Abstract
To obtain baseline data for human papillomavirus (HPV) screening and vaccination in Japan, we analyzed HPV DNA data from 2282 Japanese women (1517 normal cytology, 318 cervical intraepithelial neoplasia [CIN] grade 1, 307 CIN2–3, and 140 invasive cervical cancer [ICC]) that visited the University of Tsukuba Hospital or Ibaraki Seinan Medical Center Hospital for screening or treatment of cervical diseases between 1999 and 2007. An L1‐based PCR method was used for individual HPV genotyping. The most common HPV types in ICC were, in order of decreasing prevalence, HPV16 (40.5%), HPV18 (24.4%), HPV52 (8.4%), HPV58 (3.1%), and HPV33 (3.1%). Based on the comparison of HPV type distributions between normal cytology and CIN2–3 and ICC, estimated risk of disease progression varied considerably by genotype: HPV16, HPV18, HPV31, HPV33, HPV35, HPV52, and HPV58 (prevalence ratio, 1.92; 95% confidence interval 1.58–2.34); other oncogenic types (0.31, 95% confidence interval 0.19–0.50); and non‐oncogenic types (0.09, 95% confidence interval 0.03–0.43). HPV16 and/or HPV18, including coinfections with other types, contributed to 67.1% of ICC and 36.2% of CIN2–3 among Japanese women. More importantly, the overall prevalence of HPV16 and/or HPV18 varied greatly according to the women's age: highest in women aged 20–29 years (ICC, 90.0%; CIN2–3, 53.9%), decreasing with age thereafter, and lowest in women aged 60 years or older (ICC, 56.3%; CIN2–3, 25.0%). In conclusion, type‐specific HPV testing may help identify Japanese women at high risk of progression to CIN2–3 and cancer. In Japan, current HPV vaccines are estimated to provide approximately 70% protection against ICC and may be more useful in reducing the incidence of cervical cancer and precancer in young women of reproductive age. (Cancer Sci 2009; 100: 1312–1316)
Persistent infection with oncogenic human papillomaviruses (HPV), most commonly types 16 and 18, leads to cervical cancer, the second most common cancer in women worldwide.( 1 ) Therefore, oncogenic HPV testing combined with cytology was approved for primary screening in the USA, because of sensitivity and cost‐effectiveness.( 2 ) In addition, HPV vaccines have been licensed in the USA, Australia, and European and other countries, because of their efficacy and safety. Clinical studies of HPV vaccines have demonstrated close to 100% protection against HPV16‐ and HPV18‐related infections and diseases,( 3 , 4 , 5 ) implying possible cross‐protection against HPV45, HPV31, and HPV52.( 4 , 5 ) Based on evidence from clinical trials,( 3 , 4 , 5 , 6 , 7 ) these two tools targeting HPV (detection assay and vaccine) are becoming increasingly attractive for cervical cancer prevention worldwide. In Japan, however, HPV DNA testing is still unavailable in mass screening and no HPV vaccine has yet been licensed. Type‐specific and age‐related data of HPV prevalence, both for women with normal cytology and for women with cervical diseases, are prerequisites to make a well‐judged decision about the future role of HPV screening and vaccination in cervical cancer prevention, but these data are missing in Japan. A meta‐analysis of Japanese HPV studies provided representative data of HPV type distribution, but no information about age‐specific prevalence.( 8 )
In the present study, we analyzed HPV DNA data from 2282 Japanese women to obtain the prevalence data of HPV among women across a broad age range. Our data may help provide models for further evaluating potential impact and cost effectiveness of HPV screening and vaccination in Japan.
Materials and Methods
Study subjects. Our study subjects consisted of 2282 Japanese women (1517 normal cytology, 318 cervical intraepithelial neoplasia [CIN] grade 1, 307 CIN2–3, and 140 invasive cervical cancer [ICC]) who visited the University of Tsukuba Hospital or Ibaraki Seinan Medical Center Hospital for cervical cancer screening, treatment of cervical diseases, or other reasons between 1999 and 2007. Foreign women were excluded from the present study, based on self‐reported ethnicity. Both hospitals are located in the south‐west area of Ibaraki prefecture, approximately 50 km north of Tokyo. Histological diagnosis was made using hematoxylin–eosin‐stained sections according to the World Health Organization classification. Written informed consent was obtained from all patients. The institutional ethical and research review board of each hospital approved the study protocol.
HPV detection and genotyping. Exfoliated cells from the ectocervix and endocervix were collected into a tube containing 1 mL PBS and stored at –30°C until DNA extraction. We detected HPV DNA in cervical samples by PCR‐based methodology described previously.( 9 ) In brief, total cellular DNA was extracted from cervical samples by a standard sodium dodecyl sulfate–proteinase K procedure. HPV DNA was amplified by PCR using consensus primers (L1C1 and L1C2 + L1C2 M) for the HPV L1 region. Direct comparisons of HPV detection methodology have demonstrated that the sensitivity of our PCR assay is higher than that of PCR assays using MY09 and MY11 and GP17 and GP18 primers.( 10 , 11 ) A reaction mixture without template DNA was included in every set of PCR runs as a negative control. Also, primers for a fragment of the β‐actin gene were used as a control to rule out false‐negative results for samples in which HPV DNA was not detected. To avoid contamination, we used disposable utensils and discarded them after a single use. We also used aliquoted reagents and maintained separate locations for different stages of the assay. HPV types were identified by restriction fragment length polymorphism, which has been shown to identify at least 26 types of genital HPV. HPV detection and genotyping were carried out blinded to the clinical data collected from the study subjects. In the present study, we considered HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, and HPV68 as oncogenic types. These 13 genotypes are detected by Hybrid Capture 2 (HC2) test (Digene, Gaithersburg, MD, USA). All other HPV types were classified as non‐oncogenic types.
Statistical analysis. To estimate HPV genotype‐specific risks for progression from HPV infections to CIN 2–3 or worse, prevalence ratios and odds ratios were calculated using JMP 7.0 J statistics package (SAS Institute, Cary, NC, USA). In addition, χ2‐test for trend was used to analyze the age‐related prevalence of HPV16 and HPV18 in women with CIN 2–3 and ICC. Two‐sided P‐values were calculated throughout and considered to be significant at less than 0.05.
Results
This analysis included 2282 Japanese women (1517 normal cytology, 318 CIN1, 307 CIN2–3, and 140 ICC). The mean age of the study subjects was 35.9 years (range, 15–84 years); 35.0 years (range, 15–78 years) for women with normal cytology; 34.6 years (range, 15–75 years) for CIN1; 35.5 years (range, 18–78 years) for CIN2–3; and 49.2 years (range, 25–84 years) for ICC. HPV prevalence was 22.5% in women with normal cytology, 88.4% in CIN1, 94.8% in CIN2–3, and 93.6% in ICC. A total of 20 types were detected in women with normal cytology and the number of detected genotypes decreased according to disease severity (19 types in CIN1, 14 types in CIN2–3, and 12 types in ICC). HPV45 was not detected among our study subjects.
Age‐related prevalence in cytologically normal women. In women with normal cytology, HPV prevalence peaked (35.9%) in women aged 15–19 years (n = 167), followed by a gradual decline in prevalence through 54 years; 28.9% among women aged 20–29 years (n = 499); 22.3% among women aged 30–39 years (n = 337); and 11.4% among women aged 40–54 years (n = 367) (Fig. 1). Interestingly, a second peak of HPV prevalence was observed in women aged 55 years or older (n = 147). Similarly, the age‐related prevalence of vaccine types (HPV16 and HPV18) and HC2 (13 oncogenic types) was highest in women aged 15–19 years, with a second peak observed in women aged 55 years or older. A similar trend of age‐specific HPV prevalence was observed for each HPV genotype separately (data not shown).
Figure 1.
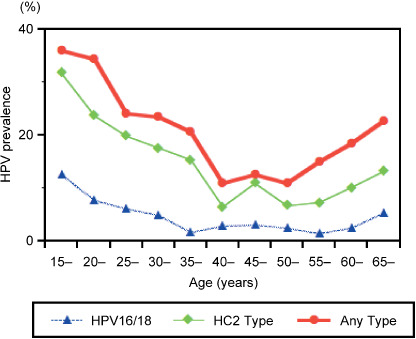
Age‐related human papillomavirus (HPV) prevalence among cytologically normal women in Japan. In women with normal cytology, HPV prevalence peaked in women aged 15–19 years, followed by a gradual decline in prevalence through 54 years. A second peak of HPV prevalence was observed in women aged 55 years or older. A similar trend of age‐specific prevalence was observed for vaccine types (HPV16 and HPV18) and Hybrid Capture 2 (HC2) types (13 oncogenic types).
When the age‐specific prevalence in cytologically normal women was applied to the age structure of the Japanese female population aged 15 years or older (56 869 466 women) reported in the 2005 Population Census,( 12 ) approximately 12 million women (20.6%) were estimated to be carriers of HPV DNA, of whom 21.6% were estimated to be infected with vaccine types, and 46.1% with the other oncogenic types.
HPV genotype‐specific risk for cervical cancer in Japan. To assess the progressive potential of each genotype, HPV type distributions among HPV‐positive women were shown according to disease severity in Table 1. Among women with normal cytology, HPV16 and HPV51 (11.7%) were most frequently detected, followed by HPV52 (9.4%), HPV58 (7.0%), HPV56 (5.8%), and HPV18 (5.6%). In CIN1, HPV51 was the most common genotype (12.5%), followed by HPV52 (11.4%), HPV16 (9.6%), HPV56 (8.9%), HPV18 (6.8%), and HPV58 (6.8%). In CIN2–3, HPV16 was most prevalent (24.1%), followed by HPV52 (17.5%), HPV58 (10.6%), HPV18 (6.9%), HPV51 (6.5%), and HPV31 (4.5%). In ICC, the most common HPV types were, in order of decreasing prevalence, HPV16 (40.5%), HPV18 (24.4%), HPV52 (8.4%), HPV33 (3.1%), HPV58 (3.1%), HPV31 (1.5%), HPV39 (1.5%), and HPV53 (1.5%). Based on the comparison of HPV type distributions between normal cytology and CIN2–3 and ICC, estimated risks for progression from viral infection to CIN 2–3 or ICC was highest in HPV31 (prevalence ratio, 3.04), followed by HPV16 (2.49), HPV18 (2.22), HPV35 (2.02), HPV52 (1.57), HPV33 (1.42), HPV58 (1.18), HPV82 (0.81), HPV53 (0.45), HPV51 (0.41), HPV56 (0.32), HPV39 (0.22), HPV59 (0.16), HPV70 (0.16), and HPV68 (0.12), suggesting that the seven genotypes of HPV16, HPV18, HPV31, HPV33, HPV35, HPV52, and HPV58 (prevalence ratio >1.0) are ‘higher‐risk’ types in Japan. The estimated risk was statistically significant for HPV16 and HPV18, but not for HPV31 because of limitations imposed by the low prevalence of this genotype. The prevalence of these seven types increased according to disease severity (normal cytology, 37.7%; CIN1, 41.6%; CIN2–3, 68.7%; ICC, 71.0%; prevalence ratio, 1.92). Conversely, the prevalence of the other oncogenic types decreased with disease severity (normal cytology, 27.2%; CIN1, 25.3%; CIN2–3, 10.7%; ICC, 3.1%; prevalence ratio, 0.31). A similar trend was observed for non‐oncogenic types (normal cytology, 16.1%; CIN1, 8.9%; CIN2–3, 3.8%; ICC, 1.5%; prevalence ratio, 0.09).
Table 1.
Human papillomavirus (HPV) type prevalence and risks for progression from viral infection to cervical intraepithelial neoplasia (CIN) 2–3 or invasive cervical cancer (ICC) in Japan
| HPV type | Normal cytology | CIN1 | CIN2–3 | ICC | Prevalence ratios | Odds ratio‡ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (n = 342) | (n = 281) | (n = 291) | (n = 131) | CIN2–3 + ICC: NL | CIN2–3 + ICC: NL | |||||
| n | % † | n | % | n | % | n | % | (95% CI) | (95% CI) | |
| Oncogenic types (HC2 types) | 222 | 64.9 | 188 | 66.9 | 231 | 79.4 | 110 | 84.0 | 1.24 (1.10–1.41) | 72.2 (47.9–113.7) |
| HPV16/18/31/33/35/52/58 | 129 | 37.7 | 117 | 41.6 | 200 | 68.7 | 106 | 80.9 | 1.92 (1.58–2.34) | 111.5 (72.7–178.1) |
| HPV39/45/51/56/59/68 | 93 | 27.2 | 71 | 25.3 | 31 | 10.7 | 4 | 3.1 | 0.31 (0.19–0.50) | 17.7 (10.2–31.1) |
| HPV16 | 40 | 11.7 | 27 | 9.6 | 70 | 24.1 | 53 | 40.5 | 2.49 (1.61–3.87) | 144.5 (86.3–251.3) |
| HPV18 | 19 | 5.6 | 19 | 6.8 | 20 | 6.9 | 32 | 24.4 | 2.22 (1.12–4.41) | 128.6 (68.0–254.6) |
| HPV31 | 4 | 1.2 | 6 | 2.1 | 13 | 4.5 | 2 | 1.5 | 3.04 (0.73–12.7) | 176.2 (59.3–653.3) |
| HPV33 | 8 | 2.3 | 7 | 2.5 | 10 | 3.4 | 4 | 3.1 | 1.42 (0.44–4.61) | 82.2 (32.4–223.3) |
| HPV35 | 2 | 0.6 | 7 | 2.5 | 5 | 1.7 | 0 | 0 | 2.02 (0.24–17.1) | 117.5 (24.1–848.2) |
| HPV39 | 11 | 3.2 | 2 | 0.7 | 1 | 0.3 | 2 | 1.5 | 0.22 (0.01–1.15) | 12.8 (2.8–44.1) |
| HPV45 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| HPV51 | 40 | 11.7 | 35 | 12.5 | 19 | 6.5 | 1 | 0.8 | 0.41 (0.20–0.83) | 23.5 (12.0–45.9) |
| HPV52 | 32 | 9.4 | 32 | 11.4 | 51 | 17.5 | 11 | 8.4 | 1.57 (0.90–2.74) | 91.1 (51.7–166.0) |
| HPV56 | 20 | 5.8 | 25 | 8.9 | 8 | 3 | 0 | 0 | 0.32 (0.12–0.97) | 18.8 (7.2–45.6) |
| HPV58 | 24 | 7.0 | 19 | 6.8 | 31 | 10.7 | 4 | 3.1 | 1.18 (0.59–2.38) | 68.5 (36.1–133.9) |
| HPV59 | 15 | 4.4 | 4 | 1.4 | 3 | 1.0 | 0 | 0 | 0.16 (0.03–0.77) | 9.4 (2.1–30.8) |
| HPV68 | 7 | 2.0 | 5 | 1.8 | 0 | 0 | 1 | 0.8 | 0.12 (0.01–1.34) | NA |
| Non‐oncogenic (non‐HC2 types) | 55 | 16.1 | 25 | 8.9 | 11 | 3.8 | 2 | 1.5 | 0.09 (0.03–0.43) | 11.1 (5.3–22.6) |
| HPV6/11 | 11 | 3.2 | 6 | 2.1 | 0 | 0 | 0 | 0 | NA | NA |
| HPV53 | 16 | 4.7 | 13 | 4.6 | 7 | 2.4 | 2 | 1.5 | 0.45 (0.15–1.38) | 26.4 (10.3–64.8) |
| HPV54 | 4 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 | NA | NA |
| HPV61 | 11 | 3.2 | 3 | 1.1 | 0 | 0 | 0 | 0 | NA | NA |
| HPV66 | 5 | 1.5 | 3 | 1.1 | 0 | 0 | 0 | 0 | NA | NA |
| HPV70 | 5 | 1.5 | 0 | 0 | 1 | 0.3 | 0 | 0 | 0.16 (0.01–2.12) | 9.4 (0.5–61.2) |
| HPV82 | 3 | 0.9 | 0 | 0 | 3 | 1.0 | 0 | 0 | 0.81 (0.10–9.00) | 47.0 (8.4–265.1) |
| Undetermined § | 24 | 7.0 | 16 | 5.7 | 16 | 5.5 | 6 | 4.6 | 0.65 (0.21–2.02) | 43.1 (21.4–87.7) |
| Multiple | 41 | 12.0 | 52 | 18.5 | 33 | 11.3 | 13 | 9.9 | 0.83 (0.37–1.81) | 52.7 (29.9–95.4) |
Percentage among HPV‐positive women.
† For estimation of odds ratios and 95% confidence intervals (CI), patients with cervical cancer who were negative for HPV (n = 9) and control individuals who were negative for HPV (n = 1175) were used as reference categories.
Undetermined HPV types denote that HPV types were unclassified or not determined due to weak reactions. NA, not available; NL, nonual cytology.
The prevalence of multiple infections did not increase according to disease severity (prevalence ratio, 0.83), suggesting no association between multiple infections and disease progression.
We also calculated odds ratios to estimate type‐specific risks of progression to CIN2–3 or ICC, although these analyses showed similar results (Table 1).
Estimating the impact of HPV16 and HPV18 vaccines in Japan. Clinical studies of HPV vaccines have demonstrated close to 100% protection against HPV16‐ and HPV18‐related infection and diseases.( 3 , 4 , 5 ) The prevalence of HPV16 and HPV18 was specifically analyzed to estimate the potential impact of current HPV vaccine against HPV16 and HPV18. When multiple infections were classified into each genotype, HPV16 and/or HPV18 were detected in 23.9% of CIN1, 36.2% of CIN2–3, and 67.1% of ICC. In women with CIN2–3 and ICC, the prevalence of HPV16 and HPV18 was highest among women aged 20–29 years, significantly decreasing with age thereafter (Table 2, χ2 test for trend, P < 0.05). In ICC cases, HPV16 and/or HPV18 were associated with 90.0% of women aged 20–29 years and 75.9% of women aged 30–39 years. Also, HPV16 and/or HPV18 contributed to 64.9% of infections detected in squamous cell carcinoma and 84.7% of infections in adenocarcinoma.
Table 2.
Age‐related prevalence of human papillomavirus (HPV) 16 and HPV18 in women with cervical intraepithelial neoplasia (CIN) 2–3 and invasive cervical cancer (ICC)
|
(A) Women with CIN2–3 | ||||||
|---|---|---|---|---|---|---|
| Detection of HPV16 and 18 | Age (years) | |||||
| 20–29 (n = 78) | 30–39 (n = 133) | 40–49 (n = 55) | 50–59 (n = 30) | 60– (n = 4) | All (n = 307) | |
| Single infection | ||||||
| HPV16 | 26 (33.3%) | 30 (22.6%) | 9 (16.4%) | 3 (10.0%) | 1 (25.0%) | 70 (22.8%) |
| HPV18 | 6 (7.7%) | 10 (7.5%) | 2 (3.6%) | 2 (6.7%) | 0 (0.0%) | 20 (6.5%) |
| Multiple infection | ||||||
| HPV16 and HPV18 only | 1 (1.3%) | 0 (0.0%) | 0 (0.0%) | 1 (3.3%) | 0 (0.0%) | 2 (0.7%) |
| HPV16, HPV18, and others | 9 (11.5%) | 5 (3.8%) | 2 (3.6%) | 1 (3.3%) | 0 (0.0%) | 19 (6.2%) |
| Total | 42 (53.8%) | 45 (33.8%) | 13 (23.6%) | 7 (23.3%) | 1 (25.0%) | 111 (36.2%) |
|
(B) Women with ICC | ||||||
|---|---|---|---|---|---|---|
| Detection of HPV16 and 18 | Age (years) | |||||
| 20–29 (n = 10) | 30–39 (n = 29) | 40–49 (n = 44) | 50–59 (n = 25) | 60– (n = 32) | All (n = 140) | |
| Single infection | ||||||
| HPV16 | 1 (10.0%) | 9 (31.0%) | 20 (45.5%) | 11 (44.0%) | 12 (37.5%) | 53 (37.9%) |
| HPV18 | 6 (60.0%) | 9 (31.0%) | 8 (18.2%) | 5 (20.0%) | 4 (12.5%) | 32 (22.9%) |
| Multiple infection | ||||||
| HPV16 and HPV18 only | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| HPV16, HPV18, and others | 2 (20.0%) | 4 (13.8%) | 1 (2.3%) | 0 (0.0%) | 2 (6.3%) | 9 (6.4%) |
| Total | 9 (90.0%) | 22 (75.9%) | 29 (65.9%) | 16 (64.0%) | 18 (56.3%) | 94 (67.1%) |
To quantify the population‐based impact of HPV vaccine in Japan, the age‐specific prevalence of HPV16 and HPV18 in ICC cases was applied to the age distribution data of ICC case based on the Japanese cancer registration in 2002.( 13 ) When excluding HPV16‐ and HPV18‐positive women from the data, the number of ICC cases was estimated to decrease from 8779 to 3074, suggesting that HPV16 and HPV18 vaccines may provide 65% protection against ICC in Japan.
Discussion
Our data showed that HPV infections are commonly detected among Japanese women, particularly of reproductive age, suggesting that approximately 12 million Japanese women may be carriers of HPV DNA. In the present study, the overall HPV prevalence in Japanese women with normal cytology (22.5%) was much higher than results from previous studies in Japan (range, 9.7–14.6%).( 14 , 15 , 16 ) In a meta‐analysis including these HPV studies, HPV prevalence in cytologically normal women was only 10.2%.( 8 ) Because the meta‐analysis data were from case‐control studies, this discrepancy may be explained by the older age of control women that were selected to match the age of the case women with cancer or precursor lesions. For instance, Asato et al. reported that HPV DNA was detected in 10.2% of control women with normal cytology,( 14 ) but they (average age, 52.4 years) were much older than our study subjects (average age, 35.0 years). In the present study, HPV prevalence in cytologically normal women aged 40–54 years was 11.4%. One may speculate that our data may be biased because the study subjects visited hospitals on their own initiative. However, our result was similar to results from recent large‐scale population‐based studies in the USA (26.8%), Denmark (22.9%), and Costa Rica (22.4%).( 17 , 18 , 19 ) With regard to age‐specific prevalence, HPV infection was most frequently detected in young women aged 15–25 years and a second peak was observed in women aged 55 years or older, which is also consistent with results from African, American, and European populations,( 20 ) although the reason for the second peak is unknown.
In the present study, type‐specific prevalence data in women with ICC and precursor lesions were very similar to those from the meta‐analysis of previous Japanese studies:( 8 ) for instance, HPV16 and HPV18 were less frequently identified in ICC cases in Japan compared with Southeast Asia, North America, and Europe,( 21 ) with HPV31, HPV33, HPV52, and HPV58 accounting for approximately 20% of ICC; HPV45 was rarely detected in Japan. However, the prevalence of HPV18 in ICC cases was far higher in this study (24%) compared with previous studies conducted by us (14%)( 22 ) and other groups (8–11%).( 15 , 16 ) In a meta‐analysis of Japanese HPV studies, HPV18 was identified in 12% of women with ICC.( 8 ) This discrepancy may be explained by the difference in age of the study subjects. Our study subjects (mean age, 49 years) were much younger than the women participating in the previous studies: the mean age of ICC cases was 57 years in the data shown by Nakagawa et al. in 1996( 22 ) and 53 years in the study reported by Asato et al. in 2004.( 16 ) This was consistent with a recent report showing the increased incidence of young women with cervical cancer in Japan.( 23 ) As shown in our previous( 22 ) and present studies, HPV18 was more frequently identified among young ICC cases. Thus, the higher HPV18 prevalence in this study may be explained by the younger age of the study subjects. Although HPV18 is more commonly detected in adenocarcinoma than in squamous cell carcinoma, the incidence of adenocarcinoma did not differ between the present and previous studies.
Our data suggested that risks of progression to CIN2–3 and ICC vary greatly by HPV genotype, with seven types of HPV (HPV16, HPV18, HPV31, HPV33, HPV35, HPV52, and HPV58) accounting for approximately 80% of ICC and 70% of CIN2–3. This was consistent with the results of a meta‐analysis of Japanese HPV studies.( 8 ) In a large‐scale prospective cohort study of Japanese women with low‐grade squamous intraepithelial lesion, the cumulative risk of CIN3 within 5 years was: 19.8% for HPV16, HPV18, HPV31, HPV33, HPV35, HPV52, and HPV58; 6.7% for other oncogenic types; and 3.1% for non‐oncogenic types (P = 0.0001; K. Matsumoto, unpublished data, 2009). These observations suggest that testing for a specific subset of oncogenic HPV types may be very useful for identifying populations at increased or decreased risk for disease progression in the follow up of women with cervical precursor lesions. Characterizing a woman's risk more precisely by partial or full genotyping may reduce the number of follow‐up smears and colposcopy referrals, although further analyses of cost‐utility are needed.
To date, clinical trials of HPV vaccines have demonstrated close to 100% efficacy in preventing infection and disease associated with types included in the vaccines.( 3 , 4 , 5 ) Our preliminary calculations excluding HPV16‐ and HPV18‐positive infections from the data suggested that the reduction in CIN2–3 and ICC that can be achieved by prophylactic HPV16 and HPV18 vaccination would be 36.2 and 67.1%, respectively, in Japan. Actually, the reduction may be smaller because some HPV16‐ and HPV18‐positive infections include coinfections with other types, whereas cross‐protection against HPV45, HPV31, and HPV52 may offer an additional prevention effect. A clinical trial of HPV16 and HPV18 vaccine demonstrated that vaccine efficacy against HPV45, HPV31, and HPV52 was 60, 36, and 32%, respectively.( 5 ) Based on these data, the reduction in CIN2–3 and ICC that can be achieved by HPV16 and HPV18 vaccination would be 44.3 and 71.0%, respectively. These estimates suggested that current HPV vaccines would contribute substantially to reducing the incidence of cervical cancer and high‐grade precursor lesions in Japan, but screening will remain necessary to detect approximately 60% of CIN2–3 and 30% of ICC cases that cannot be prevented by HPV16 and HPV18 vaccination. Equally important, the overall prevalence of HPV16 and/or HPV18 decreased significantly with the women's age: highest in women aged 20–29 years (CIN2–3, 53.9%; ICC, 90.0%) decreasing with age thereafter and lowest in women aged 60 years or older (CIN2–3, 25.0%; ICC, 56.3%). This may imply that HPV16 and HPV18 have an advantage of progressing rapidly to cervical cancer and pre‐cancer compared with other oncogenic types, in accordance with recent reports.( 24 , 25 ) Although data are limited with regard to the age‐specific prevalence of HPV16 and HPV18 in cervical cancer and pre‐cancer worldwide, a previous study also reported a high prevalence of HPV16 and HPV18 in young Japanese women with ICC.( 22 ) These observations suggest that current HPV vaccines will be more useful in reducing the incidence of cervical cancer and pre‐cancer in young women aged 20–39 years in Japan. Therefore, HPV vaccination could substantially reduce the number of young women receiving surgical treatment (cone biopsy or hysterectomy) that may result in negative consequences for pregnancy outcomes or inability to bear a child.( 26 )
In conclusion, our data suggest that risks of progression to CIN2–3 and ICC vary considerably by HPV genotype. Accordingly, testing for a specific subset of oncogenic HPV types may help identify Japanese women at particularly high risk of CIN2–3 and cancer. In Japan, current HPV vaccines are estimated to provide approximately 70% protection against ICC and may be more useful in reducing the incidence of cervical cancer and pre‐cancer in young women of reproductive age.
Acknowledgments
We thank Chizuko Fukui and Yoko Kuno for assistance. Also, we are grateful to all the women that participated in the study. This work was supported by the grants from the Ministry of Education, Science, Sports, and Culture of Japan (grant numbers 16591637, 17591722, and 19390424) and the Fund‐in‐Trust for Cancer Research from the Governor of IBARAKI Prefecture, Japan.
References
- 1. Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370: 890–907. [DOI] [PubMed] [Google Scholar]
- 2. Wright TC Jr, Schiffman M, Solomon D et al . Interim guidance for the use of human papillomavirus DNA testing as an adjunct to cervical cytology for screening. Obstet Gynecol 2004; 103: 304–9. [DOI] [PubMed] [Google Scholar]
- 3. Future II Study Group . Quadrivalent vaccine against human papillomavirus to prevent high‐grade cervical lesions. N Engl J Med 2007; 356: 1915–27. [DOI] [PubMed] [Google Scholar]
- 4. Harper DM, Franco EL, Wheeler CM et al . Sustained efficacy up to 4.5 years of a bivalent L1 virus‐like particle vaccine against human papillomavirus types 16 and 18: follow‐up from a randomised control trial. Lancet 2006; 367: 1247–55. [DOI] [PubMed] [Google Scholar]
- 5. Paavonen J, Jenkins D, Bosch FX et al . Efficacy of a prophylactic adjuvanted bivalent L1 virus‐like‐particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double‐blind, randomised controlled trial. Lancet 2007; 369: 2161–70. [DOI] [PubMed] [Google Scholar]
- 6. Mayrand MH, Duarte‐Franco E, Rodrigues I et al . Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357: 1579–88. [DOI] [PubMed] [Google Scholar]
- 7. Naucler P, Ryd W, Törnberg S et al . Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357: 1589–97. [DOI] [PubMed] [Google Scholar]
- 8. Miura S, Matsumoto K, Oki A et al . Do we need a different strategy for HPV screening and vaccination in East Asia? Int J Cancer 2006; 119: 2713–15. [DOI] [PubMed] [Google Scholar]
- 9. Yoshikawa H, Kawana T, Kitagawa K, Mizuno M, Yoshikura H, Iwamoto A. Detection and typing of multiple genital human papillomaviruses by DNA amplification with consensus primers. Jpn J Cancer Res 1991; 82: 524–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kado S, Kawamata Y, Shino Y et al . Detection of human papillomaviruses in cervical neoplasias using multiple sets of generic polymerase chain reaction primers. Gynecol Oncol 2001; 81: 47–52. [DOI] [PubMed] [Google Scholar]
- 11. Harnish DG, Belland LM, Scheid EE, Rohan TE. Evaluation of human papillomavirus‐consensus primers for HPV detection by the polymerase chain reaction. Mol Cell Probes 1999; 13: 9–21. [DOI] [PubMed] [Google Scholar]
- 12. Statistical Survey Department . Japan population census 2005 (Website on the internet). Tokyo: Statistics Bureau, Ministry of Internal Affairs and Communications, 2005. [Cited 5 March 2009] Available from URL: http://www.stat.go.jp/english/data/kokusei/index.htm.
- 13. Matsuda T, Marugame T, Kamo KI et al . Cancer incidence and incidence rates in Japan in 2002: Based on data from 11 population‐based cancer registries. Jpn J Clin Oncol 2008; 38: 641–8. [DOI] [PubMed] [Google Scholar]
- 14. Yoshikawa H, Nagata C, Noda K et al . Human papillomavirus infection and other risk factors for cervical intraepithelial neoplasia in Japan. Br J Cancer 1999; 80: 621–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sasagawa T, Basha W, Yamazaki H, Inoue M. High‐risk and multiple human papillomavirus infections associated with cervical abnormalities in Japanese women. Cancer Epidemiol Biomarkers Prev 2001; 10: 45–52. [PubMed] [Google Scholar]
- 16. Asato T, Maehama T, Nagai Y, Kanazawa K, Uezato H, Kariya K. A large case‐control study of cervical cancer risk associated with human papillomavirus infection in Japan, by nucleotide sequencing‐based genotyping. J Infect Dis 2004; 189: 1829–32. [DOI] [PubMed] [Google Scholar]
- 17. Dunne EF, Unger ER, Sternberg M et al . Prevalence of HPV infection among females in the United States. JAMA 2007; 297: 813–19. [DOI] [PubMed] [Google Scholar]
- 18. Kjaer SK, Breugelmans G, Munk C, Junge J, Watson M, Iftner T. Population‐based prevalence, type‐ and age‐specific distribution of HPV in women before introduction of an HPV‐vaccination program in Denmark. Int J Cancer 2008; 123: 1864–70. [DOI] [PubMed] [Google Scholar]
- 19. Herrero R, Castle PE, Schiffman M et al . Epidemiologic profile of type‐specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J Infect Dis 2005; 191: 1796–807. [DOI] [PubMed] [Google Scholar]
- 20. De Sanjosé S, Diaz M, Castellsagué X et al . Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta‐analysis. Lancet Infect Dis 2007; 7: 453–9. [DOI] [PubMed] [Google Scholar]
- 21. Muñoz N, Bosch FX, Castellsagué X et al . Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer 2004; 111: 278–85. [DOI] [PubMed] [Google Scholar]
- 22. Nakagawa S, Yoshikawa H, Onda T, Kawana T, Iwamoto A, Taketani Y. Type of human papillomavirus is related to clinical features of cervical carcinoma. Cancer 1996; 78: 1935–41. [PubMed] [Google Scholar]
- 23. Konno R, Shin HR, Kim YT et al . Human papillomavirus infection and cervical cancer prevention in Japan and Korea. Vaccine 2008; 26(Suppl. 12): M30–42. [DOI] [PubMed] [Google Scholar]
- 24. Khan MJ, Castle PE, Lorincz AT et al . The elevated 10‐year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type‐specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97: 1072–9. [DOI] [PubMed] [Google Scholar]
- 25. Vinokurova S, Wentzensen N, Kraus I et al . Type‐dependent integration frequency of human papillomavirus genomes in cervical lesions. Cancer Res 2008; 68: 307–13. [DOI] [PubMed] [Google Scholar]
- 26. Kyrgiou M, Koliopoulos G, Martin‐Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta‐analysis. Lancet 2006; 367: 489–98. [DOI] [PubMed] [Google Scholar]


