Abstract
We investigated the feasibility, safety, biological activity and therapeutic efficacy of adenovirus‐mediated p53 gene transfer in patients with chemoradiation resistant advanced esophageal carcinoma. Eligible patients were not surgical candidates and had measurable, advanced squamous cell carcinoma of the esophagus that was resistant to chemoradiation therapy. On a 28‐day cycle, intratumoral injections of Ad5CMV‐p53 (INGN 201; ADVEXIN®) were administered on days 1 and 3 at four dose levels (10 × 1011 particles to 25 × 1011 particles) and treated for up to five cycles. Ten patients received a total of 26 cycles with no dose‐limiting toxicity. Administration of multiple courses was feasible and well‐tolerated. Local tumor responses revealed stable disease in nine cases and progressive disease in one case. The overall responses were stable in six and progressive in four cases. Using polymerase chain reaction (PCR) analyses, gene transfer and p53 specific transgene expression were detected in tumor biopsy tissue from all patients. mRNA levels of p53, p21 and MDM2 increased in all but one case. Three patients showed absence of disease upon repeat biopsies. Substantial improvement in swallowing was observed in one patient with stenotic lesions. Intratumoral injection of Ad5CMV‐p53 is safe, feasible and biologically active when administered in multiple doses to patients with esophageal cancer. Observations from this study indicate that this treatment results in local antitumor effects in chemoradiation resistant esophageal squamous cell carcinoma. (Cancer Sci 2006; 97)
Esophageal squamous cell carcinoma (SCC) is a highly malignant neoplasm in which disease progression is frequently observed, even at initial examination. Neoadjuvant chemoradiation therapy is used for tumor down‐staging, increasing the resection rate, and possibly improving survival.( 1 ) Although combination therapy( 2 ) of radiation and the anticancer drugs 5‐FU and cisplatinum is widely used, satisfactory treatment has yet to be established due to significant toxicities and the acquisition of resistance.( 3 , 4 )
Esophageal SCC exhibits a high frequency of mutations in the p53 and p16 tumor suppressor genes. Based on the concepts of genetic alteration in carcinogenesis,( 5 , 6 ) cancer gene therapy has been developing as an alternative or adjunct to conventional cancer therapy.( 7 ) Recently, several reports showed good clinical results with adenoviral mediated wild‐type p53 gene therapy for non‐small cell lung cancer,( 8 , 9 ) bladder cancer,( 10 ) ovarian cancer,( 11 ) and head and neck cancer( 12 ) with few side‐effects. In these reports, up to 3 × 1012 viral particles (VP) of adenoviruses per injection were given to patients safely. We previously reported growth inhibitory effects of wild‐type p53 gene transfer into esophageal squamous carcinoma cell lines.( 13 ) We herein report further investigation into the safety, feasibility and biological activity of adenovirus‐mediated wild‐type p53 gene transfer in patients with esophageal SCC. We conducted a phase I/II study of Ad5CMV‐p53 (INGN 201)( 14 ) delivered via intratumoral administration to patients with esophageal SCC.( 15 ) Patients were not eligible for surgery. In this study, we also assessed the feasibility of endoscopic tissue sampling for monitoring the biological effects of investigational therapy.
Materials and Methods
Patient eligibility
Study patients included men and women aged between 20 and 80 years with pathologically documented SCC of the esophagus. Patients were eligible for the study if they were not candidates for radical surgery. Patients with nonresectable advanced esophageal SCC that was resistant to definitive chemoradiotherapy, more than 60Gy, were eligible. Tumors revealing stable disease (SD) or progressive disease (PD) after completion of chemoradiation were defined as chemoradiation‐resistant tumors. Tumors that started to grow after partial response (PR) or complete response (CR) were also defined as chemoradiation‐resistant tumors. Patients were judged to be able to survive for 12 weeks or more with performance status (PS) of 0–2 defined as follows: PS0, normal activity, asymptomatic; PS1, symptomatic, fully ambulatory; PS2, symptomatic, in bed less than 50% of the time; PS3, symptomatic, in bed more than 50% of the time but not bedridden. Patients who refused previous treatment were not enrolled. Patients provided written informed consent. Eligibility criteria included ability to administer Ad5CMV‐p53 into tumors under endoscopy or ultrasonography. Tumors (≤10 cm in its major axis) were detected and evaluated by physical examinations, endoscopy, ultrasonography, X‐rays or computed tomography scanning. Normal bone marrow counts, liver enzymes and renal function were maintained by adherence to the following criteria: neutrophil count >1500 m3; platelet count ≥100,000/mm3; hemoglobin ≥8 g/dL; total bilirubin ≤1.5 times the upper limit of normal (ULN); alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤2 times the ULN; alkaline phosphatase ≤5 times the ULN; creatinine ≤1.5 mg/dL; and prothrombin time (PT) and activated partial thromboplastin (APTT) within the normal range. The presence of a p53 mutation in the tumor was not a requirement for study entry. Clinical classification of the primary tumor, the degree of lymph node involvement and organ metastasis were examined according to the TNM/UICC classification.( 16 )
Study design and treatment plan
This was a single‐center, phase I/II study of Ad5CMV‐p53 intratumoral injection. The primary objective was to determine the feasibility and safety of this therapy. The secondary objective was to observe biological responses and antitumor effects. Duration of efficacy, time to tumor progression and survival period were also determined. The study vector was supplied by Introgen Therapeutics (Houston, TX, USA). The protocol was approved by Chiba University Graduate School of Medicine Institutional Review Board and the Recombinant DNA Advisory Committee of the National Institutes of Health Office of Biotechnology Activities. Written informed consent was obtained from all participants.
The replication‐defective adenoviral vector Ad5CMV‐p53 contains the cytomegalovirus promoter, wild‐type human p53 cDNA, and a SV40 polyadenylation signal in a minigene cassette inserted into the E1‐deleted region of modified adenovirus‐5.( 14 ) Individual doses were 10 to 25 × 1011 VP dependent on tumor size. The vector was suspended in phosphate‐buffered saline, which was kept on ice until the time of administration. On a 28‐day cycle, intratumoral injections of Ad5CMV‐p53 were administered on days 1 and 3 and treated for up to five cycles. The dose level for each patient was determined based on the estimated tumor size (sum of major axis of all measurable lesions). According to the results of clinical trials on head and neck cancer and lung cancer,( 8 , 9 , 12 ) dose levels were fixed as follows: ≤2 cm, 5 × 1011VP; 2–4 cm, 10 × 1011VP; 4–6 cm, 15 × 1011VP; 6–8 cm, 20 × 1011VP; and 8–10 cm, 25 × 1011VP. The dosing volume was adjusted using a diluter to be 0.1–0.2 mL/cm3. This dosing volume was approximately 20% (10–30%) of the estimated tumor volume. An endoscope was inserted under pharyngeal anesthesia, and a prepared Ad5CMV‐p53 solution was injected using a fine needle. In the case of lesions that allowed passage of the endoscope, injections were made at 1‐cm intervals so that the entire lesion was covered. Biopsies were taken before installation from tumor, and the subsequent intratumoral injections were performed through an endoscope when the treatment coincided with a follow‐up endoscopic examination on day 3. All treatments were performed in a negative‐pressure environment with biosafety precautions. Patients who were free of progression at day 56 were offered additional treatment up to a maximum of five cycles. In the case of lymph nodes, injections were made under ultrasonographic guidance.
Objective responses
A patient was regarded as a responder if CR or PR was obtained during the study, treatment period, or during the 12‐month follow‐up period, and if responses lasted for at least 4 weeks. An evaluation was made to determine if the efficacy was attributable to Ad5CMV‐p53 and not due to adjuvant therapy prohibited by the protocol (e.g. surgery, chemotherapy, radiotherapy). The ‘Guidelines for Clinical and Pathological Studies on Carcinoma of the Esophagus’( 16 ) were applied to measurable tumors and immeasurable tumors for tumor efficacy assessment.
Reverse transcription‐polymerase chain reaction (RT‐PCR) and real‐time PCR
Tissue samples were either formalin‐fixed or fresh‐frozen at the time of resection. For RT‐PCR, RNA was extracted from thawed, homogenized and DNase‐digested tissue. After reverse transcription, PCR was performed using primers specific to the Ad5CMV‐p53 vector or to glyceraldehyde phosphate dehydrogenase (GAPDH), as described previously.( 16 ) The esophageal cancer cell line T.Tn was transfected in vitro and used as a positive control.( 13 ) To determine the copy number of Ad5CMV‐p53 virus, viral DNA extracted from Ad5CMV‐p53 was used as an absolute standard, and the GAPDH gene was used as a reference gene to count cell numbers. The vector‐p53 transcript contains viral sequences from the CMV promoter, which serve to distinguish it from endogenous p53 mRNA.( 8 , 11 ) For RT‐PCR detection of vector‐p53 gene expression, primers were constructed to bridge the viral and human transcribed sequences as follows: forward primer, 5′‐TGGAGGAGCCGCAGTCAGAT‐3′; reverse primer, 5′‐ATATCGTCCGGGGACAGC‐3′; and probe, 5′‐TGCCGTCCCAAGCAATGGATGA‐3′. For detection of p21 gene expression, primers were constructed as follows: forward primer, 5′‐CACTGGAGGGTGACTTCG‐3′; reverse primer, 5′‐CGTTTGGAGTGGTAGAAATC‐3′; and probe, 5′‐CCTTGGCCTGCCCAAGCTCT‐3′. For detection of MDM2 gene expression, primers were constructed as follows: forward primer, 5′‐CTCACAGATTCCAGCTTCGG‐3′; reverse primer, 5′‐ACAGAGAAGCTTGGCACGC‐3′; and probe, 5′‐GGTTAGACCAAAGCCATTGCTTTTGAAG‐3′. Expression of mRNA was analyzed by quantitative real‐time PCR using the Light‐Cycler System (Roche, Basel, Switzerland). The PCR products were detected by measuring the fluorescence of SYBR Green I, which selectively bound to double‐stranded DNA and emitted greatly enhanced fluorescence. PCR products were resolved on 1% agarose gels and visualized by ethidium bromide staining.
Serological diagnosis
Serum IgG or IgM antibodies against adenovirus were determined by enzyme immunoassay kits (Adenovirus IgG/IgM ELISA; IBL‐Hamburg GmbH, Hamburg, Germany) according to the manufacturer's instructions as follows. Pipette 100 µL of each diluted sample into the respective wells of the microtiter plate; cover plate with adhesive foil; incubate for 60 min at 18–25°C; wash plate three times with 300 µL of diluted wash buffer; and remove excess solution by tapping the inverted plate on a paper towel. Following this, pipette 100 µL of enzyme conjugate into each well; cover plate with new adhesive foil; incubate for 30 min at 18–25°C then wash plate three times with 300 µL of diluted wash buffer; pipette 100 µL of Tetramethylbenzidine (TMB) substrate solution into each well; and incubate for 20 min at 18–25°C in the dark. Stop the substrate reaction by adding 100 µL of TMB stop solution into each well. Briefly mix contents by gently shaking the plate so the color changes from blue to yellow. Measure optical density with a photometer at 450 nm (reference wavelength: 600–650 nm) within 60 min after pipetting of the stop solution.
Clinical monitoring
Patients were monitored for adverse events for a minimum of 56 days. A follow‐up endoscopy was performed on days 3, 29 and 57 for all patients. Patients were seen for tumor assessment every month, which included endoscopic examination, until there was evidence of progression. Hematology, serum chemistry (including electrolytes, ALT, AST, lactate dehydrogenase, total bilirubin, urea nitrogen and creatinine) and urinalysis were performed before treatment and during follow‐up. Follow‐up information was collected from the time of study entry until patient death or December 1, 2005. Toxicity was graded according to the National Cancer Institute's (NCI) common toxicity criteria (version 1.0). Toxicity not included in the toxicity scale was scored as grade 3 if hospitalization was required and grade 4 if toxicity was regarded as life‐threatening. Overall survival was defined as the interval between the first treatment and death or last follow‐up visit. Time to progression was defined as the interval between first treatment and the appearance of a new metastasis, increased size of an established metastasis, or increased size of an established esophageal tumor (25% or greater increase in the product or sum of axis of measurable lesions). Any changes in tumor dimension, radiographical appearance or endoscopic appearance of an index lesion were noted.
The objective of this study was to evaluate the safety and therapeutic efficacy in patients with nonresectable esophageal cancer after injection of a normal p53 gene expressing adenovirus vector into tumors. Safety was monitored for 28 days after completion of the final cycle of Ad5CMV‐p53 treatment. Long‐term follow‐up was conducted every 2 months until either patient death, other treatments became necessary, or expiration of a 1‐year period after starting treatment.
Results
Patient characteristics
From December 2000 to July 2004, a total of 10 patients were registered who met the eligibility criteria. Patients consisted of nine males and one female (Table 1). The date of last follow‐up was December 1, 2005. All patients had histologically confirmed SCC of the esophagus. Patients had been previously treated with definitive chemoradiation therapy, which included more than 60Gy of radiation and concurrent 5‐fluorouracil plus cisplatin. Initial response to chemoradiation revealed CR in three, PR in two and stable disease in four patients. The other patient (#10) received chemoradiation because of a positive margin in endoscopic resected specimen. At the time of starting gene therapy, five patients had tumor invasion into adjacent organs (patients 3, 4, 7, 9, 10) and four had high risk factors for surgery (patients 1, 2, 5, 8). The remaining patient (#6) had a T3 tumor with multiple lymph node metastases. Pretreatment tissue samples were obtained from all patients. Eight patients had tumors with p53 mutations among exons 5–8.
Table 1.
Characteristics of patients with esophageal squamous cell carcinoma treated with Ad.CMV‐p53
| Patien no. | Age and Sex | Local response to CRT | Timing a of viral injection | UICC stage | Injection site b | Site of p53 mutation | Viral particles | Treatment cycles | Tumor size | Local and overall response e | Prognosis after GT f |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64 male | CR | 7M/16M | IIB | E | Exon 5 | 15 × 1011 | 2 | 43 mm | SD + SD | 19 M dead |
| 2 | 71 male | PR | 7M/15M | III | E | Exon 7 | 10 × 1011 | 5 | 25 mm | SD + SD | 15 M dead |
| 3 | 62 male | SD | 5M/6M | IVA | E | Negative c | 15 × 1011 | 3 | 50 mm | SD + PD | 3 M dead |
| 4 | 78 male | CR | 2M/10M | IVA | E | Exon 8 | 10 × 1011 | 2 | 30 mm | PD + PD | 6 M dead |
| 5 | 66 male | CR | 2M/4M | IIA | E | Exon 7 | 10 × 1011 | 3 | 40 mm | SD + SD | 47 M alive |
| 6 | 60 male | SD | 5M/9M | IVB | E | Exon 7 | 20 × 1011 | 1 | 70 mm | SD + PD | 2 M dead |
| 7 | 67 female | SD | 3M/6M | IVA | E+N | Exon 5 | 10 × 1011 | 2 | 38mm d | SD + SD | 13 M dead |
| 8 | 58 male | SD | 8M/10M | IIA | E | Exon 6 | 10 × 1011 | 2 | 40 mm | SD + SD | 15 M dead |
| 9 | 48 male | PR | 2M/3M | IVA | E | Exon 7 | 25 × 1011 | 4 | 100 mm | SD + SD | 12 M dead |
| 10 | 77 male | NA b | 12M/17M | IVA | N | Negative c | 20 × 1011 | 2 | 68 mm | SD + PD | 2 M dead |
Time to tumor progression and time to initial viral injection after completion of chemoradiation therapy.
Location of tumor injected with Ad.CMV‐p53.
c No mutation among exon 5, 6, 7 and 8.
d Tumor size was the sum of esophageal tumor and lymph node.
Treatment response was determined 4 weeks after completion of therapy by an external review board.
Survival months after first injection of Ad.CMV‐p53.
CRT, chemoradiation (performed because the pathological margin was positive in endoscopic resected specimen); E, esophageal tumor; GT, gene therapy; M, months; N, lymph node; PD, progressive disease; SD, stable disease.
Nine of 10 patients completed at least two cycles or four injections of treatment according to the protocol. Treatment cycles ranged from one to five cycles with the median number of cycles being two. Patient 6 did not undergo a second cycle of treatment because he showed rapid progression of distant metastases after the first cycle. Injected lesions were large and ranged from 25 to 100 mm (median, 43 mm in its longest axis).
Adverse events
Adverse events attributed to treatment with Ad.5CMV‐p53 generally were mild to moderate. The most common adverse events were fever and local pain (NCI grade 1 or 2). Fever was observed in all patients and pain in 30% (Table 2). Overall, drug administration was feasible and well‐tolerated. In one patient, the study had to be discontinued prematurely because of disease progression. This patient died 61 days after starting treatment (59 days after the second and last dose), but death was not related to treatment with Ad5CMV‐p53. Three patients showed hyperglycemia, which was likely attributable to total parental nutrition. Two patients revealed hypocalcemia and one patient each experienced partial thromboplastin time elongation, an increase in serum amylase or creatinine. There were no other significant laboratory abnormalities detected on follow‐up evaluations.
Table 2.
Highest grade adverse event observed in 10 patients during Ad.CMV‐p53
| Adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| Fever (%) | 1 (10) | 9 (90) | 0 | 0 | 10 (100) |
| Pain (%) | 1 (10) | 2 (20) | 0 | 0 | 3 (30) |
| Hyperglycemia (%) | 3 (30) | 0 | 0 | 0 | 3 (30) |
| Hypocalcemia (%) | 2 (20) | 0 | 0 | 0 | 2 (20) |
| APTT elongation (%) | 1 (10) | 0 | 0 | 0 | 1 (10) |
| High serum amylase (%) | 1 (10) | 0 | 0 | 0 | 1 (10) |
| High serum creatinine (%) | 1 (10) | 0 | 0 | 0 | 1 (10) |
APTT, activated partial thromboplastin time.
Detection of p53 transgene, p21 induction and MDM2 induction
Tissue samples were obtained and fresh‐frozen in liquid nitrogen before and after treatment. All patients demonstrated successful p53 gene transfer by DNA‐PCR on day 3 (Table 3), whereas all pretreatment samples were negative. DNA levels were quantitiated in biopsies from patients 1–3 and demonstrated an average of 8.2 × 104 copies/105 gene copies. Post‐treatment biopsies were obtained on day 3, day 29 and day 57. We found vector‐specific p53 expression in post‐treatment esophageal biopsies from all patients. Expression levels increased in seven patients, but did not increase on day 3 in the other three patients (patients 3, 7, 8) (Table 3). The expression level of p53 on day 29 was higher in all but two patients (Fig. 1A). P21 and MDM2 expression levels were increased in six patients on day 3 (patients 1, 4, 5, 6, 7, 10) (Fig. 1B,C). Although the peak of average mRNA levels of each gene were slightly different, all three levels increased after treatment (Fig. 1D). The ratio of expression between p21 and MDM2 increased on day 29 (Fig. 1E). Biopsies taken on day 29 showed no evidence of disease in three patients (2, 3, 5). Patients 2 and 3 showed the highest levels of gene transfer.
Table 3.
Detection of squamous cell carcinoma (SCC), Ad.CMV‐p53 vector‐specific DNA and p53 mRNA in biopsy specimens during treatment
| Patient no. | SCCa day 28 | p53 DNA day 3 | Quantity /105 gene copies | p53 mRNA (log copies/µg total RNAc) | |
|---|---|---|---|---|---|
| Preinjection | Post‐injection | ||||
| 1 | Positive | Positive | 2.60 × 104 | 4.93 | 5.88 |
| 2 | Negative | Positive | 3.74 × 104 | 4.88 | 5.05 |
| 3 | Negative | Positive | 1.73 × 105 | 4.33 | 4.07 |
| 4 | Positive | Positive | NAb | 4.21 | 5.77 |
| 5 | Negative | Positive | NA | 4.29 | 5.11 |
| 6 | Positive | Positive | NA | 5.06 | 5.62 |
| 7 | Positive | Positive | NA | 5.61 | 5.1 |
| 8 | Positive | Positive | NA | 5.07 | 4.51 |
| 9 | Positive | Positive | NA | 4.93 | 5.13 |
| 10 | Positive | Positive | NA | 4.53 | 6.68 |
NA, not applicable.
Figure 1.
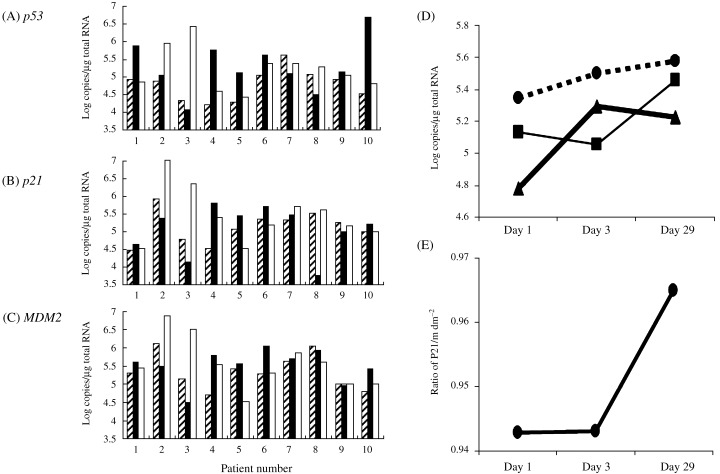
Changes in mRNA levels in each patient of marker genes before and after treatment (A) p53 (B) p21 (C) MDM2. (D) Changes in average mRNA levels of all patients, MDM2 (‐‐‐), p21 (‐) and p53 (▀). (E) Ratio of mRNA level of p21 to MDM2. (▨) Day1 (baseline); (▪) day 3; (□) day 29.
Virus studies
None of the patients were carriers of wild‐type adenovirus before they received the injection of Ad5CMV‐p53. For all serological tests, an increase in titer by at least two dilutions was considered significant. All patients had significant elevation of the antibody titers IgG and IgM to adenovirus on day 7 after injection and levels remained high during treatment (Fig. 2).
Figure 2.
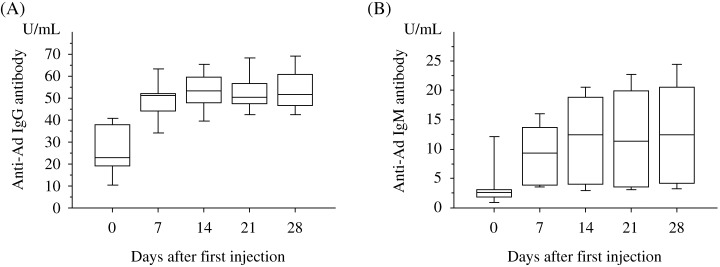
Anti‐adenovirus serological assays. (A) Anti‐adenovirus IgG antibodies. (B) Anti‐adenovirus IgM antibodies.
Long‐term follow up
Nine of the 10 eligible patients died of disease. One patient had local tumor progression and four patients had systemic progression during the initial 56 days. Six of 10 patients were alive for more than 1 year (Table 1). Three patients showed no evidence of tumor on multiple biopsies after treatment (patients 2, 3, 5). Patient 2 had a tumor with deep ulcer before treatment (Fig. 3A). After three or four cycles, the ulcer changed to a mild shape (Fig. 3B). No viable cancer cells were observed in biopsy specimens (Fig. 3E,F). Stable local tumor and no residual SCC were found on esophageal biopsies during the five cycles of treatment. Patient 3 had a large 50 mm mass and was unable to swallow at the initiation of gene therapy (Fig. 4A). Analysis of biopsies after the first cycles of treatment showed mild dysplasia and necrotic tissue (Table 3). After two injections, the patient was able to swallow liquid and meals (Fig. 4B). Although the endoscope could not pass through the stenotic portion of the lesion before treatment, it passed through after treatment (Figs 4C,D). Although the local tumor was controlled during treatment, distant metastases occurred and the patient died at 3 months. Patient 5 did not show tumor progression for 24 months after initial gene therapy. Although slight viable cancer cells were observed in biopsy specimens at 24 months, the tumor was well controlled by argon‐plasma coagulation.( 17 ) This patient remained progression‐free and alive 47 months after completion of treatment. He received a total of three courses with 1011 viral particles on days 1 and 3 of each course. With an intent‐to‐treat analysis, overall survival rate was 60% at 1 year (Fig. 5). Four patients developed clinically diagnosed distant metastases (two bone metastasis, one liver and lymph node, and one lung). Three of these four patients with development of distant metastases died of disease within 3 months after initial viral treatment. Median time to local as well as distant progression was 6 months.
Figure 3.
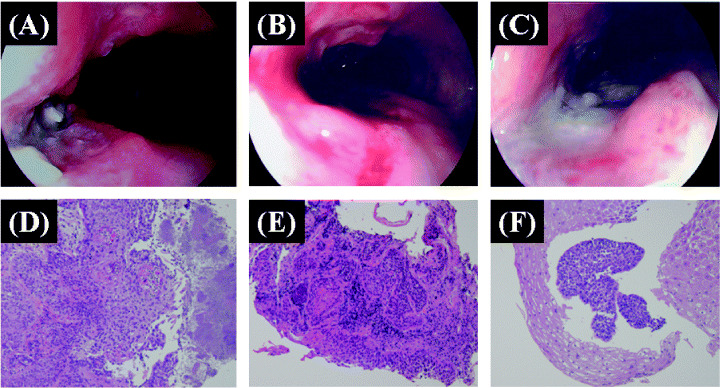
Patient 2: Middle thoracic esophageal cancer unable to undergo surgery because of multiple complications. (A) Esophagoscopy showed deep ulcer before p53 gene transfer. The ulcer changed into a mild shape after three cycles of treatment (B) and four cycles of treatment (C). Pretreatment histological section shows invasive squamous cell carcinoma (D). Only severe dysplasia was observed after three cycles of treatment (E) and only atypical squamous epithelium was observed after four cycles of treatment (F).
Figure 4.
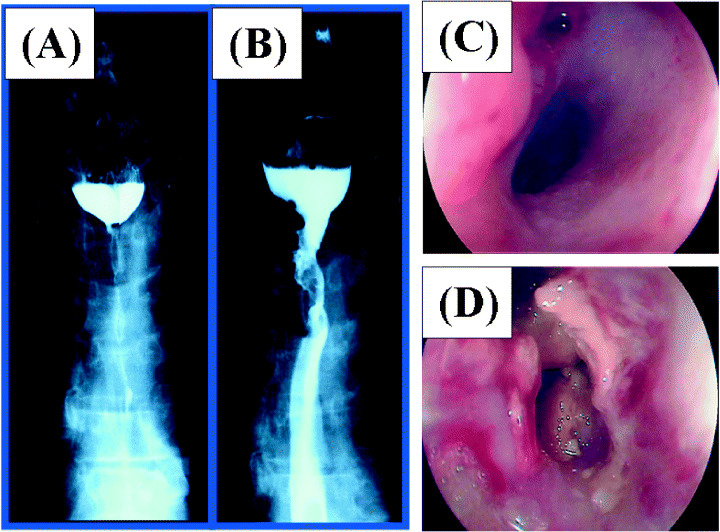
Patient 3: Upper thoracic esophageal cancer unable to undergo surgery because of tracheal invasion. (A) Esophagography shows complete obstruction before p53 gene transfer. (B) Esophageal obstruction was reduced after treatment. Esophagoscopic view before treatment (C) and after treatment (D).
Figure 5.
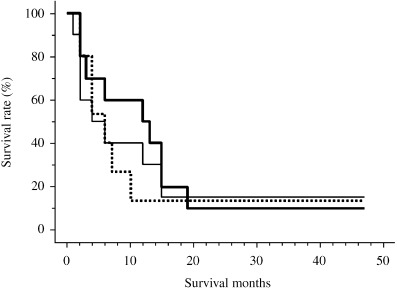
(▀) Overall survival (A), (‐‐‐) time to local progression (B); (‐) and time to metastatic progression of all patients (C).
Discussion
Our results demonstrate that administration of Ad5CMV‐p53 via intratumoral injection is feasible, safe and well‐tolerated in a population of patients with chemoradiation resistant esophageal SCC. Acute NCI grade 1 or 2 toxicities, including local pain and fever, were acceptable and provided further evidence of biological activity from the treatment. Although local tumors were stable, three patients died of disease within 12 weeks due to distant metastases. Their tumors revealed rapid metastases beyond our estimation before viral treatment.
Transduction of the vector‐p53 gene into tumor tissue was confirmed by vector‐specific DNA‐PCR analysis in all patients’ biopsy specimens. Although 80% of patients showed increased p53 mRNA after treatment and 75% of these showed elevated p21 and MDM2 downstream markers, the increase of RT‐PCR signals was more minor than we expected. This could partly be explained by the differences in biopsy sites.
Although response was a secondary end‐point of this study, all patients were evaluated for evidence of antitumor activity and clinical benefit. Six patients had stable disease at both the local site as well as systemically and survived more than 1 year after starting treatment. No significant difference was observed between time to local progression and distant progression in this study because the number of patients was small.
Possible mechanisms of antitumor effect include p53 gene expression as well as a non‐specific inflammatory effect of the adenoviral vector. Several other factors can limit the efficiency of adenovirus‐mediated gene transfer. When we compared the changes in mRNA levels between p21 and MDM2, there was good correlation in these two p53‐target genes. Boulay JL et al. reported that p21 appeared to be upregulated after p53 gene transfer and was the most sensitive marker for biological response to gene therapy in non‐small cell lung cancers.( 18 ) Because of the invasive nature of the biopsy procedure, we were not able to perform a detailed kinetic analysis of p53 target gene expression. Thus it is likely that the kinetics of p21 and MDM2 differ from that of p53 peak expression.
Other factors that may confound adenoviral gene transfer studies include: uneven distribution of the vector; a neutralizing antibody response to the adenovirus; and low levels of coxsackie and adenovirus receptor expression on target cells.( 19 ) Although secretion of infectious adenoviral particles could not be detected during treatment (data not shown), high titers of adenovirus‐reacting antibodies and neutralizing anti‐adenoviral antibodies were evident in all assessable patients 7 days after starting gene therapy.
Recently, Swisher et al. reported that p53 gene therapy combined with radiation therapy induced a high response rate (71%, 12/17) in patients with non‐small cell lung cancer.( 9 ) Because the response rate of esophageal SCC treated with chemoradiation therapy is significantly higher than that of radiation therapy alone, p53 gene therapy combined with chemoradiation therapy should be evaluated for locally advanced esophageal SCC. The response rate of T4 tumors, tumors that invade adjacent organs, may increase by combining chemoradiation with p53 gene therapy. This may result in improved resection rates and survival of those patients.
We have demonstrated that intratumoral administration of Ad5CMV‐p53 is a feasible and well‐tolerated method for therapeutic p53 gene transfer in patients with esophageal cancer. PCR analyses of tissue samples confirmed that esophageal cancer cells were successfully transduced and downstream genes were activated. Although improvement in swallowing was observed in one patient, the objective clinical benefit of p53 gene mono‐therapy was limited. Therefore, future studies should be conducted to combine chemoradiation therapy with p53 gene transfer for patients with T4 esophageal carcinoma.
Acknowledgments
We thank Drs Takeshi Tomonaga, Fumio Nomura and Takaki Hiwasa for their guidance and support. We also thank Ms Ayumi Shioya for her excellent assistance in sample analyses. This work was supported in part by a 21st Century Center of Excellence (COE) Programs Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Isono K, Ochiai T. Recent advances of treatment of cancer of the esophagus. Ann Cancer Res Ther 1992; 1: 9–16. [Google Scholar]
- 2. Nabeya Y, Ochiai T, Matsubara H et al. Neoadjuvant chemoradiotherapy followed by esophagectomy for initially respectable squamous cell carcinoma of the esophagus with multiple lymph node metastasis. Dis Esophagus 2005; 18: 388–97. [DOI] [PubMed] [Google Scholar]
- 3. Shimada H, Kitabayashi H, Nabeya Y et al. Treatment response and prognosis of patients after recurrence of esophageal cancer. Surgery 2003; 133: 24–31. [DOI] [PubMed] [Google Scholar]
- 4. Shimada H, Hoshino T, Okazumi S et al. Expression of angiogenic factors predicts response to chemoradiotherapy and prognosis of oesophageal squamous‐cell cancer. Br J Cancer 2002; 86: 552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hollstein MC, Metcalf RA, Welsh JA et al. Frequent mutation of the p53 gene in human esophageal cancer. Proc Natl Acad Sci 1990; 87: 9958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kobayashi S, Koide Y, Endo M et al. The p53 mutation is of prognostic value in esophageal squamous cell carcinoma patients in unified stage of curability. Am J Surg 1999; 177: 497–502. [DOI] [PubMed] [Google Scholar]
- 7. Roth JA, Nguyen D, Lawrence DD et al. Retrovirus‐mediated wild‐type p53 gene transfer to tumors of patients with lung cancer. Nature Med 1996; 2: 985–91. [DOI] [PubMed] [Google Scholar]
- 8. Swisher SG, Roth JA, Nemunaitis J et al. Adenovirus‐mediated p53 gene transfer in advanced non‐small‐cell lung cancer. J Natl Cancer Inst 1999; 91: 763–71. [DOI] [PubMed] [Google Scholar]
- 9. Swisher SG, Roth JA, Komaki R et al. Induction of p53‐regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res 2003; 9: 93–101. [PubMed] [Google Scholar]
- 10. Pagliaro LC, Keyhani A, Williams D et al. Repeated intravesical instillations of an adenoviral vector in patients with locally advanced bladder cancer: a phase I study of p53 gene therapy. J Clin Oncol 2003; 21: 2247–53. [DOI] [PubMed] [Google Scholar]
- 11. Wolf JK, Bodurka DC, Gano JB et al. A phase I study of Adp53 (INGN 201; ADVEXIN) for patients with platinum‐ and paclitaxel‐resistant epithelial ovarian cancer. Gynecol Oncol 2004; 94: 442–8. [DOI] [PubMed] [Google Scholar]
- 12. Clayman GL, El‐Naggar AK, Lippman SM et al. Adenovirus‐mediated p53 gene transfer in patients with advanced recurrent head and neck squamous cell carcinoma. J Clin Oncol 1998; 16: 2221–32. [DOI] [PubMed] [Google Scholar]
- 13. Shimada H, Shimizu T, Ochiai T et al. Preclinical study of adenoviral p53 gene therapy for esophageal cancer. Surg Today 2002; 31: 597–604. [DOI] [PubMed] [Google Scholar]
- 14. Zhang WW, Fang X, Mazur W et al. High‐efficiency gene transfer and high‐level expression of wild‐type p53 in human lung cancer cells mediated by recombinant adenovirus. Cancer Gene Ther 1994; 1: 5–13. [PubMed] [Google Scholar]
- 15. Shimada H, Matsubara H, Ochiai T. Invited review: p53 gene therapy for esophageal cancer. J Gastroenterol 2002; 37: 87–91. [DOI] [PubMed] [Google Scholar]
- 16. Japanese Society for Esophageal Diseases . Guidelines for clinical and pathological studies on carcinoma of the esophagus, ninth edition: part II. Esophagus 2004; 1: 107–25.
- 17. Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol 1994; 2: 42–6. [PubMed] [Google Scholar]
- 18. Boulay JL, Perruchoud AP, Reuter J, Bolliger C, Herrmann R, Rochlitz C. P21 gene expression as an indicator for the activity of adenovirus‐p53 gene therapy in non‐small cell lung cancer patients. Cancer Gene Ther 2000; 7: 1215–9. [DOI] [PubMed] [Google Scholar]
- 19. Yu L, Takenobu H, Shimozato O et al. Increased infectivity of adenovirus type 5 bearing type 11 or type 35 fibers to human esophageal and oral carcinoma cells. Oncol Rep 2005; 14: 831–5. [PubMed] [Google Scholar]


