Abstract
Sphingolipids display a wide spectrum of biological activities, including cell growth, differentiation and apoptosis. However, precise mechanisms by which these compounds exert anticancer or cancer‐preventive effects are not known. In the present study, we evaluated the preventive efficacy of enriched dietary monoglucosylceramide 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine (G1CM) on 1,2‐dimethylhydrazine (DMH)‐induced aberrant crypt foci (ACF) and β‐catenin‐accumulated crypt (BCAC) formation in F344 rats during initiation stage. We also examined whether G1CM affects cell proliferation and apoptosis in these lesions. Pure G1CM was isolated from rice bran. Forty‐two rats were divided randomly into five experimental groups. Rats in groups 1–3 were given subcutaneous injections of DMH (40 mg/kg body weight) once a week for 2 weeks. One week before the first injection of DMH, rats in groups 2 and 3 were fed a diet containing 200 and 1000 p.p.m. G1CM, respectively, for 5 weeks. Rats in group 4 were fed a diet containing 1000 p.p.m. G1CM. Rats in group 5 were given the basal diet alone and served as untreated controls. The experiment was terminated 5 weeks after the start. Dietary G1CM at both doses (groups 2 and 3) significantly inhibited the induction of ACF and BCAC (P < 0.001) when compared to group 1 treated with DMH alone. In groups 2 and 3, the proliferating cell nuclear antigen labeling indices of epithelial cells in ACF and BCAC were also lower than in group 1 (P < 0.0001 for ACF, P < 0.05 for BCAC). These results, that dietary G1CM has possible chemopreventive effects in the present short‐term colon carcinogenesis bioassays, suggest that longer exposure may cause suppression of tumor development. (Cancer Sci 2005; 96: 876–881)
Sphingolipids are a group of structural and functional derivatives that have a long‐chain (sphingoid) base backbone and exhibit a variety of biological activities.( 1 , 2 , 3 ) In most sphingolipids, the amino group is substituted with a long‐chain fatty acid (these compounds are termed ceramide) and there is a group at position 1, such as phosphocholine in sphingomyelin (Fig. 1).( 1 , 4 ) Milk, eggs and soybeans appear to be rich sources although a limited number of foods have been analyzed for the amount and type of sphingolipids.( 1 , 4 ) Fujino et al. reported that ceramide and glucosylceramide (cerebroside) are included in the bran and endoderm of rice grains.( 5 ) Because some sphingolipids, including ceramide and glucosylceramide, display a broad range of biological activities related to cell growth, differentiation and apoptosis,( 3 ) they may play an important role in all steps of carcinogenesis.( 6 , 7 ) In fact, colonic cell proliferation and aberrant crypt foci (ACF) formation are inhibited by dietary glycosphingolipids in 1,2‐dimethylhydrazine (DMH)‐treated CF1 mice.( 8 , 9 ) In addition, synthetic sphingomyelins inhibit DMH‐induced ACF formation( 10 ) and dietary sphingomyelin suppresses colon carcinogenesis induced by DMH in CF1 mice.( 11 , 12 , 13 ) It has also been shown that sphingoid bases and ceramides enhance retinoic acid‐induced differentiation in the HL60 human leukemia cell line,( 14 ) and induce apoptosis in HT29 and HCT116 human colon carcinoma cell lines.( 15 ) Although the above‐described findings obtained with sphingolipids in experimental studies are promising, the precise mechanisms by which this complex causes tumor suppression are not known.
Figure 1.
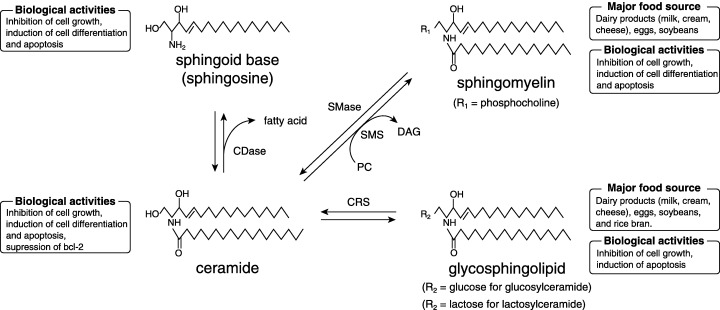
General structures, metabolism, biological activities and food sources of major sphingolipids. CDase, ceramidase; CRS, cerebrosidase; DAG, diacylglycerol; PC, phosphatidylcholine; SMase, sphingomyelinase; SMS, sphingomyelin synthase.
Aberrant crypt foci are putative preneoplastic lesions of the colonic mucosa in mice, rats and humans.( 16 , 17 , 18 , 19 , 20 , 21 ) In the rat colonic mucosa, focal lesions that display accumulation of β‐catenin protein are termed β‐catenin accumulated crypts (BCAC).( 22 , 23 ) Also, these lesions have been shown to have frequent β‐catenin gene mutations.( 22 ) Thus, BCAC are thought to be independent precursor lesions. Both ACF and BCAC have been used as valuable biomarkers for short‐term colon carcinogenesis bioassays and are also useful to examine possible chemopreventive effects of a wide variety of candidate agents.( 17 , 24 )
In the present study, we examined whether monoglucosylceramide 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine (G1CM) has short‐term chemopreventive effects on two different categories of putative preneoplastic lesions such as ACF and BCAC involved in rat colon carcinogenesis. We have also examined whether this compound affects cell growth and apoptosis by measuring proliferating cell nuclear antigen (PCNA)‐positive cells and apoptotic bodies in epithelial cells consisting of the crypts of ACF and BCAC.
Materials and Methods
Animals
Four‐week‐old male F344 rats were purchased from Shizuoka Laboratory Animal Center (Hamamatsu, Japan). All rats were housed 3–4 per wire cage with free access to tap water and basal diet (CE2; CLEA Japan, Tokyo, Japan) under controlled conditions of humidity (50 ± 10%), lighting (12:12 h L:D cycle) and temperature (23 ± 2°C). The animals were maintained in the Animal Facility of the University of the Ryukyus according to the Institutional Animal Care Guidelines.
Treatment
Pure monoglucosylceramide 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine (Fig. 2) obtained from rice bran was provided by the Oryza Oil and Fat Chemical Company, (Aichi, Japan). Purity (> 93%) was determined by carrying out high‐performance liquid chromatographic (HPLC) analysis, and three major bands were detected. Of these, the highest peak contained G1CM. The remaining two major bands contained other sphingoid compounds that have different chemical structures from G1CM. Mass spectrometric analysis was then used to evaluate purity and provide detailed information about the molecular structure of G1CM. We used 200 and 1000 p.p.m. dose levels of G1CM in the diet because at this dose range there were obvious inhibitory effects of dietary sphingomyelin or glucosylceramide on colonic ACF formation and colon tumor development.( 8 , 12 , 13 ) Forty‐two rats were assigned randomly to the experimental groups (Fig. 3). Rats in groups 1–3 were given subcutaneous injections of DMH (40 mg/kg body weight) (Sigma Chemical Company, St Louis, MO, USA) once a week for 2 weeks. One week before the first injection of DMH, rats in groups 2 and 3 were fed a diet containing 200 and 1000 p.p.m. G1CM, respectively, for 5 weeks. Rats in group 4 were fed a diet containing 1000 p.p.m. G1CM. Rats in group 5 were given the basal diet alone and served as untreated controls. The rats were observed carefully and weighed weekly during the experiment. The experiment was terminated at 5 weeks after the start of the experiment. All animals were then killed under CO2 anesthesia. Colons were removed carefully, washed with saline, opened longitudinally, and then fixed with 10% buffered formalin.
Figure 2.
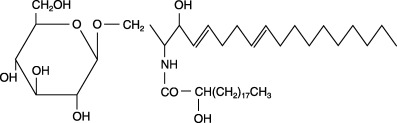
Chemical structure of 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine (G1CM).
Figure 3.
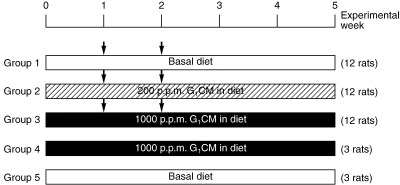
Experimental protocol. Arrow indicates 1,2‐dimethylhydrazine subcutaneous injection (40 mg/kg bodyweight). G1CM, 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine.
Identification of aberrant crypt foci and β‐catenin‐accumulated crypts
Colon tissues were stained in a 0.5% methylene blue solution for 30 s, immediately washed with distilled water and then placed on a glass plate with the mucosal surface up. Using a light microscope at a magnification of ×40, ACF were counted according to the criteria described earlier.( 25 ) After ACF counting, colon tissues were embedded in paraffin and processed for immunohistochemistry of BCAC and PCNA.( 26 ) Briefly, distal and middle segments of the colon were used for immunostaining. Serial sections (4‐µm thick) were prepared to include the middle portion between the surface and the bottom of the crypt. These sections were treated in 3% H2O2 for 20 min to block the endogenous peroxidase activity and then incubated with a primary antibody of the β‐catenin protein (1:100 dilution) (Transduction Laboratories, Lexington, KY, USA) at room temperature for 60 min. Sections were stained with a Simple Stain kit (Nichirei, Tokyo, Japan).
Immunohistochemical staining and measurement of proliferating cell nuclear antigen labeling index
Proliferating activity of cells in the colonic crypts was evaluated by measuring PCNA‐positive nuclei as described in a previous paper.( 26 ) In brief, after staining 4‐µm thick sections with an anti‐PCNA antibody (1:50 dilution) (Dako, Kyoto, Japan), at least 300 epithelial cells in ACF and BCAC were counted by a single reader who was blind to the treatment groups. PCNA labeling indices were calculated as the percentage of positive cells with respect to the total number of cells counted.
Detection of apoptosis
Apoptotic cells in ACF and BCAC were evaluated in sections stained with hematoxylin and eosin using light microscopy. Apoptotic cells were identified by cell shrinkage, nuclear condensation and apoptotic body formation.( 27 ) Apoptotic index was calculated as the percentage of apoptotic cells with respect to the total number of cells counted, as describe in a recent work.( 28 )
Statistical analysis
Statistical analyses by Student's or Welch's t‐test were carried out to determine the significance of the differences between groups. All statements of significance are P < 0.05.
Results
General observations
All rats survived to the end of the experiment and none of them developed colon tumors. To ascertain that dietary feeding of G1CM caused no toxic side‐effects on body weight gain, the rats were monitored on a routine basis. No significant effects of DMH or G1CM were seen on body, liver or kidney weight (Table 1). Food consumption of animals in the experimental groups did not vary. G1CM at 200 and 1000 p.p.m. caused no adverse side‐effects in any of the rats.
Table 1.
Body, liver and kidney weights of each experimental group at the end of the experiment
| Group | Treatment | Body weight (g) | Liver weight (g) | Kidney weight (g) | Relative liver weight (g) | Relative kidney weight (g) |
|---|---|---|---|---|---|---|
| 1 | DMH | 233 ± 14.6 | 8.53 ± 0.53 | 1.82 ± 0.13 | 3.65 ± 0.11 | 0.78 ± 0.04 |
| 2 | DMH + 200 p.p.m. G1CM | 232 ± 13.4 | 8.21 ± 0.39 | 1.74 ± 0.07 | 3.63 ± 0.15 | 0.75 ± 0.04 |
| 3 | DMH + 1000 p.p.m. G1CM | 235 ± 12.9 | 8.50 ± 0.70 | 1.79 ± 0.10 | 3.62 ± 0.20 | 0.76 ± 0.05 |
| 4 | 1000 p.p.m. G1CM | 237 ± 16.5 | 8.30 ± 0.75 | 1.79 ± 0.06 | 3.50 ± 0.09 | 0.76 ± 0.03 |
| 5 | Basal diet | 253 ± 9.1 | 9.34 ± 0.18 | 1.99 ± 0.5 | 3.74 ± 0.08 | 0.79 ± 0.07 |
Values are mean ± SD. DMH, 1,2‐dimethylhydrazine; G1CM, 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine.
Inhibitory effect of G1CM on aberrant crypt foci and β‐catenin‐accumulated crypts
In the current study, we used the well‐established and short‐term protocol of the rat colon carcinogenesis model system to examine the potential chemopreventive effect of G1CM. We chose ACF and BCAC as end points in this bioassay because ACF and BCAC are preneoplastic lesions and these lesions are regarded as useful biomarkers. All rats in groups 1–3 developed ACF and BCAC in the colonic mucosa. No ACF or BCAC were seen in any of the rats of groups 4 and 5. The number of ACF was significantly lower in DMH‐treated rats fed 200 and 1000 p.p.m. G1CM diet than that in controls (P < 0.0001; Table 2). The number of ACF containing one to three or more than four aberrant crypts was also decreased significantly in DMH‐treated rats fed both G1CM doses (P < 0.0001 and P < 0.05, respectively, Table 2). In groups 2 and 3, treatment of rats with both dose levels of G1CM caused a significant decrease in mean number of BCAC/cm2 colon, when compared with the control rats treated with DMH alone (group 1) (P < 0.05; Table 3). These results indicate that dietary G1CM significantly inhibited ACF and BCAC formation induced by DMH in F344 rats.
Table 2.
Inhibitory effect of dietary 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine (G1CM) on aberrant crypt foci (ACF) formation induced by 1,2‐dimethylhydrazine (DMH)
| Group | Treatment | No. ACF/colon | No. ACF containing one to three aberrant crypts/colon | No. ACF containing more than four aberrant crypts/colon |
|---|---|---|---|---|
| 1 | DMH | 196.6 ± 32.4 | 186.3 ± 29.2 | 10.4 ± 4.8 |
| 2 | DMH + 200 p.p.m. G1CM | 107.1 ± 15.0 † | 101.7 ± 14.3 † | 5.4 ± 4.4 ‡ |
| 3 | DMH + 1000 p.p.m. G1CM | 116.6 ± 21.7 † | 109.9 ± 22.3 † | 6.7 ± 3.1 ‡ |
| 4 | 1000 p.p.m. G1CM | 0 | 0 | 0 |
| 5 | Basal diet | 0 | 0 | 0 |
Significntly different from goup 1 by Student's t‐test, P < 0.0001.
Significntly different from goup 1 by Welch's t‐test, P < 0.0001. Values are mean ± SD.
Table 3.
Inhibitory effect of dietary 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine (G1CM) on β‐catenin‐accumulated crypt (BCAC) formation induced by 1,2‐dimethylhydrazine (DMH)
| Group | Treatment | Mean no. BCAC/cm2 colon |
|---|---|---|
| 1 | DMH | 0.63 ± 0.16 |
| 2 | DMH + 200 p.p.m. G1CM | 0.21 ± 0.05 † |
| 3 | DMH + 1000 p.p.m. G1CM | 0.20 ± 0.06 † |
| 4 | 1000 p.p.m. G1CM | 0 |
| 5 | Basal diet | 0 |
Significantly different from group 1 by Welch's t‐test, P < 0.05. Values are mean ± SD.
Inhibition of proliferating cell nuclear antigen labeling index by G1CM in aberrant crypt foci and β‐catenin‐accumulated crypts
To determine whether G1CM affects cell proliferation in ACF and BCAC, we measured the PCNA labeling index in these lesions by immunohistochemistry. In groups 2 and 3, treatment of rats with both dose levels of G1CM resulted in a significant decrease in the labeling index of PCNA in ACF and BCAC when compared to group 1 treated with DMH alone (P < 0.0001 for ACF and P < 0.05 for BCAC; Table 4). Moreover, the PCNA labeling index in BCAC of rats treated with DMH alone and in normal colonic mucosa of rats without treatment was 10.91 (Table 4) and 5.9 (data not shown), respectively. However, in groups 2 and 3 the PCNA labeling index was 5.44 and 5.56 in DMH‐treated rats fed 200 and 1000 p.p.m. G1CM, respectively (Table 4). These findings suggest that dietary G1CM can normalize elevation of cell proliferation induced by DMH.
Table 4.
Inhibition of proliferating cell nuclear antigen (PCNA) labeling index by dietary 1‐O‐β‐glucosyl‐N‐2′‐hydroxyarachidoyl‐4,8‐sphingadienine (G1CM) in the preneoplastic lesions aberrant crypt foci (ACF) and β‐catenin‐accumulated crypts (BCAC)
| Group | Treatment | PCNA labeling index in ACF | PCNA labeling index in BCAC | ||
|---|---|---|---|---|---|
| % | Crypts examined (n) | % | Crypts examined (n) | ||
| 1 | DMH | 3.00 ± 4.73 | 325 | 10.91 ± 9.39 | 51 |
| 2 | DMH + 200 p.p.m. G1CM | 1.22 ± 2.35 † | 246 | 5.44 ± 4.89 ‡ | 20 |
| 3 | DMH + 1000 p.p.m. G1M | 1.37 ± 2.89 † | 222 | 5.56 ± 6.57 § | 16 |
Significantly different from group 1 by Welch's t‐test, † P < 0.001, ‡ P < 0.005. §Significantly different from group 1 by Student's t‐test, P < 0.05. Values are mean ± SD.
Effect of G1CM on apoptosis.
Because G1CM is able to normalize elevation of cell proliferation in ACF and BCAC, we investigated whether this inhibition is due to the induction of apoptosis in these lesions. There was a clear tendency that an increase in apoptotic index of BCAC is dose dependent of G1CM compared to the control, but this was not statistically significant (Table 5). Regarding the apoptotic index of ACF, no clear tendency was seen. Apoptotic index in untreated normal mucosa was similar to that of group 3 (data not shown).
Table 5.
Apoptotic index in aberrant crypt foci (ACF) and β‐catenin‐accumulated crypts (BCAC)
| Group | Treatment | ACF | BCAC | ||
|---|---|---|---|---|---|
| % | Crypts examined (n) | % | Crypts examined (n) | ||
| 1 | DMH | 0.08 ± 0.49 | 325 | 0.47 ± 1.14 | 51 |
| 2 | DMH + 200 p.p.m. G1CM | 0.05 ± 0.47 | 246 | 0.62 ± 1.28 | 20 |
| 3 | DMH + 1000 p.p.m. G1CM | 0.03 ± 0.40 | 222 | 1.36 ± 2.19 | 16 |
Values are mean ± SD.
Discussion
There has been considerable interest in sphingolipids, including ceramide and glucosylceramide, in the prevention or therapy of malignant neoplasms. Despite the reported benefits of these compounds in experimental animal models or cell culture systems, it remains unclear whether these agents have cancer‐preventive properties and whether there is a molecular basis to their growth inhibitory effects on carcinoma cells. In parallel studies, we have also found possible cancer‐preventive effects of crude glycosphingolipid using a carcinogen‐induced rat colon carcinogenesis model (unpublished data). Therefore, we have focused on the effect of rice bran G1CM, a purified derivative of sphingolipids, on the earliest morphological changes of colonic cells to neoplasms such as ACF and BCAC, cell proliferation and apoptosis. We carried out the present studies to provide further evidence to support the use of this compound in cancer prevention. In the present study, G1CM was extracted from rice bran and purified using HPLC analysis. We found that dietary G1CM caused significant inhibition of ACF and BCAC formation in F344 rats. G1CM also normalized the elevation of DMH‐induced cell proliferation in ACF and BCAC. This result is in accordance with that of a previous report by Lemonnier et al.( 11 ) DMH is a commonly used carcinogen and enhances proliferation of cells constructing crypts in the rat colon.( 8 ) This agent also induces ACF and BCAC formation and tumor development in the rat colon.( 26 ) Cell proliferation is more enhanced in ACF, BCAC and tumor than in normal colonic mucosa.( 29 ) Taken together, cell proliferation appears to be an important aspect in the occurrence of these lesions. Dietary sphingolipids isolated from milk or soy suppress DMH‐induced ACF formation and cell proliferation of colonic crypts in CF1 mice.( 8 , 9 , 10 , 12 ) Also, dietary sphingomyelin and glucosylceramide inhibit the development of adenomas in multiple intestinal neoplasia (min) mice,( 12 ) and DMH‐induced adenocarcinomas in CF1 mice.( 11 , 13 ) Sphingosine, sphinganine and C2‐ceramide cause dose‐dependent growth inhibition and induce apoptosis in HT29 and HCT116 human colon carcinoma cell lines.( 15 ) Therefore, cell proliferation may play a critical role in suppression of ACF and BCAC formation by sphingolipids, including G1CM. In view of the above‐described results and discussions, G1CM may have a potent chemopreventive effect in this short‐term colon carcinogenesis bioassay system. In the present study, we did not find an appreciable contribution of G1CM to the induction of apoptosis but G1CM appears to increase the rate of apoptosis to the normal level. This aspect warrants further investigation, including long‐term carcinogenesis studies. In general, a 100–250 p.p.m. dose range of potent chemopreventive agents has been used in many experimental animal models.( 30 ) However, it is not known whether this is the maximal effect of G1CM because our data did not display a clear dose–response relationship in the inhibition of ACF and BCAC formation and cell proliferation. This is presumably due to the maximum tolerant dose of this compound and the small number of animals involved. To address these questions, more extensive dose–response studies should be conducted in a larger number of animals.
Schmelz et al. reported that up to approximately 88% of dietary sphingomyelin is degraded to ceramide or sphingoid bases in the small intestine, and the remaining 12% of dietary sphingomyelin is found in the cecum and colon.( 31 ) By this mechanism, colonic epithelial cells could be exposed to ceramide and sphingoid bases. These bioactive metabolites are taken up by cells in the colonic epithelium and affect a broad range of target molecules in cells through regulation of growth, differentiation and apoptosis.( 1 , 3 , 6 , 7 ) Also, most sphingolipids are hydrophobic, and are thus insoluble in aqueous solution. There have been no reports investigating the cancer‐preventive effects of G1CM. Therefore, whether this effect is confined to colon cancer requires further investigation. In the present study, we found that G1CM inhibited proliferation of epithelial cells in ACF and BCAC. However, G1CM did not inhibit the growth of SW480 human colon carcinoma and HL60 human leukemia cell lines (unpublished data). Therefore, further studies are in progress to examine whether rat colonic tissues are exposed to the effective concentrations of G1CM or to examine cell permeability in vitro.
The specific targets of dietary sphingolipids have not yet been identified because sphingolipids affect several signaling pathways.( 3 , 32 ) Schmelz et al.( 33 ) demonstrated that sphingolipids cause a marked redistribution of the β‐catenin protein from a diffuse (cytoplasm plus membrane) to a more normal (intercellular junctions) distribution in intestinal epithelial cells of C57BL/6JMin/+mice. We earlier demonstrated that the β‐catenin signaling pathway plays a major role in short‐term and long‐term colon carcinogenesis models induced by a specific carcinogen.( 22 , 34 , 35 ) In the present study, we found that dietary G1CM inhibited DMH‐induced colonic BCAC formation. Under microscopic examination in a pilot study, BCAC is likely to display junctional localization of the β‐catenin protein in crypt epithelial cells of BCAC exposed with G1CM (unpublished data). Thus, more detailed studies are required to further characterize possible effect of G1CM on the β‐catenin signaling pathway.
Acknowledgments
This work was supported in part by a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare, a Grant‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, a Grant‐in‐Aid for Regional New Consortium Projects (Regional R&D Proposal‐Based Program) from the Ministry of Economy, Trade and Industry of Japan, and a grant from the Takeda Science Foundation.
References
- 1. Vesper H, Schmelz EM, Nikolova‐Karakashian MN, Dillehay DL, Lynch DV, Merrill AH Jr. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr 1999; 129: 1239–50. [DOI] [PubMed] [Google Scholar]
- 2. Merrill AH Jr, Schmelz EM, Dillehay DL et al. Sphingolipids − the enigmatic lipid class: biochemistry, physiology, and pathophysiology. Toxicol Appl Pharmacol 1997; 142: 208–25. [DOI] [PubMed] [Google Scholar]
- 3. Spiegel S, Merrill AH Jr. Sphingolipid metabolism and cell growth regulation. FASEB J 1996; 10: 1388–97. [DOI] [PubMed] [Google Scholar]
- 4. Zeisel SH, Char D, Sheard NF. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr 1986; 116: 50–8. [DOI] [PubMed] [Google Scholar]
- 5. Fujino Y, Ohnishi M, Ito S. Molecular species of ceramide and mono‐, di‐, tri‐, and tetraglycosylceramide in bran and endosperm of rice grains. Agric Biol Chem 1985; 49: 2753–62. [Google Scholar]
- 6. Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest 2002; 110: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmelz EM, Merrill AH Jr. Ceramides and ceramide metabolites in cell regulation: evidence for dietary sphingolipids as inhibitors of colon carcinogenesis. Nutrition 1998; 14: 717–19. [DOI] [PubMed] [Google Scholar]
- 8. Schmelz EM, Sullards MC, Dillehay DL, Merrill AH Jr. Colonic cell proliferation and aberrant crypt foci formation are inhibited by dairy glycosphingolipids in 1,2‐dimethylhydrazine‐treated CF1 mice. J Nutr 2000; 130: 522–7. [DOI] [PubMed] [Google Scholar]
- 9. Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J, Merrill AH Jr. Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2‐dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res 1996; 56: 4936–41. [PubMed] [Google Scholar]
- 10. Schmelz EM, Bushnev AS, Dillehay DL, Liotta DC, Merrill AH Jr. Suppression of aberrant colonic crypt foci by synthetic sphingomyelins with saturated or unsaturated sphingoid base backbones. Nutr Cancer 1997; 28: 81–5. [DOI] [PubMed] [Google Scholar]
- 11. Lemonnier LA, Dillehay DL, Vespremi MJ, Abrams J, Brody E, Schmelz EM. Sphingomyelin in the suppression of colon tumors: prevention versus intervention. Arch Biochem Biophys 2003; 419: 129–38. [DOI] [PubMed] [Google Scholar]
- 12. Symolon H, Schmelz EM, Dillehay DL, Merrill AH Jr. Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2‐dimethylhydrazine‐treated CF1 mice and ApcMin/+ mice. J Nutr 2004; 134: 1157–61. [DOI] [PubMed] [Google Scholar]
- 13. Dillehay DL, Webb SK, Schmelz EM, Merrill AH Jr. Dietary sphingomyelin inhibits 1,2‐dimethylhydrazine‐induced colon cancer in CF1 mice. J Nutr 1994; 124: 615–20. [DOI] [PubMed] [Google Scholar]
- 14. Stevens VL, Winton EF, Smith EE, Owens NE, Kinkade JM Jr, Merrill AH Jr. Differential effects of long‐chain (sphingoid) bases on the monocytic differentiation of human leukemia (HL‐60) cells induced by phorbol esters, 1 alpha, 25‐dihydroxyvitamin D3, or ganglioside GM3. Cancer Res 1989; 49: 3229–34. [PubMed] [Google Scholar]
- 15. Ahn EH, Schroeder JJ. Sphingoid bases and ceramide induce apoptosis in HT‐29 and HCT‐116 human colon cancer cells. Exp Biol Med (Maywood) 2002; 227: 345–53. [DOI] [PubMed] [Google Scholar]
- 16. Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett 1995; 93: 55–71. [DOI] [PubMed] [Google Scholar]
- 17. Corpet DE, Tache S. Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr Cancer 2002; 43: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pretlow TP, Barrow BJ, Ashton WS et al. Aberrant crypts: putative preneoplastic foci in human colonic mucosa. Cancer Res 1991; 51: 1564–7. [PubMed] [Google Scholar]
- 19. Roncucci L, Stamp D, Medline A, Cullen JB, Bruce WR. Identification and quantification of aberrant crypt foci and microadenomas in the human colon. Hum Pathol 1991; 22: 287–94. [DOI] [PubMed] [Google Scholar]
- 20. Roncucci L. Early events in human colorectal carcinogenesis. Aberrant crypts and microadenoma. Ital J Gastroenterol 1992; 24: 498–501. [PubMed] [Google Scholar]
- 21. Jen J, Powell SM, Papadopoulos N et al. Molecular determinants of dysplasia in colorectal lesions. Cancer Res 1994; 54: 5523–6. [PubMed] [Google Scholar]
- 22. Yamada Y, Yoshimi N, Hirose Y et al. Frequent beta‐catenin gene mutations and accumulations of the protein in the putative preneoplastic lesions lacking macroscopic aberrant crypt foci appearance, in rat colon carcinogenesis. Cancer Res 2000; 60: 3323–7. [PubMed] [Google Scholar]
- 23. Yamada Y, Yoshimi N, Hirose Y et al. Sequential analysis of morphological and biological properties of beta‐catenin‐accumulated crypts, provable premalignant lesions independent of aberrant crypt foci in rat colon carcinogenesis. Cancer Res 2001; 61: 1874–8. [PubMed] [Google Scholar]
- 24. Hirose Y, Kuno T, Yamada Y et al. Azoxymethane‐induced beta‐catenin‐accumulated crypts in colonic mucosa of rodents as an intermediate biomarker for colon carcinogenesis. Carcinogenesis 2003; 24: 107–11. [DOI] [PubMed] [Google Scholar]
- 25. Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett 1987; 37: 147–51. [DOI] [PubMed] [Google Scholar]
- 26. Nabandith V, Suzui M, Morioka T et al. Inhibitory effects of crude alpha‐mangostin, a xanthone derivative, on two different categories of colon preneoplastic lesions induced by 1,2‐dimethylhydrazine in the rat. Asian Pac J Cancer Prev 2004; 5: 433–8. [PubMed] [Google Scholar]
- 27. Samaha HS, Kelloff GJ, Steele V, Rao CV, Reddy BS. Modulation of apoptosis by sulindac, curcumin, phenylethyl‐3‐methylcaffeate, and 6‐phenylhexyl isothiocyanate: apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res 1997; 57: 1301–5. [PubMed] [Google Scholar]
- 28. Suzui M, Inamine M, Kaneshiro T et al. Indole‐3‐carbinol inhibits the growth of human colon carcinoma cells but enhances the tumor multiplicity and volume of azoxymethane‐induced rat colon carcinogenesis. Int J Oncol 2005; 27: 1391–9. [PubMed] [Google Scholar]
- 29. Kohno H, Tanaka T, Kawabata K et al. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane‐induced colon carcinogenesis in male F344 rats. Int J Cancer 2002; 101: 461–8. [DOI] [PubMed] [Google Scholar]
- 30. Tanaka T. Chemoprevention of human cancer: biology and therapy. Crit Rev Oncol Hematol 1997; 25: 139–74. [DOI] [PubMed] [Google Scholar]
- 31. Schmelz EM, Crall KJ, Larocque R, Dillehay DL, Merrill AH Jr. Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J Nutr 1994; 124: 702–12. [DOI] [PubMed] [Google Scholar]
- 32. Merrill AH Jr, Sweeley CC. Sphingoid Metabolism and Cell Signaling, 2nd edn. Amsterdam: Elsevier Science Publishers, 1996. [Google Scholar]
- 33. Schmelz EM, Roberts PC, Kustin EM et al. Modulation of intracellular beta‐catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res 2001; 61: 6723–9. [PubMed] [Google Scholar]
- 34. Suzui M, Ushijima T, Dashwood RH et al. Frequent mutations of the rat beta‐catenin gene in colon cancers induced by methylazoxymethanol acetate plus 1‐hydroxyanthraquinone. Mol Carcinog 1999; 24: 232–7. [DOI] [PubMed] [Google Scholar]
- 35. Dashwood RH, Suzui M, Nakagama H, Sugimura T, Nagao M. High frequency of beta‐catenin (ctnnb1) mutations in the colon tumors induced by two heterocyclic amines in the F344 rat. Cancer Res 1998; 58: 1127–9. [PubMed] [Google Scholar]


