Abstract
Urinary 8‐hydroxydeoxyguanosine (8‐OH‐dG) and 7‐methylguanine (m7Gua) were measured by a column‐switching high performance liquid chromatography method as markers of oxidative and methylating DNA damage, respectively. We investigated the associations between urinary 8‐OH‐dG or m7Gua and various lifestyle and demographic factors, such as age and sex. The urinary 8‐OH‐dG excretion level was positively correlated with cigarette smoking, but inversely correlated with fruit consumption, physical activity and total energy consumed per day. A multiple regression analysis revealed that daily physical activity and healthy meal combinations decreased the urinary 8‐OH‐dG level, whereas alcohol consumption increased it. In terms of the urinary m7Gua measurement, cigarette smoking, age and consumption of meat, fish, egg, soybean, etc. were positively correlated with the urinary m7Gua level, whereas body weight, BMI, physical activity, and dietary index score, which indicates good nutritional balance, were negatively correlated with the amount of m7Gua. Based on a multiple regression analysis, cigarette smoking and age correlated with the m7Gua level, while high BMI and healthy meal combinations have significant reducing effects on m7Gua level. Therefore, the urinary m7Gua level is considered to be a useful marker of DNA methylation, not only from smoking, but also from aging and unhealthy dietary habits. (Cancer Sci 2009; 100: 715–721)
Oxygen radicals are formed in cells by oxygen metabolism and various environmental agents, and they damage DNA, RNA, and proteins.( 1 ) Among the many types of oxidative DNA damage, 8‐hydroxydeoxyguanosine (8‐OH‐dG) is a major product and is frequently analyzed as a marker of cellular oxidative stress related to carcinogenesis,( 2 , 3 ) because 8‐OH‐dG induces mutations,( 4 , 5 ) is excreted in the urine, and it has been analyzed by high performance liquid chromatography‐electrochemical detection (HPLC‐ECD),( 6 , 7 ) liquid chromatography‐tandem mass spectrometry (LC‐MS),( 8 ) gas chromatography‐mass spectrometry (GC‐MS),( 9 ) and enzyme linked immunosorbent assay (ELISA).( 10 ) However, the reproducibility and accuracy of its measurement are much higher with the HPLC‐ECD and LC‐MS/MS methods, as compared to the ELISA method.( 11 , 12 ) We have reported that higher 8‐OH‐dG levels were observed in the lung DNA of smokers,( 13 ) the liver DNA of chronic hepatitis patients,( 14 ) and in the stomach DNA of patients infected with Helicobacter pylori.( 15 ) It has also been reported that the urinary 8‐OH‐dG level is higher in cancer patients than in healthy people,( 16 ) higher in smokers than in non‐smokers,( 17 ) and lower in people who exercise moderately.( 17 ) In addition, the urinary 8‐OH‐dG level was higher in men than in women,( 7 ) and it negatively correlated to body mass index (BMI).( 7 ) As an explanation for the relationship between a lean BMI and high urinary 8‐OH‐dG excretion, it has been suggested that lean persons have a higher metabolic rate than obese persons,( 18 ) and therefore have higher oxidative stress. Thus, various factors affect the 8‐OH‐dG levels in humans.
On the other hand, 7‐methylguanine (m7Gua) is a biomarker of DNA damage induced by methylating agents. m7Gua may serve as a good biomarker of DNA damage caused by nitrosamines in tobacco smoke,( 19 ) and other environmental methylating agents, such as methyl bromide.( 20 ) It is also possible that m7Gua is formed in cellular DNA by an endogenous methylating agent, S‐adenosylmethionine.( 21 ) m7Gua is also a degradation product from RNA,( 22 , 23 ) and is known as a metabolic rate marker. Urinary m7Gua was measured by several researchers,( 24 ) as a product of DNA damage. For instance, the amount of m7Gua excreted in the urine is increased after exposure to methylating agents in laboratory animals.( 25 , 26 ) Higher levels of m7Gua excretion have been reported among patients with colon cancer,( 27 ) although not in patients with gastric cancer.( 28 ) In particular, the urinary excretion of m7Gua has been shown to be higher among smokers than non‐smokers.( 29 )
Therefore, urinary 8‐OH‐dG and m7Gua seem to be useful biomarkers of DNA damage caused by oxidation and methylation, respectively. Measuring the two markers may be very meaningful, because the mechanisms of mutagenesis and carcinogenesis due to DNA oxidation and methylation are different. Therefore, it would be beneficial if the amounts of 8‐OH‐dG and m7Gua in human urine could be analyzed simultaneously. Recently, we developed a new HPLC method to analyze 8‐OH‐dG and m7Gua simultaneously, based on an anion exchange and reverse phase column‐switching system.( 30 ) This HPLC method was further modified to measure 8‐OH‐dG and m7Gua in not only human urine samples, but also those from rat and mouse.( 30 ) In this study, with this new HPLC method, we examined the influence of various lifestyle factors on the levels of urinary 8‐OH‐dG and urinary m7Gua among a sample of 361 Japanese healthy male employees.
Materials and Methods
Urine collection and questionnaire investigation. After informed consent was obtained, urine samples were collected from 578 healthy employees in a steel‐manufacturing company. At the same time, each individual's information on age, height and weight (for BMI), sex, status of cigarette smoking and alcohol drinking, status of dietary habits (for dietary score), status of rest (for rest score), and status of daily physical activity was obtained through a questionnaire. However, in the present study we only selected the participants who answered all of the items in the questionnaire, to avoid bias as much as possible. Consequently, the data from 361 male subjects (aged 18–59 years, mean 36.3 ± 10.3) were analyzed.
With regard to the questionnaire, the total scores of rest and meals (rest index, dietary index) were expressed as the sum of each score. For example, the rest index score is the sum of the scores (1–3) of sleeping hours, frequency of holidays, feeling of fatigue, rhythm of daily life, and ability to refresh (Table 1). Therefore, a low rest index score means insufficient rest, whereas a high score shows sufficient rest status. Similarly, the status of the dietary habits (dietary index score) is the total score of 10 items consisting of meal size, healthy combinations of meals, frequency of skipping meals, intake of light‐colored vegetables, green‐ and yellow‐colored vegetables, fruits, milk, edible oil, seaweed, and intake of meat, fish, egg, soybean, etc. Consequently, a high dietary index score means good nutritional balance. Physical activity was calculated by two different methods (physical activity‐1, ‐2), based on resting metabolic rate (RMR) and physical activity by commuting, working and sports, etc. Namely, ‘physical activity‐1’ was calculated by the ratio of (physical activity by commuting, working and sports/RMR) and was categorized into four groups (scores from 1 to 4). A higher value means high physical activity. ‘Physical activity‐2’ was calculated by physical activity due to commuting and sports, age, sex and weight and was expressed by kCal/day. Total energy consumed per day was calculated from height, age, sex and physical activity by commuting, working and sports, and was expressed by kCal/day. As continuous variables, age, weight, BMI, total energy consumed (KCal/day), physical activity‐2 (kCal/day), alcohol drinking (number of glasses drunk per day: converted to Japanese sake), cigarette smoking (number of cigarettes smoked per day) and Brinkman index obtained through the questionnaire were used.
Table 1.
The characteristics of categorical lifestyle factors and urinary 8‐hydroxydeoxyguanosine (8‐OH‐dG) levels and urinary 7‐methylguanine (m7Gua) levels in 361 male subjects
| Variables | Category | n | % | Urinary markers † | |||
|---|---|---|---|---|---|---|---|
| 8‐OH‐dG | P * | m7Gua | P * | ||||
| Sleep | Deficient | 10 | 2.8 | 4.37 ± 0.25 | 0.53 | 8.03 ± 0.47 | 0.26 |
| Slightly deficient | 181 | 50.1 | 4.19 ± 0.12 | 8.98 ± 0.21 | |||
| Sufficient | 170 | 47.1 | 4.20 ± 0.11 | 8.60 ± 0.19 | |||
| Holiday | Little or none | 2 | 0.6 | 5.41 ± 1.20 | 1.00 | 9.49 ± 0.12 | 0.15 |
| Once a week | 51 | 14.1 | 3.96 ± 0.21 | 9.42 ± 0.40 | |||
| Twice a week | 308 | 85.3 | 4.23 ± 0.08 | 8.66 ± 0.15 | |||
| Fatigue | Always | 18 | 5.0 | 3.66 ± 0.32 | 0.86 | 8.07 ± 0.36 | 0.37 |
| Sometimes | 256 | 70.9 | 4.17 ± 0.09 | 8.87 ± 0.17 | |||
| Rarely | 87 | 24.1 | 4.39 ± 0.17 | 8.62 ± 0.25 | |||
| Rhythm | Irregular | 66 | 18.3 | 4.05 ± 0.19 | 0.25 | 8.28 ± 0.27 | 0.24 |
| Mostly regular | 215 | 59.6 | 4.20 ± 0.10 | 8.87 ± 0.19 | |||
| Regular | 80 | 22.2 | 4.30 ± 0.16 | 8.91 ± 0.28 | |||
| Refreshing | Difficult | 13 | 3.6 | 4.40 ± 0.38 | 0.48 | 7.85 ± 0.56 | 0.36 |
| Moderate | 255 | 70.6 | 4.13 ± 0.09 | 8.86 ± 0.17 | |||
| Easy | 93 | 25.8 | 4.33 ± 0.17 | 8.67 ± 0.23 | |||
| Size of a meal | Full stomach every time | 24 | 6.6 | 3.87 ± 0.27 | 0.85 | 8.84 ± 0.55 | 0.55 |
| No pattern | 198 | 54.8 | 4.29 ± 0.11 | 8.90 ± 0.20 | |||
| Moderation every time | 139 | 38.5 | 4.12 ± 0.11 | 8.58 ± 0.20 | |||
| Healthy | Rerely | 46 | 12.7 | 4.25 ± 0.20 | 0.11 | 8.57 ± 0.37 | 0.72 |
| Meal | Consider sometimes | 187 | 51.8 | 4.33 ± 0.12 | 8.87 ± 0.20 | ||
| Combination | Consider every time | 128 | 35.5 | 3.99 ± 0.11 | 8.70 ± 0.23 | ||
| Skipping meals | One meal every day | 63 | 17.5 | 4.37 ± 0.20 | 0.62 | 9.32 ± 0.39 | 0.10 |
| 2 or 3 meals a week | 103 | 28.5 | 4.09 ± 0.15 | 8.88 ± 0.28 | |||
| Rarely | 195 | 54.0 | 4.20 ± 0.10 | 8.53 ± 0.16 | |||
| Light‐colored | Rarely | 17 | 4.7 | 4.32 ± 0.42 | 0.78 | 9.30 ± 0.94 | 0.35 |
| Vegetable | Once a day | 268 | 74.2 | 4.22 ± 0.09 | 8.83 ± 0.16 | ||
| Each meal | 76 | 21.1 | 4.09 ± 0.14 | 8.43 ± 0.29 | |||
| Green‐ and yellow‐collored vegetables | Rarely | 26 | 7.2 | 4.10 ± 0.32 | 0.75 | 9.93 ± 0.68 | 0.06 |
| 2 or 3 times a week | 244 | 67.6 | 4.21 ± 0.10 | 8.74 ± 0.16 | |||
| Everyday | 91 | 25.2 | 4.18 ± 0.12 | 8.53 ± 0.25 | |||
| Fruit | Rarely | 140 | 38.8 | 4.24 ± 0.12 | 0.03 | 8.54 ± 0.22 | 0.06 |
| 2 or 3 times a week | 187 | 51.8 | 4.28 ± 0.11 | 9.07 ± 0.20 | |||
| Everyday | 34 | 9.4 | 3.57 ± 0.19 | 8.10 ± 0.36 | |||
| Meat, fish, egg, etc. | Rarely | 11 | 3.0 | 4.72 ± 0.49 | 0.16 | 8.15 ± 0.55 | 0.05 |
| Twice a day | 194 | 53.7 | 4.25 ± 0.11 | 9.08 ± 0.19 | |||
| Each meal | 156 | 43.2 | 4.09 ± 0.11 | 8.43 ± 0.21 | |||
| Milk | Rarely | 118 | 32.7 | 4.32 ± 0.16 | 0.23 | 8.86 ± 0.24 | 0.68 |
| 2 or 3 times a week | 171 | 47.4 | 4.10 ± 0.10 | 8.82 ± 0.21 | |||
| Everyday | 72 | 19.9 | 4.23 ± 0.15 | 8.53 ± 0.29 | |||
| Oil | Rarely | 9 | 2.5 | 4.41 ± 0.58 | 0.55 | 9.30 ± 1.20 | 0.26 |
| 2 or 3 times a week | 192 | 53.2 | 4.15 ± 0.12 | 8.95 ± 0.19 | |||
| Eeveryday | 160 | 44.3 | 4.24 ± 0.10 | 8.52 ± 0.20 | |||
| Seaweed | Rarely | 58 | 16.1 | 3.99 ± 0.18 | 0.75 | 8.42 ± 0.34 | 0.50 |
| 2 or 3 times a week | 266 | 73.7 | 4.26 ± 0.09 | 8.86 ± 0.16 | |||
| Everyday | 37 | 10.2 | 4.04 ± 0.21 | 8.69 ± 0.49 | |||
| Physical activity‐1 | Light | 192 | 53.2 | 4.39 ± 0.11 | 0.03 | 8.71 ± 0.17 | 0.35 |
| Moderate | 119 | 33.0 | 4.09 ± 0.12 | 9.07 ± 0.28 | |||
| Moderately heavy | 12 | 3.3 | 3.85 ± 0.48 | 8.23 ± 0.67 | |||
| Heavy | 38 | 10.5 | 3.68 ± 0.19 | 8.32 ± 0.41 | |||
One‐way analysis of variance.
Data are mean ± SE: 8‐OH‐dG (µg/g creatinine), m
7 Gua (mg/g creatinine).
Analysis of m7Gua, 8‐OH‐dG and creatinine. Urinary m7Gua and 8‐OH‐dG were determined by the method previously described,( 31 ) Briefly, a human urine sample was mixed with the same volume of a dilution solution containing the ribonucleoside marker, 8‐hydroxyguanosine. A 20‐µL aliquot of the diluted urine sample was injected into HPLC‐1 (MCI GEL CA08F, 7 µm, 1.5 × 120 mm; elution, 2% acetonitrile in 0.3 mM sulfuric acid, 50 µL/min, 65°C), via the guard column (1.5 × 40 mm), and the chromatograms were recorded by a Gilson UV detector (UV/VIS‐155 with 0.2 mm light path cell). Creatinine and m7Gua were detected at 245 and 305 nm, respectively. The 8‐OH‐dG fraction was collected, depending on the relative elution position from the peak of the added marker, 8‐OH‐G, and was automatically injected into the HPLC‐2 column. The 8‐OH‐dG fraction was fractionated by the HPLC‐2 column (Shiseido, Capcell Pak C18, 5 µm, 4.6 × 250 mm; elution, 10 mM sodium phosphate buffer [pH 6.7] containing 5% methanol and an antiseptic Reagent MB [100 µL/L], 1 mL/min, 40°C). The 8‐OH‐dG was detected by a Coulochem II EC detector (ESA Inc., Chemsford, MA, USA) with a guard cell (5020) and an analytical cell (5011) (applied voltage: guard cell, 350 mV; E1, 170 mV; E2, 300 mV).
Statistics. The relationships between the urinary 8‐OH‐dG levels and the urinary m7Gua levels with categorical and continuous variables were analyzed by using oneway analysis of variance (ANOVA) and Kendall's rank correlation coefficients, respectively. In addition to the ANOVA analysis, multiple comparisons between groups were conducted with Scheffe's test. Since the distributions of 8‐OH‐dG and m7Gua were skewed, the log‐transformed values of 8‐OH‐dG and m7Gua, which showed normal distributions, were used in the multiple regression analysis. P‐values less than 0.05 (two‐tailed) were considered to indicate significant differences. All data were analyzed using the SPSS statistical package (SPSS, Chicago, IL, USA) for Windows 14.0.
Results
The mean level of urinary 8‐OH‐dG (µg/g creatinine) in the 361 male subjects was 4.20 ± 1.47 (SD). A 19.4‐fold interindividual variation was found (0.53–10.28 µg/g creatinine). The mean level of m7Gua normalized to creatinine (mg/g creatinine) was 8.77 ± 2.61 (SD), and a 4.80‐fold interindividual variation was found (3.94–18.93 mg/g creatinine). The relationships between the 16 categorical lifestyle factors and the urinary 8‐OH‐dG level or the urinary m7Gua level are shown in Table 1. The ANOVA analysis revealed that the urinary 8‐OH‐dG level was significantly negatively related to fruit consumption (P = 0.03) and physical activity‐1 (P = 0.03). It is noteworthy that the urinary 8‐OH‐dG levels of the ‘rarely’ and ‘two or three times per week’ groups were significantly higher than that of the ‘everyday’ group (P = 0.03) in the fruits item. The results of the Scheffe's test also indicated that fruit consumption significantly reduced the urinary 8‐OH‐dG level. On the other hand, only the intake of meat, fish, egg, soybean, etc. significantly influenced the m7Gua excretion (P < 0.05). Although the Scheffe's test was conducted to facilitate multiple comparisons of the urinary m7Gua levels between the groups, no significant differences were observed in all categorical variables.
Table 2 shows the correlations of the continuous variables to the 8‐OH‐dG (µg/g creatinine) and m7Gua (mg/g creatinine) levels. Significant positive correlations were observed between the urinary 8‐OH‐dG level and the average number of cigarettes smoked per day (r = 0.088, P = 0.023) and the Brinkman index (r = 0.082, P = 0.024), whereas there were significant inverse correlations between the urinary 8‐OH‐dG level and physical activity‐2 (r = –0.069, P = 0.049). In contrast, more factors affect the m7Gua levels. Namely, significant positive correlations were observed between the urinary m7Gua level and age (r = 0.190, P < 0.001), the average number of cigarettes smoked per day (r = 0.247, P < 0.001) and the Brinkman index (r = 0.278, P < 0.001), whereas significant inverse correlations were observed between the urinary m7Gua level and weight (r = –0.094, P = 0.008), BMI (r = –0.078, P = 0.028), total energy consumed (r = –0.086, P = 0.015), and the total score obtained from the meal index (r = –0.077, P = 0.036). In particular, the relationships of age, cigarettes smoked per day and Brinkman index with urinary m7Gua excretion were remarkable, as shown in 1, 2, 3, respectively.
Table 2.
Association of 8‐hydroxydeoxyguanosine (8‐OH‐dG) and 7‐methylguanine (m7Gua) with continuous variables
| Variables | Mean ± SE | Min | Max | Correlation coefficient | |||
|---|---|---|---|---|---|---|---|
| 8‐OH‐dG | P | m7Gua | P | ||||
| Age | 36.28 ± 0.54 | 18 | 59 | –0.014 | 0.698 | 0.190 | <0.001 |
| Weight | 67.14 ± 0.52 | 45.6 | 104 | –0.033 | 0.343 | –0.094 | 0.008 |
| BMI | 22.63 ± 0.16 | 15.4 | 34.0 | –0.065 | 0.066 | –0.078 | 0.028 |
| Energy consumed | 2487.10 ± 21.72 | 1892 | 3916 | –0.069 | 0.049 | –0.086 | 0.015 |
| Physical activity‐2 | 82.92 ± 5.90 | 3 | 1207 | 0.040 | 0.258 | –0.057 | 0.11 |
| Alcohol drinking | 0.89 ± 0.05 | 0 | 4.3 | 0.065 | 0.073 | 0.047 | 0.191 |
| Smoking | 15.50 ± 0.57 | 0 | 40 | 0.088 | 0.023 | 0.247 | <0.001 |
| Brinkman index | 259.79 ± 14.45 | 0 | 1520 | 0.082 | 0.024 | 0.278 | <0.001 |
| Rest index score | 11.75 ± 0.09 | 6 | 15 | 0.069 | 0.071 | –0.005 | 0.902 |
| Dietary index score | 21.56 ± 0.16 | 13 | 30 | –0.011 | 0.757 | –0.077 | 0.036 |
Figure 1.
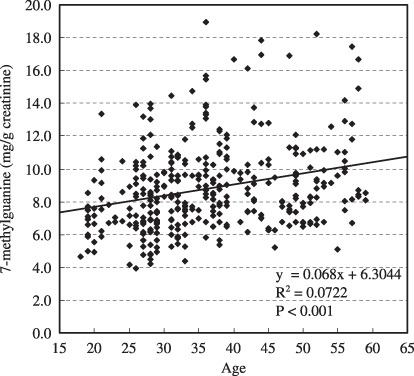
Association between age and urinary 7‐methylguanine level.
Figure 2.
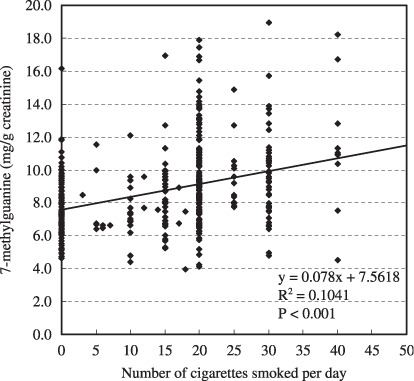
Association between cigarettes smoked per day and urinary 7‐methylguanine level.
Figure 3.
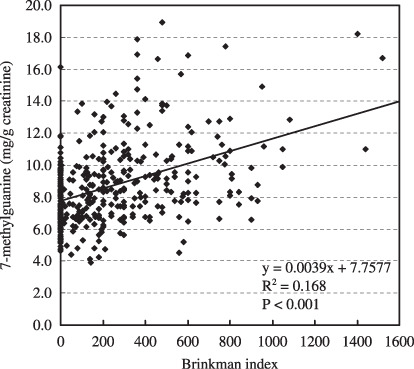
Association between Brinkman index and the urinary 7‐methylguanine level.
The results of the multiple regression analysis of 8‐OH‐dG by the stepwise method in the 361 male subjects are shown in Table 3. Due to the significant correlation between fatigue and rest score (r = –0.728, P < 0.001), the fatigue item was not used in the analysis, to avoid collinearity. Similarly, there was a significant correlation between cigarettes smoked per day and Brinkman index (r = 0.725, P < 0.001), so the Brinkman index item was not included in the analysis. Accordingly, the following 24 items were used in the analysis as the independent variables for the subjects with complete data: 15 categorical variables consisting of sleep, holiday, rhythm of daily life, ability to refresh, size of a meal, healthy meal combination, frequency of skipping meals, consumption of light‐colored vegetables, green‐ and yellow‐colored vegetables, fruit, meat, milk, oil, seaweed, and physical activity‐1, and nine continuous variables consisting of age, weight, BMI, energy consumption, total energy consumed, alcohol drinking, cigarette smoking, rest index score, and dietary index score. The results of the multiple regression analysis by the stepwise method indicated that physical activity‐1 and healthy meal combination decreased the urinary 8‐OH‐dG level, whereas alcohol drinking significantly increased it. The consumption of meat, fish, egg, soybean, etc. showed a tendency to reduce the 8‐OH‐dG level, and the intakes of green‐ and yellow‐colored vegetables showed a tendency of increasing it. These five independent factors obtained from the multiple regression analysis (Table 3) explain only 5.6% of the total variance. On the other hand, the total energy consumed, cigarette smoking, and BMI were not correlated with the urinary 8‐OH‐dG level.
Table 3.
Multiple regression analysis of log (8‐OH‐dG) against related factors in 361 male subjects
| Independent variables Male subjects (n = 361, R 2 = 0.056) | Partial r | SE | Beta | P |
|---|---|---|---|---|
| Physical activity‐1 | –0.065 | 0.020 | –0.169 | 0.001 |
| Alcohol drinking | 0.060 | 0.020 | 0.156 | 0.003 |
| Healthy meal combination | –0.065 | 0.030 | –0.117 | 0.034 |
| Intakes of meat, fish, egg, soybean, etc. | –0.066 | 0.035 | –0.100 | 0.064 |
| Green‐ and yellow‐colored vegetable consumption | 0.680 | 0.038 | 0.101 | 0.076 |
8‐OH‐dG, 8‐hydroxydeoxyguanosine. Note: Statistical analysis was conducted by a stepwise multiple regression analysis. Partial r indicates partial regression coefficient. Beta indicates standardized partial regression coefficient.
Table 4 shows the results of the multiple regression analysis using m7Gua as the dependent variable. The 24 items as above were used in the regression analysis as the independent variables. As a result, cigarette smoking and age were significantly correlated to the urinary m7Gua level, whereas high BMI and dietary index score (healthy meal style) were negatively correlated to it. These four independent factors obtained from the multiple regression analysis explain 19.6% of the entire variation.
Table 4.
Multiple regression analysis of log (m7Gua) against related factors in 361 male subjects
| Independent variables Male subjects (n = 361, R 2 = 0.196) | Partial r | SE | Beta | P |
|---|---|---|---|---|
| Smoking | 0.070 | 0.001 | 0.281 | <0.001 |
| Age | 0.080 | 0.001 | 0.281 | <0.001 |
| BMI | –0.012 | 0.005 | –0.125 | 0.010 |
| Dietary index score | –0.010 | 0.005 | –0.113 | 0.026 |
| Frequency of holiday | –0.069 | 0.036 | –0.092 | 0.058 |
m7Gua, 7‐methylguanine. Note: Statistical analysis was conducted by a stepwise multiple regression analysis. Partial r indicates partial regression coefficient. Beta indicates standardized partial regression coefficient.
Discussion
In this article, we analyzed how the urinary 8‐OH‐dG and m7Gua levels are related to various lifestyle factors. In the univariate analysis of the urinary 8‐OH‐dG level by the lifestyle and demographic variables, we found a decrease in the urinary 8‐OH‐dG level with fruit consumption and daily physical activity. Many studies have shown significant relationships between the dietary consumption of fruits and vegetables and the low urinary excretion of 8‐OH‐dG,( 7 , 32 , 33 ) although other studies found no associations between fruits and vegetables and 8‐OH‐dG.( 34 , 35 )
According to Kendall's rank correlation coefficients (Table 2), the urinary 8‐OH‐dG level was inversely correlated with the total energy consumed. On the other hand, factors positively related to the urinary 8‐OH‐dG level were cigarettes smoked per day and Brinkman index. Significant relationships between the urinary 8‐OH‐dG level and cigarette smoking have been observed not only in urine,( 17 ) but also in leukocytes,( 36 ) and lung tissue,( 13 ) However, alcohol consumption was not significantly correlated to urinary 8‐OH‐dG excretion. Similarly, we did not obtain a significant association between the urinary 8‐OH‐dG level and the rest index, while good correlations were reported between the urinary 8‐OH‐dG level and the average number of working hours per day,( 37 ) and the working conditions.( 17 )
In our previous work,( 17 , 37 ) and the report by Loft et al.( 7 ) there were significant negative correlations between 8‐OH‐dG and BMI. In the present study, the same tendency was observed in the univariate analysis (r = –0.065, P = 0.066) (Table 2), but a significant association was not observed in the multiple regression analysis. This discrepancy may be due to differences in statistical calculation methods and in other lifestyle and demographic factors between the present and previous studies. Our present results are consistent with those reported by Pilger et al.( 38 )
In the multiple regression analysis (Table 3), physical activity‐1, which includes physical activity by working, showed a strong negative correlation to the urinary 8‐OH‐dG. This is in good agreement with our previous findings that physical exercise reduced the 8‐OH‐dG levels in rat organs (liver, lung and heart),( 39 ) human urine,( 17 ) and human leukocyte,( 40 ) although high‐intensity exercise has been shown to increase 8‐OH‐dG excretion.( 41 , 42 , 43 ) Alcohol drinking also correlated with the 8‐OH‐dG level in the multiple regression analysis. Many studies have shown a significant relationship between alcohol consumption and 8‐OH‐dG generation in peripheral leukocytes,( 44 , 45 ) esophageal tissues,( 46 ) liver,( 47 ) and urine.( 48 , 49 ) Cigarette smoking was not related to the urinary 8‐OH‐dG level, whereas it was correlated with the urinary 8‐OH‐dG level, according to the calculation with continuous variables (Kendall's correlation coefficients, Table 2). The discrepancy between the current results and those from other investigations can be explained by variations in sample size, sample composition, methods of urinary 8‐OH‐dG measurement and statistical methods.
In terms of urinary m7Gua measurement results, the categorical lifestyle item related to the elevation of urinary m7Gua excretion was the intake of meat, fish and other protein‐rich foods. It is possible that N‐nitroso compounds that methylate DNA are produced by the consumption of these foods.( 50 , 51 )
In the analysis of continuous variables, age, cigarette smoking and Brinkman index were positively correlated with the urinary m7Gua level, whereas weight, BMI, total energy consumed, and total meal index score were negatively correlated to the amount of m7Gua. Particularly, the multiple regression analysis showed that cigarette smoking, age, BMI and meal index score were related to the urinary amount of m7Gua. These factors explain 19.6% of the entire variation. The inverse correlation between m7Gua and BMI can be explained by the fact that m7Gua is a marker of the metabolic rate, and it is lower in people with a high BMI, mainly due to the lower physiological production of heat to maintain body temperature.( 52 )
Previous studies have shown the strong link between cigarette smoking and the urinary m7Gua level. For instance, methylated DNA adducts were detected in animal and human tissues, as a result of exposure to tobacco smoke.( 19 , 53 ) In other studies, the urinary excretion of m7Gua was shown to be higher in smokers than in non‐smokers.( 29 ) Furthermore, the m7Gua level in human urine decreased after smoking cessation.( 54 ) Therefore, our results confirmed those of previous studies. Moreover, considering that tobacco‐specific nitrosamines are a group of carcinogens present in tobacco smoke,( 55 ) the urinary m7Gua level can be analyzed to monitor DNA methylation and to assess the risk of lung cancers. The measurment of urinary m7Gua levels would be useful not only to assess the harmful effects of smoking, but also the effects of environmental tobacco smoke.
The present analyses revealed that the urinary m7Gua level was linked to age, food‐related items, such as meat intake, weight, BMI, total energy consumed, and the meal index score. With respect to the effect of age, m7Gua may be increased due to lower glutathione (GSH) concentration in aged people,( 56 ) because GSH may be involved in scavenging alkylating agents. Ames and collaborators( 57 ) reported that the m7Gua levels in rat liver DNA were increased 2.5‐fold in old rats (24 months old) as compared to the levels in young rats (6 months old). Our results are compatible with their data. It has been argued that age can affect the overall DNA repair capacity. Thus, the amount of m7Gua in DNA could reflect a balance between methylating stress and DNA repair activity. However, urinary m7Gua may be related to the total amount of m7Gua released from DNA, by repair and by spontaneous depurination due to the labile glycosylic bond.
In our study, the creatinine value was used to normalize the urinary m7Gua level. Urinary creatinine excretion is influenced by muscle mass. This may be a possible explanation for the higher levels of m7Gua normalized to creatinine with increasing age. To clarify this point, we conducted a correlation analysis between creatinine and age. Although significant associations were obtained not only between age and m7Gua, but also between age and creatinine, the association between age and m7Gua (r 2 = 0.08) was stronger than the association between age and creatinine (r 2 = 0.01). Considering statistical values (r 2), we decided that the present results are not remarkably influenced by the association between age and creatinine.
We also found a significant correlation between the 8‐OH‐dG and m7Gua concentrations when the Pearson's correlation coefficient was calculated (r = 0.122, r 2 = 0.015, P = 0.02). This may be explained by the fact that some factors, such as energy consumed, physical activity and smoking, have similar effects on the 8‐OH‐dG and m7Gua levels (Table 2). However, the coefficient of determination (r 2) explains only 1.5% of the entire variation. Therefore, 8‐OH‐dG and m7Gua can be considered as independent markers affected by many factors.
The present study suggested that the amount of m7Gua excreted in the urine is a very sensitive marker in response to aging and lifestyle, such as smoking or dietary habits. Lifestyle has a more significant effect on urinary m7Gua than 8‐OH‐dG, based on all statistical analyses. The urinary excretion of m7Gua has not been extensively investigated as a biomarker,( 58 ) except for the influences of smoking.( 29 , 54 ) In this study, urine samples from male working subjects at a specific company were analyzed. In the future, in order to prove the usefulness of urinary m7Gua as a biomarker, we should confirm the reliability and validity of the present findings, according to appropriately designed large‐scale studies.
We demonstrated that urinary m7Gua is a useful biomarker for DNA methylation in humans, in addition to 8‐OH‐dG, a form of oxidative DNA damage. The urinary m7Gua excretion value can be a useful marker not only for the assessment of lung cancer risk, but also for evaluating the aging process and various lifestyles.
Acknowledgments
This work was supported in part by a grant from the Smoking Research Foundation.
References
- 1. Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet 1994; 10: 721–4. [DOI] [PubMed] [Google Scholar]
- 2. Kasai H. Analysis of a form of oxidative DNA damage, 8‐hydroxy‐2′‐deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 1997; 387: 147–63. [DOI] [PubMed] [Google Scholar]
- 3. Shigenaga MK, Ames BN. Assays for 8‐hydroxy‐2′‐deoxyguanosine: a biomarker of in vivo oxidative DNA damage. Free Radic Biol Med 1991; 10: 211–6. [DOI] [PubMed] [Google Scholar]
- 4. Wood ML, Dizdaroglu M, Gajewski E, Essigmann JM. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8‐hydroxyguanine (7‐hydro‐8‐oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry 1990; 29: 7024–32. [DOI] [PubMed] [Google Scholar]
- 5. Cheng KC, Cahill DS, Kasai H, Nishimura S, Loeb LA. 8‐Hydroxyguanine, an abundant form of oxidative DNA damage, causes G→T and A→C substitutions. J Biol Chem 1992; 5: 166–72. [PubMed] [Google Scholar]
- 6. Kasai H. A new automated method to analyze urinary 8‐hydroxydeoxyguanosine by a high‐performance liquid chromatography‐electrochemical detector system. J Radiat Res 2003; 44: 185–9. [DOI] [PubMed] [Google Scholar]
- 7. Loft S, Vistisen K, Ewertz M, Tjonneland A, Overvad K, Poulsen HE. Oxidative DNA damage esitimated by 8‐hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Calcinogenesis 1992; 13: 2241–7. [DOI] [PubMed] [Google Scholar]
- 8. Ravanat JL, Duretz B, Guiller A, Douki T, Cadet J. Isotope dilution high‐performance liquid chromatography‐electrospray tandem mass spectrometry assay for the measurement of 8‐oxo‐7,8‐dihydro‐2′‐deoxyguanosine in biological samples. J Chromatogr B Biomed Sci Appl 1998; 18: 349–56. [DOI] [PubMed] [Google Scholar]
- 9. Holmberg I, Stal P, Hamberg M. Quantitative determination of 8‐hydroxy‐2′‐deoxyguanosine in human urine by isotope dilution mass spectrometry: normal levels in hemochromatosis. Free Radic Biol Med 1999; 26: 129–35. [DOI] [PubMed] [Google Scholar]
- 10. Witherell HL, Hiatt RA, Replogle M, Parsonnet J. Helicobacter pylori infection and urinary excretion of 8‐hydroxy‐2‐deoxyguanosine, an oxidative DNA adduct. Cancer Epidemiol Biomarkers Prev 1998; 7: 91–6. [PubMed] [Google Scholar]
- 11. Shimoi K, Kasai H, Yokota N, Toyokuni S, Kinae N. Comparison between high‐performance liquid chromatography and enzyme‐linked immunosorbent assay for the determination of 8‐hydroxy‐2′‐deoxyguanosine in human urine. Cancer Epidemiol Biomarkers Prev 2002; 11: 767–70. [PubMed] [Google Scholar]
- 12. Yoshida R, Ogawa Y, Kasai H. Urinary 8‐oxo‐7,8‐dihydro‐2′‐deoxy‐ guanosine values measured by an ELISA correlated well with measurements by high‐performance liquid chromatography with electrochemical detection. Cancer Epidemiol Biomarkers Prev 2002; 11: 1076–81. [PubMed] [Google Scholar]
- 13. Asami S, Manabe H, Miyake J et al . Cigarette smoking induces an increase in oxidative DNA damage, 8‐hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis 1997; 18: 1763–6. [DOI] [PubMed] [Google Scholar]
- 14. Shimoda R, Nagashima M, Sakamoto M et al . Increased formation of oxidative DNA damage, 8‐hydroxydeoxyguanosine, in human livers with chronic hepatitis. Cancer Res 1994; 15: 3171–2. [PubMed] [Google Scholar]
- 15. Baik SC, Youn HS, Chung MH et al . Increased oxidative DNA damage in Helicobacter pylori‐infected human gastric mucosa. Cancer Res 1996; 15: 1279–82. [PubMed] [Google Scholar]
- 16. Tagesson C, Källberg M, Klintenberg C, Starkhammar H. Determination of urinary 8‐hydroxydeoxyguanosine by automated coupled‐column high performance liquid chromatography: a powerful technique for assaying in vivo oxidative DNA damage in cancer patients. Eur J Cancer 1995; 31: 934–40. [DOI] [PubMed] [Google Scholar]
- 17. Kasai H, Iwamoto‐Tanaka N, Miyamoto T et al . Life style and urinary 8‐hydroxydeoxyguanosine, a marker of oxidative DNA damage: effects of exercise, working conditions, meat intake, body mass index, and smoking. Jpn J Cancer Res 2001; 92: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shar M, Miller DS, Geissler CA. Lowere metabolic rate of post obese versus lean women: thermogenesis, basal metabolic rate and genetics. Eur J Nutr 1988; 42: 741–52. [PubMed] [Google Scholar]
- 19. Hecht SS. DNA adduct formation from tobacco‐specific N‐nitrosamines. Mutat Res 1999; 424: 127–42. [DOI] [PubMed] [Google Scholar]
- 20. Guillemin MP, Hillier RS, Bernhard CA. Occupational and environmental hygiene assessment of fumigations with methyl bromide. Ann Occup Hyg 1990; 34: 591–607. [DOI] [PubMed] [Google Scholar]
- 21. Rydberg B, Lindahl T. Nonenzymatic methylation of DNA by the intracellular methyl group donor S‐adenosyl‐L‐methionine is a potentially mutagenic reaction. EMBO J 1982; 1: 211–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Topp H, Schöch G. Whole‐body degradation rates of transfer‐, ribosomal‐, and messenger ribonucleic acids and resting metabolic rate in 3‐ to 18‐year‐old humans. Pediatr Res 2000; 47: 163–8. [DOI] [PubMed] [Google Scholar]
- 23. Sander G, Topp H, Heller‐Schöch G, Wieland J, Schöch G. Ribonucleic acid turnover in man. RNA catabolites in urine as measure for the metabolism of each of the three major species of RNA. Clin Sci 1986; 71: 367–74. [DOI] [PubMed] [Google Scholar]
- 24. Shuker DE, Farmer PB. Relevance of urinary DNA adducts as markers of carcinogen exposure. Chem Res Toxicol 1992; 5: 450–60. [DOI] [PubMed] [Google Scholar]
- 25. Shaikh B, Huang SS, Pontzer NJ. Urinary excretion of methylated purines and 1‐methyl‐nicotinamide following administration of methylating carcinogens. Chem Biol Interact 1980; 30: 253–6. [DOI] [PubMed] [Google Scholar]
- 26. Farmer PB, Shuker EG, Bird I. DNA and protein adducts as indicators of in vivo methylation by nitrosatable drugs. Carcinogenesis 1986; 7: 49–52. [DOI] [PubMed] [Google Scholar]
- 27. Porcelli B, Muraca LF, Frosi B et al . Fast‐atom bombardment mass spectrometry for mapping of endogenous methylated purine bases in urine extracts. Rapid Commun Mass Spectrom 1997; 11: 398–404. [DOI] [PubMed] [Google Scholar]
- 28. Wishnok JS, Tannenbaum SR, Stillwell WG, Glogowski JA, Leaf CD. Urinary markers for exposures to alkylating or nitrosating agents. Environ Health Perspect 1993; 99: 155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stillwell WG, Glogowski J, Xu HX et al . Urinary excretion of nitrate, N‐nitrosoproline, 3‐methyladenine, and 7‐methylguanine in a Colombian population at high risk for stomach cancer. Cancer Res 1991; 1: 190–4. [PubMed] [Google Scholar]
- 30. Svoboda P, Kasai H. Simultaneous HPLC analysis of 8‐hydroxydeoxyguanosine and 7‐methylguanine in urine from humans and rodents. Anal Biochem 2004; 334: 239–50. [DOI] [PubMed] [Google Scholar]
- 31. Kasai H, Svoboda P, Yamasaki S, Kawai K. Simultaneous determination of 8‐hydroxydeoyguanosine, a marker of oxidative stress, and creatinine, a standardization compound, in urine. Ind Health 2005; 43: 333–6. [DOI] [PubMed] [Google Scholar]
- 32. Thompson HJ, Heimendinger J, Haegele A et al . Effect of increased vegetable and fruit consumption on markers of oxidative cellular damage. Carcinogenesis 1999; 20: 2261–6. [DOI] [PubMed] [Google Scholar]
- 33. Kiefer I, Prock P, Lawrence C et al . Supplementation with mixed fruit and vegetable juice concentrates increased serum antioxidants and folate in healthy adults. J Am Coll Nutr 2004; 23: 205–11. [DOI] [PubMed] [Google Scholar]
- 34. Loft S, Poulsen HE. Antioxidant intervention studies related to DNA damage, DNA repair and gene expression. Free Radic Res 2000; 33: 67–83. [PubMed] [Google Scholar]
- 35. Møller P, Vogel U, Pedersen A, Dragsted LO, Sandström B, Loft S. No effect of 600 grams fruit and vegetables per day on oxidative DNA damage and repair in healthy nonsmokers. Cancer Epidemiol Biomarkers Prev 2003; 12: 1016–22. [PubMed] [Google Scholar]
- 36. Asami S, Hirano T, Yamaguchi R, Tomioka Y, Itoh H, Kasai H. Increase of a type of oxidative DNA damage, 8‐hydroxyguanine, and its repair activity in human leukocytes by cigarette smoking. Cancer Res 1996; 56: 2546–9. [PubMed] [Google Scholar]
- 37. Irie M, Tamae K, Iwamoto‐Tanaka N, Kasai H. Occupational and lifestyle factors and urinary 8‐hydroxydeoxyguanosine. Cancer Sci 2005; 96: 600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pilger A, Germadnik D, Riedel K, Meger‐Kossien I, Scherer G, Rudiger HW. Longitudinal study of urinary 8‐hydroxy‐2′‐deoxyguanosine excretion in healthy adults. Free Radic Res 2001; 35: 273–80. [DOI] [PubMed] [Google Scholar]
- 39. Asami S, Hirano T, Yamaguchi R, Tsurudome Y, Itoh H, Kasai H. Effects of forced and spontaneous exercise on 8‐hydroxydeoxyguanosine level in rat organ. Biochem Biophys Res Commun 1998; 243: 678–82. [DOI] [PubMed] [Google Scholar]
- 40. Asami S, Hirano T, Yamaguchi R, Itoh H, Kasai H. Reduction of 8‐hydroxyguanine in human leukocyte DNA by physical exercise. Free Rad Res 1998; 29: 581–4. [DOI] [PubMed] [Google Scholar]
- 41. Poulsen HE, Loft S, Vistisen K. Extreme exercise and oxidative DNA modification. J Sports Sci 1996; 14: 343–6. [DOI] [PubMed] [Google Scholar]
- 42. Radák Z, Pucsuk J, Boros S, Josfai L, Taylor AW. Changes in urine 8‐hydroxydeoxyguanosine levels of super‐marathon runners during a four‐day race period. Life Sci 2000; 24: 1763–7. [DOI] [PubMed] [Google Scholar]
- 43. Møller P, Loft S, Lundby C, Olsen NV. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J 2001; 15: 1181–6. [DOI] [PubMed] [Google Scholar]
- 44. Nakajima M, Takeuchi T, Takeshita T, Morimoto K. 8‐hydroxydeoxyguanosine in human leukocyte DNA and daily health practice factors: effects of individual alcohol sensitivity. Environ Health Perspect 1996; 104: 1336–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Irie M, Asami S, Nagata S, Miyata M, Kasai H. Psychosocial factors as a potential trigger of oxidative DNA damage in human leukocytes. Jpn J Cancer Res 2001; 92: 367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Asami S, Hirano T, Yamaguchi R, Tsurudome Y, Itoh H, Kasai H. Increase in 8‐hydroxyguanine and its repair activity in the esophagi of rats given long‐term ethanol and nutrition‐deficient diet. Jpn J Cancer Res 2000; 91: 973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cahill A, Wang X, Hoek JB. Increased oxidative DNA damage to mitochondrial DNA following chronic ethanol consumption. Biochem Biophys Res Commun 1997; 235: 286–90. [DOI] [PubMed] [Google Scholar]
- 48. Wong RH, Yeh CY, Hsueh YM, Wang JD, Lei YC, Cheng TJ. Association of hepatitis virus infection, alcohol consumption and plasma vitamin A level with urinary 8‐hydroxydeoxyguanosine in chemical workers. Mutat Res 2003; 535: 181–6. [DOI] [PubMed] [Google Scholar]
- 49. Kuo HW, Chang SF, Wu KY, Wu FY. Chromium (VI) induced oxidative damage to DNA; increase of urinary 8‐hydroxydeoxyguanosine concentration (8‐OHdG) among electroplating workers. Occup Environ Med 2003; 60: 590–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hughes R, Cross AJ, Pollock JR, Bingham S. Dose‐dependent effect of dietary meat on endogenous colonic N‐nitrosation. Carcinogenesis 2001; 22: 199–202. [DOI] [PubMed] [Google Scholar]
- 51. Chen CS, Pignatelli B, Malaveille C et al . Levels of direct‐acting mutagens, total N‐nitroso compounds in nitrosated fermented fish products, consumed in a high‐risk area for gastric cancer in southern China. Mutat Res 1992; 265: 211–21. [DOI] [PubMed] [Google Scholar]
- 52. Shah M, Miller DS, Geissler CA. Lower metabolic rates of post‐obese versus lean women: Thermogenesis, basal metabolic rate and genetics. Eur J Clin Nutr 1988; 42: 741–52. [PubMed] [Google Scholar]
- 53. Mustonen R, Hemminki K. 7‐Methylguanine levels in DNA of smokers’ and non‐smokers’ total white blood cells, granulocytes and lymphocytes. Carcinogenesis 1992; 13: 1951–5. [DOI] [PubMed] [Google Scholar]
- 54. Ichiba M, Matsumoto A, Kondoh T, Horita M, Tomokuni K. Decreasing urinary PAH metabolites and 7‐methylguanine after smoking cessation. Int Arch Occup Environ Health 2006; 79: 545–9. [DOI] [PubMed] [Google Scholar]
- 55. Hecht SS, Hoffmann D. Tobacco‐specific nitrosamines, an important group of carcinogens in tobacco and tobacco smoke. Carcinogenesis 1988; 9: 875–84. [DOI] [PubMed] [Google Scholar]
- 56. Loguercio C, Taranto D, Vitale LM, Beneduce F, Del Vecchio Blanco C. Effect of liver cirrhosis and age on the glutathione concentration in the plasma, erythrocytes, and gastric mucosa of man. Free Rad Biol Med 1996; 20: 483–8. [DOI] [PubMed] [Google Scholar]
- 57. Park JW, Ames BN. 7‐Methylguanine adducts in DNA are normally present at high levels and increase on aging: analysis by HPLC with electrochemical detection. Proc Natl Acad Sci 1988; 85: 7467–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Loft S, Svoboda P, Kasai H et al . Prospective study of urinary excretion of 7‐methylguanine and the risk of lung cancer: Effect modification by mu class glutathione‐S‐transferases. Int J Cancer 2007; 121: 1579–84. [DOI] [PubMed] [Google Scholar]


