) and monoclonal anti‐HIF‐1 Ab (1:300; BD Biosciences, San Jose, CA, USA). Tissues were incubated in biotinlabeled goat antirabbit serum (1:200) for 30 min, and incubated with avidin–biotin–peroxidase complex for 1 h. Results were evaluated by a previously described formula( 10 ) in which staining score = the intensity of immunoreactivity (IR) × the proportion of positively staining cells. Immunoreactivity (IR) intensity was stratified into four categories: 0, no IR; 1, weak IR; 2, moderate IR; and 3, strong IR. The proportion of positive cells was classified into four groups: 0, 0–5% tumor cells exhibiting IR; 0.33, 5–33% of the tumor cells exhibiting IR; 0.67, 33–67% of the tumor cells exhibiting IR; and 1, 67–100% of the tumor cells exhibiting IR.
Immunofluorescence microscopy. SGC7901cells were plated on sterile coverslips. After 18 h, the cells were incubated in serum‐free medium under hypoxic (1.0% O2) or normoxic (21% O2) conditions at the indicated times. Cultures were incubated with antibodies specific for HIF‐1α or 67LR for 18 h, followed by secondary antibody conjugated with secondary antibodies (with fluorescein isothiocyanate for HIF‐1 and with tetramethylrhodamin isothiocyanate for 67LR; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h, and then incubated 10 min with DAPI (Invitrogen, Carlsbad, CA, USA). Images were obtained using a 63× oil‐immersion objective (NA 1.4) and a Hamamatsu digital camera with Simple PCI software at room temperature. Deconvolution was performed using Simple PCI software.
Adhesion assay. Adhesion to Matrigel was determined in 24‐well plates as described by others.( 11 ) After 0.5, 1, 2, or 4 h of incubation at 37°C under normoxic or hypoxic (1% O2) conditions, adhesive cells were washed with PBS twice, then counted under a microscope at ×200 magnification from 10 random fields in each well. Each experiment was performed in triplicate.
Invasion assays. Cell invasion assays were performed as described previously( 12 ) using Transwells (8‐mm pore size; Corning Costar, Acton, MA, USA). SGC7901/67LR‐si, SGC7901/psi cells, or SGC7901 or MKN‐45 cells were suspended at 2 × 105/mL in DMEM containing 1% bovine serum In some cases, inhibitors (U0126 or SP600125) were also added for 2 h. The cell suspension (150 μL) was placed in upper compartments and the cells were allowed to invade for 24 h at 37°C under normoxic or hypoxic conditions. After incubation, cells invading the bottom surface of the filter were fixed and stained with hematoxylin. The invasiveness was determined by counting the penetrating cells under a microscope at ×200 magnification from 10 random fields per well. Each experiment was performed in triplicate.
Tail vein metastatic assay. Mice were handled using best humane practices and were cared for in accordance with NIH Animal Care and Use Committee guidelines. Mice were injected with 1 × 106 cells in 0.1 mL PBS through the tail vein. At 4 weeks after injection, the mice were sacrificed and the lung tissues were observed with the naked eye to count the number of visible tumors on the lung surface. Lung tissues were made into serial sections before being hematoxylin–eosin (H&E) dyed and observed under a light microscope. Each experimental group contained six to 10 mice.
Plasmid construction and cell transfection. The siRNA expression vector for HIF‐1, and stable‐transfection subclones were constructed as previously reported.( 13 ) pSilencer3.0 (Ambion, Austin, TX, USA) was used according to the manufacturer’s protocol for construction of human 67LR siRNA vectors 67LRsi1 and 67LRsi2. Cell transfection was performed with Lipofectamine2000 (Invitrogen) as described in the manufacturer’s protocol. For transient transfection, cells were harvested for further experiments after 48 h of transfection. For stable transfecion, G418 (400 μg/mL) was added. Mixed clones were screened and expanded for an additional 6 weeks. MKN‐45 transfected with 67LRsi1, 67LRsi2, and pSilencer was designated as MKN‐45‐67LRsi1, MKN‐45‐67LRsi2, and MKN‐45‐psi, respectively. SGC7901 transfected with 67LRsi1, 67LRsi2, or pSilencer, was designated as SGC7901‐67LRsi1, SGC7901‐ 67LRsi2, or SGC7901‐psi respectively. The 67LR siRNA1 targeting sequence was 5′‐ACACCGCAGCCTTCAGGG AGCCACTTGCTTGAAGCAGCCTTCAGGGAGCCACT‐3′. The 67LR siRNA2 targeting sequence was 5′‐ACACCGCAGCCTTCAGGGAG CCACTTGCTTGAAGC AGCCTTCAGGGAGCCACT‐3′.
Western blotting. Whole cells or primary gastric cancer tissue from patients were harvested and lysed on ice for 30 min in lysis buffer. Equal amounts of protein (40 μg) were loaded onto 8% sodium dodecyl sulfate–polyacrylamide gel for electrophoresis at 200 V for 50 min, and transferred to nitrocellulose. Anti‐HIF‐1α (1:1000), anti‐67LR antibody (1:200), anti‐uPA, anti‐MMP2, anti‐MMP9, anti‐TIMP1, anti‐TIMP2 (1:200; Lab vision, Fremont, CA, USA), anti‐total or anti‐phospho p38 MAPK, ERK1/2, JNK (1:1000, #9250; Cell Signaling, Danvers, MA, USA) or anti‐β‐actin (1:4000; Sigma, St. Louis, MO, USA) was added for 3 h, and species‐matched peroxidase‐conjugated secondary antibody was added (1:2000). Labeled bands from washed blots were detected by ECL (Amersham, Piscataway, NJ, USA).
Real‐time RT‐PCR analysis. Primary gastric cancer tissues or cells after 8 h of normoxia or hypoxia were collected. RNA was extracted by using RNAzol (Biogenesis Springfield, VA, USA) according to the manufacturer’s instructions. cDNA strand synthesis was performed by using a Moloney murine leukemia virus cDNA synthesis kit (Gibco BRL, Grand Island, NY, USA). Taqman PCR primers were designed for each gene based on the mRNA sequence by using Primer Express software (PerkinElmer, Waltham, MA, USA) supplied by Sigma Genosys. Sequences were as follows: for actin, GGCGGCACCACCATGTACCCT and AGGGGCCGGACTCGTCATACT; for 67LR, GCAGCAGGAACCCACTTAGG and GGCAGCA GCAA AC TTCAGC.
Statistical analysis. Statistical analysis was carried out using one‐way anova or unpaired t‐test, with P < 0.05 for n independent samples deemed statistically significant.
Results
Hypoxia‐inducible factor 1 (HIF‐1) and 67LR are overexpressed in metastatic gastric cancer. Previously, we showed that 37LRP, the 67LR precursor, is an HIF‐1 target gene and prompts multidrug resistance of gastric cancer. To explore the role of 67LR and HIF‐1 on metastasis and invasion of gastric cancer, we compared the expression of HIF‐1α and 67LR in primary sites with corresponding metastatic lymph nodes sites from 62 patients with metastatic gastric cancer. We found that 67LR was predominantly located in the cytoplasm and membrane of gastric cancer cells and HIF‐1α was predominantly located in the cytoplasm and nucleus. The average staining scores was 2.59 ± 0.26 for 67LR expression in primary sites and 2.66 ± 0.37 for corresponding metastatic lymph node sites. The average staining scores was 2.15 ± 0.41 for HIF‐1α expression in primary sites and 2.35 ± 0.30 for the corresponding metastatic lymph node sites.
We compared expression of HIF‐1 and 67LR in primary sites from 62 patients with nonmetastatic gastric cancer with those from 49 patients with metastatic gastric cancer (Fig. 1A). As shown in Table 1, the positive rate of HIF‐1 expression in metastatic gastric cancer was 91.8% (45/49), higher than the 60.5% (37/62) observed for nonmetastatic gastric cancer. A positive rate of 67LR expression in metastatic gastric cancer was 95.9% (47/49), higher than the 64.5% (40/62) seen for nonmetastatic gastric cancer. The average staining score for HIF‐1 in metastatic gastric cancer was significantly higher than that in nonmetastatic gastric cancer (2.15 ± 0.41 vs 1.19 ± 0.21, P < 0.05). The average staining score of 67LR in metastatic gastric cancer was significantly higher than for nonmetastatic gastric cancer (2.59 ± 0.26 vs 1.31 ± 0.17, P < 0.05). These results suggested that HIF‐1 and 67LR were highly expressed in metastatic gastric cancer.
Figure 1.
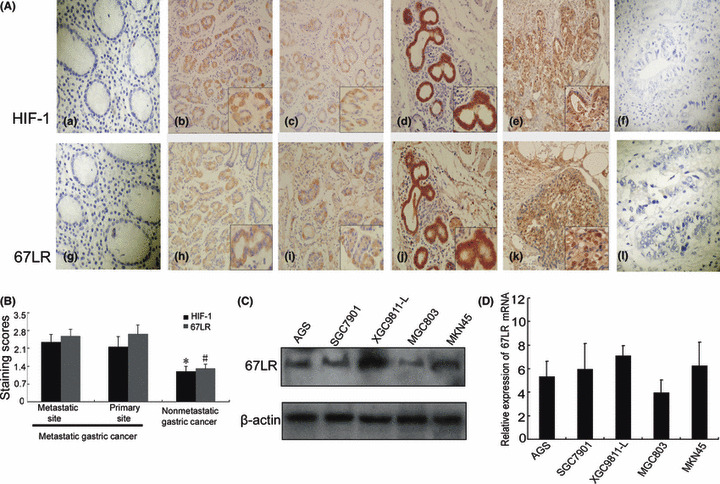
Immunohistochemical analysis of hypoxia‐inducible factor 1 (HIF‐1) and 67‐kDa laminin receptor (67LR) in metastatic and nonmetastatic gastric cancers. (A) Immunohistochemical staining with HIF‐1α and 67LR antibodies. (a,g) Noncancerous region of gastric cancer; (b,c,h,j) primary site of nonmetastatic gastric cancer; (d,j) primary site of metastatic gastric cancer; (e,k) metastatic site of metastatic gastric cancer in lymph node; (f,i) primary site of metastatic gastric cancer with no primary antibody control. (B) Immunohistochemical staining scores as described in the Materials and Methods. *P < 0.05 versus nonmetastatic gastric cancer; #P < 0.05 versus nonmetastatic gastric cancer. (C,D) Expression of 67LR in the gastric cancer cell lines were tested by western blotting and RT‐PCR.
Table 1.
Intensity of immunoreactivity of HIF‐1 and 67LR in metastatic and nonmetastatic gastric cancer
| Case | HIF‐1 | 67LR | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | ||
| Metastatic gastric cancer* | 49 | 4 | 26 | 10 | 9 | 2 | 27 | 11 | 9 |
| Nonmetastatic gastric cancer | 62 | 25 | 22 | 12 | 3 | 22 | 24 | 13 | 3 |
*P < 0.05 versus nonmetastatic gastric cancer.
67LR, 67 KDa laminin receptor; HIF‐1, hypoxia‐inducible factor 1.
Western blotting and RT‐PCR analysis also revealed that 67LR mRNA and protein were highly expressed in the highly liver‐metastatic gastric cancer cell line XGC9811‐L, compared to other gastric cancer cell lines, SGC7901, MKN‐45, AGS, and MGC803 (Fig. 1C,D). We also found that 67LR mRNA and protein were more highly expressed in the primary sites of 10 patients with metastatic gastric cancer than in nine primary sites from nonmetastatic gastric cancer patients (Fig. 2a–d). These results suggested that the 67LR has a role in gastric cancer metastasis.
Figure 2.
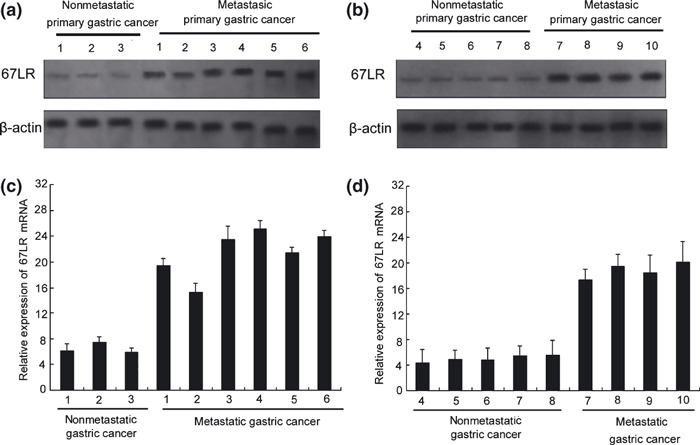
67‐kDa laminin receptor (67LR) overexpres‐sion in gastric metastasis cancer in vivo. Expression of 67LR in the gastric cancer tissues of eight patients with nonmetastatic cancer or nine patients with metastatic gastric cancer patients was assayed by western blotting (a,b) and RT‐PCR (c,d).
67‐kDa laminin receptor (67LR) promotes adhesive, invasive, and in vivo metastatic potentials of gastric cancer. To down‐regulate the expression of 67LR in gastric cancer cells, western blot analysis was first used to evaluate 67LR expression after stable transfection with siRNA1 and siRNA2. As shown in Figure 3(a,b), 67LRsi1 down‐regulated the expression of 67LR both in SGC7901 and MKN‐45 cell lines, while the effect of 67LRsi2 on 67LR expression was minimal. So to evaluate the effect of 67LR on cell adhesion, the ability of SGC7901‐67LRsi1 and MKN‐45‐67LRsi1 cells to adhere to Matrigel was investigated by adhesive assay. All the gastric cancer cells bound to Matrigel in a time‐dependent manner. However, down‐regulation of the 67LR in SGC7901 and MKN‐45 cells decreased cells’ ability to adhere to Matrigel (Fig. 3c,d). We next studied the influence of 67LR on the invasive ability of gastric cancer cells in vitro invasion assay. As shown in Figure 3(e), transfection with 67LR siRNA markedly inhibited SGC7901 and MKN‐45 invasion through Matrigel in a Boyden chamber assay (P < 0.05). Tail vein metastatic assays in nude mice were performed to test the in vivo metastatic ability of SGC7901‐67LRsi1 and MKN‐45‐67LRsi1 cells. Compared to transfection with empty vector in control cells, i.v. inoculation of SGC7901‐67LRsi1 and MKN‐45‐67LRsi1 cells led to significantly fewer visible tumors on the lung surface (Fig. 3f, both P < 0.05). Both in vitro invasion assays, and in vivo nude mice assays suggested that 67LR had the potential to promote gastric cancer metastasis.
Figure 3.
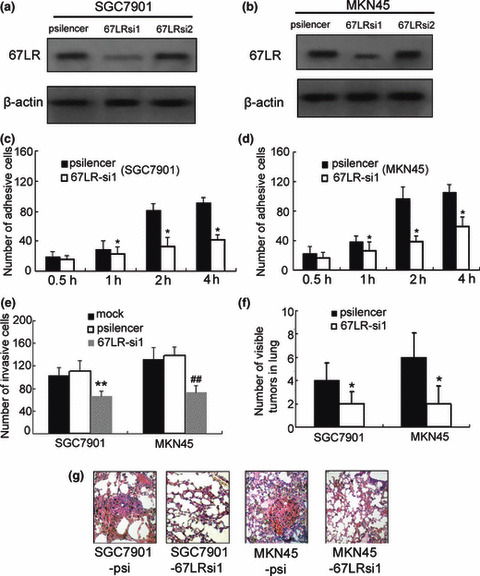
Effect of 67‐kDa laminin receptor (67LR) siRNA on the adhesive, invasive, and metastatic abilities of gastric cancer cells. (a,b) After stable transfection, the expression of 67LR was evaluated by western blotting. (c–e) Adhesion and invasion assay were used to evaluate 67LR‐si1 on invasion and adhesion abilities. *P < 0.05, **P < 0.01 versus SGC7901‐psi or MKN45‐psi. ##P < 0.01 versus MKN45‐psi or mock. (f,g) Lung tissues were observed visually, and the number of visible tumors in lung surface was counted. The lung tissues were made into serial sections before being dyed with H&E and observed under a light microscope. *P < 0.05 versus cells transfected with pSilencer3.0.
Urokinase‐type plasminogen activator (uPA), MMP9, and TIMP1 are involved in invasion of gastric cancer regulated by 67LR. To further explore the mechanism of 67LR in invasion of gastric cancer, we examined the effect of 67LR on the expression of uPA, MMP2, MMP9, TIMP1, and TIMP2 in gastric cancer cells SGC7901 and MKN‐45 after transfection with siRNA. Our data showed that the expression of MMP9 in cytoplasm (inactive form, 92 kDa) and supernatant (active form, 86 kDa) of SGC7901 and MKN‐45 cells could both be down‐regulated by 67LR si1.Similar results were found with uPA. However, TIMP1 results showed opposing effects (Fig. 4a). The expression levels of MMP2 and TIMP2 were not changed by 67LR siRNA. The levels of uPA, MMP9, and TIMP1 proteins in 67LRsi1‐transfected cells were significantly different compared to cells transfected with empty vector.
Figure 4.
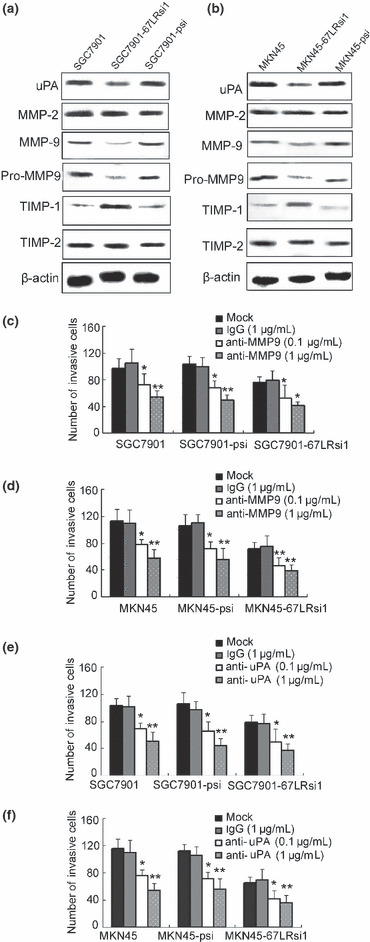
Effect of 67‐kDa laminin receptor (67LR) on urokinase‐type plasminogen activator (uPA), matrix metalloproteinase (MMP)‐9, and tissue inhibitor of matrix metalloproteinase (TIMP)‐1 in SGC7901 and MKN‐45 gastric cancer cell lines. (a,b) Expression of uPA, MMP2, and MMP9 in cytoplasm, and of active forms of MMP9 in supernatant. Tissue inhibitor of matrix metalloproteinase (TIMP)‐1 andTIMP2 were evaluated by western blot analysis. (c–f) Cells (2 × 105) were preincubated with immunoglobulin G (IgG; 1 μg/mL), uPA antibody (0.1 or 1 μg/mL), and MMP9 antibody (0.1 or 1 μg/mL) and then subjected to invasion assay, *P < 0.05; **P < 0.01 versus control and IgG treatment. Representative of three experiments with similar results.
To investigate the possible role of uPA and MMP9 in 67LR‐related invasion, SGC7901 and MKN‐45 cells transfected with psilencer or 67LR siRNA were treated with uPA or MMP9 antibody (0.1 or 1 μg/mL) before the invasion assay was carried out. The results showed that treatment with uPA or MMP9 antibody inhibited the invasive ability of both cell lines. Inhibition was 46.1% for uPA antibodies and 51.2% for MMP9 antibodies at 1 μg/mL for SGC7901‐67LRsi1 cells. For MKN‐45 67LRsi1 cells, inhibition was 44.6% with uPA antibodies, and 55.8% with MMP9 antibodies. Taken together, we suggest these results show that promotion of gastric cancer metastasis by 67LR is partially mediated by overexpression of uPA and MMP9 protein, possibly leading to subsequent degradation of the extracellular matrix.
Hypoxia‐inducible factor 1 (HIF‐1) mediates hypoxia‐induced 67LR expression. To explore 67LR effects on HIF‐1‐dependent hypoxia induced metastasis and invasion in gastric cancer, we tested HIF‐1 and 67LR expression under hypoxia. Western blot analysis and dual‐labeling immunofluorescence showed that hypoxia increased expression of 67LR protein in a time‐dependent manner, consistent with HIF‐1 protein expression. (Fig. 5a,b). To explore the relationship between HIF‐1 function and hypoxia‐mediated up‐regulation of 67LR, SGC7901 cells were transfected with either a vector containing a scrambled siRNA or a vector containing an HIF‐1α targeting sequence prior to incubation under normoxic or hypoxic conditions for 8 h, Figure 5(c,d) shows a substantial reduction expression in HIF‐1α protein expression in hypoxic‐cultured SGC7901 cells transfected with HIF‐1α siRNA, which correlated with the inhibition of 67LR protein expression and mRNA level. The data demonstrate that the up‐regulation of 67LR in hypoxia is an HIF‐1‐dependent event.
Figure 5.
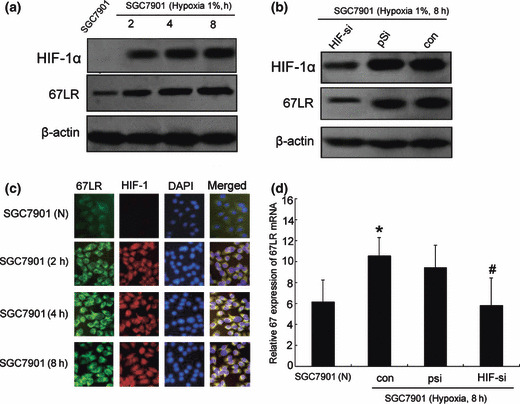
Hypoxia‐inducible factor 1 (HIF‐1)‐depen‐dent up‐regulation of 67‐kDa laminin receptor (67LR) by hypoxia. (a) Western blotting for HIF‐1 and 67LR protein expression under hypoxic (2, 4, 8 h) or normoxic conditions. (b) Dual‐immuno‐fluorescence staining against 67LR (green), HIF‐1 (red), or nuclear staining with DAPI (blue). Merged images are shown on the right in yellow. Magnification: ×40. (c) SGC7901 transiently trans‐fected with either a vector containing scrambled HIF‐1α, or a vector containing an HIF‐1α targeting sequence 48 h prior to incubation in normoxia or hypoxia for 8 h. Lysates were prepared and immunoblotted for HIF‐1α and 67LR. (d). Reverse transcription–PCR was used for analysis of 67LR mRNA levels in SGC7901 cells. Results shown are mean ± SE of three independent experiments performed in triplicate. *P < 0.05 versus SGC7901 in normoxia; #P < 0.05 versus SGC7901 transfected with scrambled sequence in hypoxia.
Extracellular signal‐regulated kinase (ERK)‐1/2 and JNK signaling are involved in 67LR, MMP‐9, and uPA expression and gastric cancer invasion induced by hypoxia. We then investigated the MAPK signaling cascade involved in hypoxia‐induced 67LR expression and 67LR‐related invasion of gastric cancer in hypoxia. Western blot analysis from MKN‐45 cell lysates showed that the p38‐specific inhibitor SB203580, the MEK‐specific inhibitor U0126, or the JNK‐specific inhibitor SP600125 inhibited the phosphorylated levels of p38, ERK, and JNK respectively, and that two other kinases were not altered. Further analysis from western blotting found that the MEK inhibitor U0126 and the JNK inhibitor SP600125 decreased the hypoxia‐induced expression of 67LR, MMP‐9, and uPA. However, the expression of 67LR, MMP‐9, and uPA was not affected by pretreatment of SGC7901 cells with the specific p38 inhibitor SB203580 (5–50 μmol/L) (Fig. 6a).
Figure 6.

Hypoxia‐induced cell adhesion and invasion via JNK, ERK1/2‐, and 67‐kDa laminin receptor (67LR)‐dependence. (a) Pretreated MKN‐45 cells with or without SB203580 (5 or 50 μM), SP600125 (5 or 50 μM), or U0126 (1 or 10 μM). Western blotting for p38 MAPK, ERK1/2, JNK phosphorylation and 67LR, matrix metalloproteinase (MMP)‐9, or urokinase‐type plasminogen activator (uPA) expression. (b,c) Adhesion and invasive assays were used to evaluate the effect of 67LR siRNA, SP600125 (5 or 50 μM) and U0126 (1 or 10 μM) on ability of gastric cancer cells exposed to hypoxia. *P < 0.05 versus MKN45 cell‐transfected scramble sequence in hypoxia. #P < 0.05 versus MKN45 cells exposed to hypoxia.
Next, adhesion and invasion assay revealed that inhibition of 67LR expression by siRNA transfection decreased the hypoxia‐induced increase in cell adhesion and invasion. Treating MKN‐45 cells with SP600125 (5–50 μM) or U0126 (1–10 μM) inhibited the hypoxia‐induced increase in cell adhesion and invasion in a dose‐dependent manner (Fig. 6b,c).
Discussion
It is well known that metastasis is a mortal factor for gastric cancer patients, but the exact mechanisms of gastric cancer metastasis remain unclear. In this study, we showed that the 67LR has an important function in hypoxia‐induced metastasis and invasion of gastric cancer.
The 67‐kDa laminin receptor (67LR) is a high‐affinity nonintegrin laminin‐binding protein which is overexpressed on the tumor cell surface and implicated in invasion and metastasis in a variety of human carcinomas including glioma cell,( 14 ) bile duct carcinoma,( 15 ) cystic carcinoma,( 16 ) breast cancer,( 17 ) urothelial tumors,( 18 ) metastatic renal cell carcinoma cell,( 19 ) and fibrosarcoma cell.( 20 ) Our results showed that 67LR was highly expressed in metastatic gastric cancers compared with nonmetastatic tissue from gastric cancer patients. Its expression level was also significantly higher in highly liver‐metastatic gastric cancer cell lines than in other gastric cancer cell lines. While protein expression was clearly strongest in liver‐metastatic XGC9811‐L cells, gene expression by RT‐PCR analysis failed to detect significant differences between gastric cancer lines SGC7901, MKN‐45, and XGC9811‐L. This could be explained by post‐transcriptional modification, and the exact mechanism needs to be explored. Furthermore, in vitro adhesion, invasion assays, and in vivo metastatic assays, found the 67LR significantly increased invasion and metastasis in gastric cancer. These data suggested 67LR participated in the process of invasion and metastasis in gastric cancer.
A hallmark of the invasion and metastasis of solid tumors is a requirement for tumor‐associated proteases that promote the dissolution of the tumor matrix and basement membrane. The proteases uPA, MMPs, and their TIMPs, have an important function in that process in gastric cancer.( 21 , 22 ) We found that inhibition of 67LR expression with siRNA reduced the uPA and MMP‐9 protein expression and increased the TIMP1 levels, suggesting that the 67LR contributed to metastasis and invasion by regulation of downstream molecules uPA and MMP‐9. However, the expression of MMP2 and TIMP2 was not influenced by alteration of 67LR. In summary, in vitro and in vivo evidence showed the potential effects of 67LR on the invasive and metastatic potential of gastric cancer through regulation of uPA, MMP9, and TIMP1expression.
We previously reported that 37LRP is directly activated by HIF‐1 through binding the hypoxia response element (HRE) at –16bp from translation start of the 37LRP gene. Other researchers reported that inhibition of 67LR inhibits hypoxia‐induced retinal neovascularization.( 23 ) To explore whether 67LR is involved in metastasis and invasion induced by hypoxia, and is HIF‐1‐dependent, we examined the different expression of 67LR under normoxic and hypoxic conditions. The data confirmed that hypoxia induced 67LR expression in gastric cancer cell lines, and inhibition of HIF‐1 expression with siRNA blocked expression of 67LR induced by hypoxia, suggesting that hypoxia‐induced 67LR expression is HIF‐1‐dependent. We also observed inconsistent blockage of HIF‐1 siRNA with 67LR. As shown in Figure 5(a), the hypoxia‐induced up‐regulation of 67LR mRNA and protein was completely blocked in HIF‐si cells. However, HIF‐si cells still express a substantial amount of HIF‐1α protein, suggesting that other transcriptional factors are involved in hypoxia‐induced 67LR expression.
Previous studies by other researchers have shown rapid and transient activation of ERK, JNK, and p38 MAPK during hypoxia.( 24 , 25 ) In this study, we showed that JNK and ERK activation is necessary for induction of 67LR and the downstream molecules MMP‐9 and uPA in MKN‐45 cells using the specific JNK inhibitor SP600125 and the ERK inhibitor U0126 in hypoxia. Pretreating MKN‐45 cells with SB203580, a specific inhibitor of p38 MAPK, did not inhibit the hypoxic induction of either 67LR or uPA and MMP‐9. Taken together, these results support the hypothesis that MAPKs, JNK, and ERK signaling pathways mediate the induction of 67LR by hypoxia.
Finally, we examined whether the hypoxia‐mediated increase in adhesion and invasion requires signaling through JNK and ERK1/2 activation, and subsequent 67LR up‐regulation in MKN‐45 cells. We demonstrated that pretreating MKN‐45 cells with siRNA against 67LR, or with SP600125 or U0126, significantly inhibited the hypoxia‐increased adhesion and invasion in a dose‐dependent manner. These data suggested that the JNK and ERK1/2 pathways were involved in hypoxia‐induced 67LR expression, resulting in an increase in cell adhesion and invasion. This partly explained the mechanism by which 67LR influenced the hypoxia‐induced invasion of gastric cancer. Our previously study showed that hypoxia increases SGC7901 cell adherence to laminin,( 26 ) although the function of 67LR in this process requires further investigation.
In conclusion, this study documented that 67LR prompted invasion and metastasis by regulating downstream molecules uPA, MMP‐9, and TIMP‐1 in gastric cancer. We also found that 67LR was involved in hypoxia‐induced metastasis and invasion. We further showed that ERK1/2 and JNK kinases regulated HIF‐dependent, hypoxia‐induced 67LR expression in gastric cancer cells. Extracellular signal‐regulated kinase (ERK)‐1/2 and JNK signaling contributed to the hypoxia‐induced activation of 67LR, uPA, and MMP‐9, and hence contributed to metastasis and invasion in the gastric cancer cell line MKN‐45. We showed that 67LR up‐regulation in gastric cancer correlated with metastasis and invasion, indicating involvement in adhesion to laminin, and also exposure to the hypoxic microenvironment in gastric cancer. These findings suggest that 67LR plays a key role in the tumor microenvironment that mediates metastasis and invasion. The results of this study may be useful in designing novel therapeutic interventions that block hypoxia‐dependent 67LR activation, resulting in reduction of MMP‐9 and uPA secretion and consequently blocking the invasion and metastasis in gastric cancer.
Acknowledgments
This study was supported by the National Basic Research Program of China (no. 2006CB504100) and National Nature Science Foundation of China (no. 30971338, 30700361), and received project funding from the China Postdoctoral Science Foundation (no. 20080441306).
References
- 1. Brahimi‐Horn MC, Chiche J, Pouysségur J. Hypoxia and cancer. J Mol Med 2007; 85: 1301–7. [DOI] [PubMed] [Google Scholar]
- 2. Chang Q, Qin R, Huang T, Gao J, Feng Y. Effect of antisense hypoxia‐inducible factor 1alpha on progression, metastasis, and chemosensitivity of pancreatic cancer. Pancreas 2006; 32: 297–305. [DOI] [PubMed] [Google Scholar]
- 3. Yoo YG, Kong G, Lee MO. Metastasis‐associated protein 1 enhances stability of hypoxia‐inducible factor‐1alpha protein by recruiting histone deacetylase 1. EMBO J 2006; 22: 1231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horwitz VG, Davidson B, Reich R. Laminin‐induced signaling in tumor cells: the role of the Mr 67,000 laminin receptor. Cancer Res 2004; 64: 3572–9. [DOI] [PubMed] [Google Scholar]
- 5. Selleri C, Ragno P, Ricci P et al. The metastasis‐associated 67‐kDa laminin receptor is involved in G‐CSF‐induced hematopoietic stem cell mobilization. Blood 2006; 108: 2476–84. [DOI] [PubMed] [Google Scholar]
- 6. Me′nard S, Castronovo V, Tagliabue E, Sobel ME. New insights into the metastasis associated 67 kD laminin receptor. J Cell Biochem 1997; 67: 155–65. [PubMed] [Google Scholar]
- 7. Liu LL, Sun L, Zhang HB et al. hypoxia‐ Mediated Up‐Regulation of MGr1‐Ag/37LRP in gastric cancers occurs via Hypoxia‐ inducible‐Factor 1‐Dependent Mechanism and contributes to Drug Resistance. Int J Cancer 2009; 124: 1707–15. [DOI] [PubMed] [Google Scholar]
- 8. Pan Y, Bi F, Liu N et al. Expression of seven main Rho family members in gastric carcinoma. Biochem Biophys Res Commun 2004; 315: 686–91. [DOI] [PubMed] [Google Scholar]
- 9. Hu S, Guo X, Xie H et al. Phage display selection of peptides that inhibit metastasis ability of gastric cancer cells with high liver‐metastatic potential. Biochem Biophys Res Commun 2006; 341: 964–72. [DOI] [PubMed] [Google Scholar]
- 10. Liu N, Bi F, Pan Y et al. Reversal of the malignant phenotype of gastric cancer cells by inhibition of RhoA expression and activity. Clin Cancer Res 2004; 15: 6239–47. [DOI] [PubMed] [Google Scholar]
- 11. Mazzieri R, Masiero L, Zanetta L et al. Control of type IV collagenase activity by components of the urokinase‐plasmin system: a regulatory mechanism with cell‐boundreactants. EMBO J 1997; 16: 2319–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin HF, Pan YL, He LJ et al. p75 Neurotrophin Receptor Inhibits Invasion and Metastasis of Gastric Cancer. Mol Cancer Res 2007; 5: 423–33. [DOI] [PubMed] [Google Scholar]
- 13. Liu L, Ning X, Sun L et al. Hypoxia‐inducible factor‐1α contributes to Hypoxia‐induced Chemoresistance in Gastric Cancer. Cancer Sci 2008; 99(1): 121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen FX, Qian YR, Duan YH et al. Down‐regulation of 67LR reduces the migratory activity of human glioma cells in vitro. Brain Res Bull 2009; 14: 402–8. [DOI] [PubMed] [Google Scholar]
- 15. Li D, Chen J, Gao Z, Li X, Yan X, Xiong Y. Wang S. 67‐kDa laminin receptor in human bile duct carcinoma. Eur Surg Res 2009; 42: 168–73. [DOI] [PubMed] [Google Scholar]
- 16. Morais Freitas V, Nogueira da Gama de Souza L, Cyreno Oliveira E, Furuse C, Cavalcanti de Araújo V, Gastaldoni Jaeger R. Malignancy‐related 67kDa laminin receptor in adenoid cystic carcinoma. Effect on migration and beta‐catenin expression. Oral Oncol 2007; 43: 987–98. [DOI] [PubMed] [Google Scholar]
- 17. Molino A, Pedersini R, Micciolo R et al. Prognostic significance of laminin, laminin receptor, and bone marrow micrometastases in breast cancer patients: are these markers of aggressive behavior and metastatic potential? Appl Immunohistochem Mol Morphol 2003; 11: 311–8. [DOI] [PubMed] [Google Scholar]
- 18. Diggle CP, Cruickshank S, Olsburgh JD et al. Identification of genes up‐regulated in urothelial tumors: the 67‐kd laminin receptor and tumor‐associated trypsin inhibitor. Am J Pathol 2003; 163: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohno Y, Izumi M, Tachibana M et al. Characterization and gene expression analysis of novel matched primary and metastatic renal cell carcinoma cell lines. Oncol Rep 2008; 20: 501–9. [PubMed] [Google Scholar]
- 20. Zuber C, Knackmuss S, Zemora G et al. Invasion of tumorigenic HT1080 cells is impeded by blocking or downregulating the 37‐KDa/67‐KDa laminin receptor. J Mol Bio 2008; 378: 530–9. [DOI] [PubMed] [Google Scholar]
- 21. Kaneko T, Konno H, Baba M, Tanaka T, Nakamura S. Urokinase‐type plasminogen activator expression correlates with tumor angiogenesis and poor outcome in gastric cancer. Cancer Sci 2003; 94: 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahabeleshwar GH, Das R, Kundum GC. Tyrosine Kinase, p56lck‐induced Cell Motility, and Urokinase‐type Plasminogen Activator Secretion Involve Activation of Epidermal Growth Factor Receptor/Extracellular Signal Regulated Kinase Pathways. J Biological Chemistry 2004; 279: 9733–42. [DOI] [PubMed] [Google Scholar]
- 23. Gebarowska D, Stitt AW, Gardiner TA, Harriott P, Greer B, Nelson J. Synthetic Peptides Interacting with the 67‐kd Laminin Receptor Can Reduce Retinal Ischemia and Inhibit Hypoxia‐Induced Retinal Neovascularization. Am J Pathology 2002; 160: 307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Comerford KM, Cummins EP, Taylor CT. c‐Jun NH2‐Terminal Kinase Activation Contributes to Hypoxia‐Inducible Factor1α–Dependent P‐Glycoprotein Expression in Hypoxia. Cancer Res 2004; 64: 9057–61. [DOI] [PubMed] [Google Scholar]
- 25. Lester RD, Jo M, Campana WM, Gonias SL. Erythropoietin promotes MCF‐7 breast cancer cell migration by an ERK/mitogen‐activated protein kinase‐dependent pathway and is primarily responsible for the increase in migration observed in hypoxia. J Biol Chem 2005; 25: 39273–7. [DOI] [PubMed] [Google Scholar]
- 26. Liu LL, Zhang HB, Sun L et al. ERK/MAPK activation contributes to Hypoxia‐Inducible Factor‐1α‐Dependent MGr1‐Ag/37LRP expression and increased resistance to Apoptosis. Int J Cancer 2009. [Epub ahead of print]. [Google Scholar]


