Abstract
Physiological recruitment of lymphocytes from the blood into lymph nodes and Peyer’s patches is mediated by high endothelial venules (HEV), specialized blood vessels found in secondary lymphoid tissues except for the spleen. The HEV are distinguished from other types of blood vessels by their tall and plump endothelial cells, and by their expression of specific chemokines and adhesion molecules, which all contribute to the selective lymphocyte trafficking across these blood vessels. The development of HEV is ontogenically regulated, and they appear perinatally in the mouse. High endothelial venules can appear ectopically, for instance in chronically inflamed tissues. Given that HEV enable the efficient trafficking of lymphocytes into tissues, the induction of HEV at a tumor site could potentiate tumor‐specific immune responses, and the artificial manipulation of HEV neogenesis might thus provide a new tool for cancer immunotherapy. However, the process of HEV development and the mechanisms by which the unique features of HEV are maintained are incompletely understood. In this review, we discuss the process of HEV neogenesis and development during ontogeny, and their molecular requirements for maintaining their unique characteristics under physiological conditions. (Cancer Sci 2010; 101: 2302–2308)
Lymphocyte recirculation is critical for the induction of efficient immune responses because it maximizes the probability that lymphocytes will encounter their specific cognate antigen. Under physiological conditions, naïve lymphocytes continuously traffic from the blood to lymphoid tissues, including the lymph nodes (LN) and Peyer’s patches (PP), through the walls of specific postcapillary venules, called high endothelial venules (HEV). In contrast to the flat endothelial cells that line other types of blood vessels, HEV endothelial cells (HEV‐EC) are almost cuboidal, and they selectively express certain tissue‐specific adhesion molecules and chemokines. The interaction of these molecules with ones expressed on the lymphocyte surface leads to the selective trafficking of lymphocytes from the blood into the LN and PP.( 1 , 2 ) High endothelial venules are mainly found in the paracortical and interfollicular areas of the LN and PP parenchyma, and possess a thick basal lamina, which is surrounded by an intricate stromal network consisting of fibroblastic reticular cells (FRC); this FRC network is thought to support lymphocyte migration from the HEV into the parenchyma and further, into the medulla.( 3 ) These morphological and functional characteristics of HEV appear to be stably maintained under physiological conditions by tissue‐specific environmental factors, including soluble and/or cellular component(s) provided from the lymph.( 4 ) Upon antigenic challenge, the number of HEV and the expression patterns of HEV‐associated genes change grossly but return to background levels over time,( 5 , 6 , 7 ) indicating that HEV have the plasticity to differentiate and dedifferentiate under certain conditions, and that the tissue microenvironment surrounding the HEV may have an important role in these processes.
Although a previous study suggested that HEV endothelial cells are derived from nonhematopoietic lineage cells,( 8 ) the cellular and molecular bases of the differentiation and proliferation of HEV endothelial cells during ontogeny are only partially understood. In this review, we summarize the current knowledge regarding the process of HEV neogenesis and development during ontogeny, and discuss extrinsic and intrinsic factors that influence HEV development and homeostasis. We also discuss the possibility of inducing HEV in tumor tissues or tumor‐draining lymph nodes for the purpose of increasing the efficacy of anti‐tumor immunotherapy.
Morphological Features of HEV
Like other kinds of blood vessels, HEV are composed of three layers: an inner endothelium of a single layer of endothelial cells; a middle layer of a few pericytes; and an outermost basal lamina. They also have several distinct characteristics (Fig. 1). First, the HEV‐EC are tall and plump with numerous mitochondria, free ribosomes, multivesicular bodies and a well‐developed Golgi apparatus, suggesting that they have high metabolic activities.( 9 ) Second, the HEV are surrounded by multiple layers of pericyte‐like cells called fibroblastic reticular cells (FRC). The FRC sheath, or perivascular sheath, creates a narrow space outside the HEV basal lamina, called the perivascular channel, through which lymphocytes appear to move from the abluminal side of the HEV to the LN parenchyma.( 3 ) Third, the FRC produce various extracellular matrix components, including fibronectin, collagen IV and laminins, which form the thick basal lamina of the HEV. Thus, HEV are easily identified by light microscopy by their unique cuboidal endothelial cell lining, thick surrounding basal lamina and sheath of FRC.
Figure 1.
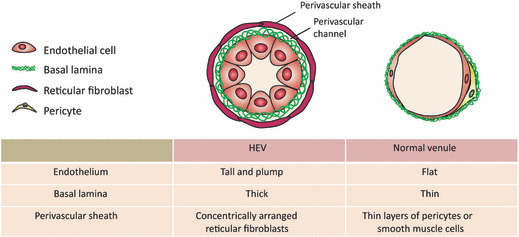
The unique morphological features of high endothelial venules (HEV). The HEV are easily distinguished from normal venules by their specialized endothelial cells, the high endothelial cells, which have a tall and plump shape. A single layer of high endothelial cells is surrounded by thick basal lamina composed of fibronectin, collagen IV, and laminins. The HEV are further enclosed by concentric layers of fibroblastic reticular cells.
High Endothelial Venules‐Associated Molecules
The HEV‐EC express a number of molecules that play critical roles in lymphocyte trafficking from the blood into the lymphatic system (Table 1). First, peripheral HEV‐EC express a group of L‐selectin ligands called peripheral node addressins (PNAd). PNAd refers to a set of sulfated and glycosylated proteins, including GlyCAM‐1,( 10 ) CD34,( 11 ) endomucin( 12 ) and nepmucin.( 13 ) To produce functional PNAd, HEV must support a series of specific post‐translational events mediated by several glycosyltransferases, such as Fuc‐TVII,( 14 )β3GlcNAcT‐3( 15 ) and GlcNAc6ST‐2.( 16 ) The PNAd is expressed along the entire HEV lumen and interacts with L‐selectin on lymphocytes, thereby mediating the lymphocyte tethering to HEV‐EC, and the subsequent lymphocyte rolling along the HEV lumen. In mesenteric LN and PP, the tethering/rolling step is mediated by another addressin, mucosal addressin cell adhesion molecule‐1 (MAdCAM‐1), which is expressed by HEV‐EC and interacts with α4β7 integrin on lymphocytes.( 17 )
Table 1.
High endothelial venules (HEV)‐associated molecules and their possible functions
| Molecule | Classification | Possible functions in lymphocyte trafficking | References |
|---|---|---|---|
| MAdCAM‐1 | Adhesion molecule (expressed in mucosal and immature HEV) | Integrin α4β7 ligand, control of lymphocyte rolling and adhesion to HEV | ( 17 ) |
| PNAd (sulfated and glycosylated molecules, i.e. GlyCAM‐1, CD34, endomucin, nepmucin) | Adhesion molecules | L‐selectin ligands, control of lymphocyte tethering and rolling on HEV | ( 10, 11, 12, 13 ) |
| ICAM‐1, ICAM‐2 | Adhesion molecules | Integrin ligands, control of lymphocyte firm adhesion to HEV | ( 2 ) |
| Fuc‐TVII, β3GlcNAcT‐3, GlcNAc6ST2 | Enzyme | Synthesis of functional L‐selectin ligand | ( 14, 15, 16 ) |
| CCL21, CCL19, CXCL13, CXCL12 | Chemokines | Activation of integrins, induction of cell adhesion and migration | ( 2 ) |
| Autotaxin | Enzyme | Activation of lysophosphatidic acid signaling | ( 19, 20 ) |
| DARC | Chemokine receptor | Chemokine immobilization, scavenging? | ( 21, 22 ) |
| Mac25/angiomodulin | Growth factor binding protein | Regulation of the local concentration of growth factors/chemokines? | ( 22, 23, 24 ) |
| LRHG | ECM and growth factor binding protein | Regulation of the local concentration of growth factors? | ( 25 ) |
DARC, Duffy antigen receptor for chemokines; ECM, extracellular matrix; LRHG, leucine‐rich HEV glycoprotein.
Second, HEV‐EC express a series of chemokines, small chemoattractant cytokines, which mediate the trafficking, activation and proliferation of many cell types. Under physiological conditions, multiple chemokines including CCL21, CCL19, CXCL10, CXCL12 and CXCL13 are expressed on HEV‐EC, and induce the activation of integrins through specific G‐protein‐coupled chemokine receptors expressed on lymphocytes.( 2 )
Finally, HEV‐EC express integrins and immunoglobulin superfamily adhesion molecules, which support the binding and migration of lymphocytes across HEV. The chemokines displayed in the lumen of HEV also accumulate in the basal lamina, and have been proposed to promote directional lymphocyte trafficking from HEV into the lymphoid tissue parenchyma.( 18 ) Besides these molecules, our group and others have identified molecules that are selectively or preferentially expressed in HEV‐EC by comparing the gene expression profile between HEV‐EC and flat EC; these include autotaxin,( 19 , 20 ) Duffy antigen receptor for chemokines (DARC),( 21 , 22 ) mac25/TAF( 22 , 23 , 24 ) and a novel HEV‐associated molecule, leucine‐rich HEV glycoprotein (LRHG),( 25 ) although the exact contributions of these molecules to HEV function remain unclear.
High Endothelial Venule Development During LN Neogenesis
Recent ontogenetic studies on mouse LN and PP by Mebius’( 26 ) group have led to a general model of lymphoid tissue organogenesis (Fig. 2). In this model, the earliest event in LN and PP organogenesis is initiated by a clustering of “inducer” cells of hematopoietic origin and “organizer” cells of mesenchymal lineage.( 27 ) The initial interaction of the two cell subsets is triggered by CXCL13 produced by the stromal organizer cells at the sites of lymphoid tissue formation; CXCL13 appears to be induced by retinoic acid and neuronal stimulation.( 28 ) CXCL13 attracts CXCR5‐expressing inducer cells, which produce cytokines essential for lymphoid tissue organogenesis, lymphotoxin (LT) α1β2. LTα and LTβ are structurally related members of the tumor necrosis factor (TNF) ligand family, which form a trimer, α1β2, that binds to a specific receptor LTβR on the cell surface. The LTα1β2 released by the inducer cells triggers LTβR signaling in the organizer cells, causing them to produce CXCL13, CCL19 and CCL21, and results in the further accumulation of lymphoid inducer cells responsive to these chemokines. In addition, CXCL13 promotes LTαβ expression on the surface of inducer cells, which amplifies the LTβR signaling in stromal organizer cells resulting in a stable feedback loop for the inducer‐organizer cell‐cluster formation. These sequential molecular events eventually give rise to larger cell clusters, which develop into functional lymphoid organs.( 26 )
Figure 2.
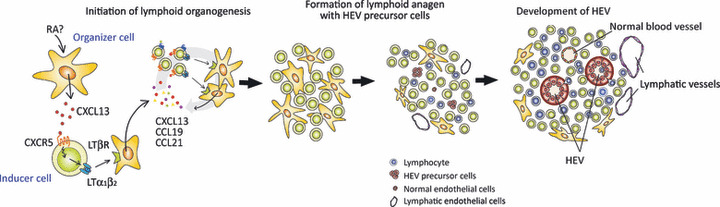
Schematic of lymphoid tissue organogenesis and high endothelial venule (HEV) development. Lymphoid organogenesis starts with expression of CXCL13 from stromal inducer cells in response to retinoic acid (RA) produced by adjacent cells, such as neurons. CXCL13 attract CXCR5+ LTα1β2 + lymphoid tissue inducer cells, which leads to the triggering of LTβR on stromal organizer cells. The stimulated organizer cells produce the chemokines CXCL13, CCL19 and CCL21, resulting in further accumulation of lymphoid inducer cells. After stable cell clustering occurs, HEV precursors differentiate into mature HEV, induced by interactions with the lymphoid tissue microenvironment.
High endothelial venules arise at some point during these processes and begin to mature functionally. In mice, HEV appear perinatally, and lymphocytes start to accumulate in the PP and LN at around 18.5 dpc( 29 ) and 1–2 days after birth,( 8 ) respectively. The final maturation of HEV is accompanied by the specific expression of the above‐described HEV‐associated molecules, which contribute to the robust accumulation of naïve lymphocytes in the secondary lymphoid tissues.
The study of HEV development in fetal LN and PP has been hampered by the lack of specific marker molecules for the HEV present during embryonic stages. One molecule that might be useful for this is MAdCAM‐1, an adhesion molecule that is preferentially expressed by mucosal HEV‐EC in adult mice. However, one study showed that MAdCAM‐1 is expressed in most veins, which lack the morphological phenotypes of HEV, in early embryos.( 30 ) In addition, in 16.5‐dpc mesenteric LN, MAdCAM‐1 is detected on vascular structures expressing the lymphatic endothelial cell marker molecule LYVE‐1,( 31 ) suggesting that MAdCAM‐1 is expressed in non‐HEV cells at this stage. Moreover, we recently found that MAdCAM‐1+/CD34 (pan‐endothelial marker)+ structures are present as cell aggregates but not as lumen‐containing blood vessels in 16.5‐dpc mesenteric LN (Fig. 3A). Furthermore, we found that a certain proportion of MAdCAM‐1+ vascular structures express LYVE‐1 but not CD34 in 16.5‐dpc mesenteric LN (MLN); these structures do not have high endothelial cells and probably represent immature lymphatic vessels. Therefore, not only does MAdCAM‐1 seem not to be a definitive HEV‐specific marker at this stage, but no HEV can be identified by morphological and/or phenotypical criteria before 16.5 dpc.
Figure 3.
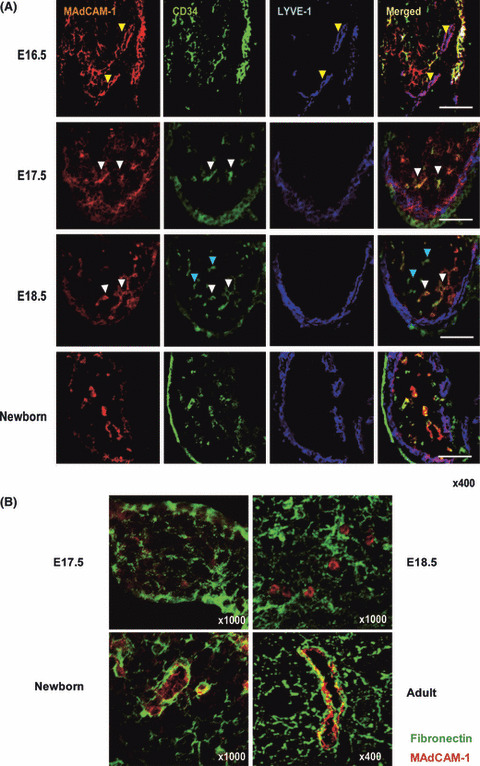
Histological demonstration of high endo‐thelial venule (HEV) neogenesis. (A) In 16.5‐dpc mesenteric lymph nodes (MLN), MAdCAM‐1+ vascular structures that express LYVE‐1 but not CD34 are observed (yellow arrowheads), indicating that lymphatic vessel formation starts at this stage. From 17.5 dpc onward, most MAdCAM‐1+ structures are CD34+ (white arrowheads). No blood vessels with a distinct lumen are observed at this time. In 18.5‐dpc MLNs, MAdCAM‐1+/CD34+ (white arrowheads) and MAdCAM‐1−/CD34+ (blue arrowheads) cell aggregates are observed; HEV‐type and non‐HEV‐type blood vessels appear at this stage. At this stage, LYVE‐1 expression is more prominent in the subcapsular region, whereas MAdCAM‐1 expression is less prominent. In newborn MLN, the expression of MAdCAM‐1 and LYVE‐1 are geographically segregated. Bars, 100 μm. (B) At 17.5 and 18.5 dpc, the expression of fibronectin (green) is scattered, and not necessarily closely associated with MAdCAM‐1+ cell aggregates (red). In newborn MLN, the basal lamina is prominent around MAdCAM‐1+ structures, sugges‐ting that the fibronectin‐expressing basal lamina typically seen in HEV develops prenatally.
In MLN at 17.5 dpc, MAdCAM‐1 colocalizes predominantly with CD34, but at 18.5 dpc, two distinct MAdCAM‐1+/CD34+ and MAdCAM‐1−/CD34+ vascular structures are observed in the MLN parenchyma. These observations are consistent with the idea that immature MAdCAM‐1+/CD34+ vascular EC differentiate into HEV‐type (MAdCAM‐1+/CD34+) and non‐HEV‐type (MAdCAM‐1−/CD34+) cells between 17.5 and 18.5 dpc in MLN. During this period, MAdCAM‐1+ LYVE‐1+ structures, which probably represent developing lymphatics, are found in the subcapsular area. In newborn MLN, the HEV‐type and non‐HEV‐type structures become more prominent, and a considerable proportion of the MAdCAM‐1+/CD34+ structures bear a high‐walled endothelium with a fibronectin‐expressing basal lamina, a hallmark of mature HEV (Fig. 3B). Thus, morphologically identifiable HEV appear only around birth.
HEV‐EC Precursor Cells
Cupedo et al. ( 8 ) reported that the subcutaneous injection of newborn LN‐derived cells into mice induced LN‐like structures, which contained HEV and lymphatic endothelium of donor origin. This study indicated that HEV precursor cells are present in newborn LN. Although such precursor cells have not been isolated as a distinct cell entity, one possible candidate is the immature endothelial cells in newborn vascular structures. Our recent DNA microarray analysis of HEV‐EC isolated from newborn and adult MLN indicated that newborn HEV‐EC preferentially express a variety of angiogenesis‐associated genes as well as those predominantly expressed in endothelial progenitor and immature endothelial cells (Table 2). The expression of these genes in HEV‐EC precursors may stimulate angiogenesis and/or the neovascularization of immature HEV, and may further support the idea that functional HEV develop during postnatal stages. The newborn and adult HEV‐EC in this microarray analysis expressed a set of pan‐endothelial genes including VEGFR1 and VEGFR2 at high levels, and also a lymphatic endothelial cell marker gene LYVE1 and VEGFR3 to a lesser extent. This was probably because the HEV‐EC used in this microarray analysis were predominantly composed of MAdCAM‐1+ vascular EC, but also contained a small number of contaminating MAdCAM‐1+ lymphatic EC, because we isolated the HEV‐EC on the basis of their MAdCAM‐1 and CD34 expression (immature lymphatic EC appear to express MAdCAM‐1, as described above). Alternatively, HEV‐EC may predominantly have the characteristics of vascular EC, yet retain the characteristics of lymphatic EC to a certain extent, given that HEV‐EC and lymphatic EC are derived from a common progenitor. Further investigation is required to resolve this issue.
Table 2.
Gene expression analysis of lymph node MAdCAM‐1+ and MAdCAM‐1− endothelial cells
| Gene | Newborn | Adult | |||
|---|---|---|---|---|---|
| MAdCAM‐1+ EC | MAdCAM‐1− EC | MAdCAM‐1+ EC | MAdCAM‐1− EC | ||
| HEV‐associated genes | GlyCAM‐1 | − | − | ++++ | +++ |
| GlcNAc6ST2 | + | − | ++ | − | |
| Autotaxin | + | + | +++ | ++ | |
| CCL21 | + | − | +++ | ++ | |
| Mac25 | +++ | ++ | +++ | +++ | |
| DARC | + | + | ++ | + | |
| Lymphotoxin β receptor | ++ | + | −† | + | |
| Lymphatic endothelial cell marker | LYVE‐1 | + | + | + | − |
| VEGFR‐3 (Flt‐4) | + | − | + | + | |
| Stem cell marker | CD133 | ++ | − | − | − |
| Early endothelial cell marker | VGFR‐2 (Flk‐1) | ++ | + | ++ | ++ |
| Conventional endothelial cell markers | CD34 | + | + | ++ | ++ |
| CD31 | ++ | − | ++ | + | |
| VE‐cadherin | + | + | + | + | |
| Endothelial cell proliferation‐related genes | FGFR‐1 | + | + | − | + |
| Tie2 | +++ | ++ | ++ | ++ | |
| VGFR‐1 (Flt‐1) | +++ | + | +++ | +++ | |
| CD105 (endoglin) | ++ | + | ++ | ++ | |
C57BL/6 mouse mesenteric lymph nodes (newborn or 6‐week‐old females) were dissected and single‐cell suspensions of the stromal fractions were obtained and stained with anti‐CD34 and anti‐MAdCAM‐1 mAbs. CD34+ MAdCAM‐1+ and CD34+ MAdCAM‐1− cells were enriched by fluorescence‐activated cell sorting and subjected to RNA extraction and microarray analysis. The transcript expression levels were scaled to an average intensity of 500 units on each chip. ++++, >100 000; +++, >10 000; ++, 1000–10 000; +, 100–1000; −, <100. †Although the signal intensity was <100 in this assay, protein expression was consistently detected by immunohistochemistry using an anti‐LTβR antibody. HEV, high endothelial venules.
Intrinsic and Extrinsic Signals That Affect HEV Development
Like other types of precursor cells, HEV‐EC precursors must both receive a number of extracellular signals and integrate them to generate the intracellular responses involved in lineage commitment, vascular neogenesis, maturation, and so on. Although the molecules responsible for the lineage specification of HEV‐EC are poorly understood, the HEV lineage commitment is likely to be regulated by cell‐type‐specific transcription factors that activate genetic programs in the immature HEV‐EC precursor cells. Such HEV lineage decision appears to occur independently of interactions with lymphoid cells, because HEV form normally in T‐cell‐deficient and B‐cell‐deficient mice.( 6 )
After the lineage commitment, HEV growth appears to be stimulated by angiogenic factors provided from cellular components in the lymphoid tissues, such as the CD11c+ dendritic cells in LN.( 32 ) In addition, hematopoietic cells and/or lymphocytes may support the final growth and functional maturation of HEV through interactions between specific adhesion molecules or by providing humoral factors such as cytokines and chemokines. As mentioned above, LTβR‐mediated signaling seems to be important in this process. Drayton et al. ( 33 ) demonstrated that the LTαβ‐LTβR system triggers the alternative NFκB signaling pathway in an IKKα‐dependent manner, which induces the expression of HEV‐specific genes such as those encoding GlyCAM‐1, GlcNAc6ST2, CCL21, CCL19 and CXCL13. Consistent with this finding, gene knockout mice lacking the LTαβ signaling components IKKα( 33 ) or NFκB2( 34 ) have only rudimentary LN with poorly developed HEV; the expression of GlyCAM‐1, CD34, CCL19, CCL21 and CXCL13 in the HEV is also significantly reduced in these mice. In addition, Stein’s group demonstrated that immunization‐induced HEV remodeling depends on the LTα1β2 signal but not the VEGF‐A signal, and that the LTα1β2 signals are largely provided by B cells.( 7 ) On the other hand, analysis of T‐ or B‐lymphocyte‐specific LTαβ‐deficient mice showed that the HEV architecture expressing CCL21 is unaffected in these mutants.( 35 ) This observation suggested that T and B cells do not necessarily provide the LTαβ for HEV development/maturation and other cell subsets expressing LTαβ( 27 ) might provide redundant signals.
High Endothelial Venule Development Under Pathological Conditions
Blood vessels with HEV‐like morphology that express HEV‐associated genes are occasionally found in chronically inflamed non‐lymphoid tissues. These HEV‐like structures are associated with lymphoid cell aggregates called tertiary lymphoid organs (TLO), which represent highly organized lymphoid tissues induced by microbial infection, autoimmunity or other pathological conditions such as atherosclerosis.( 36 ) The TLO have similar tissue components to the secondary lymphoid organs, such as T‐cell‐B‐cell compartments, organized B‐cell follicles with follicular dendritic cells, lymphatic vessels and HEV, implying that TLO formation and lymphoid neogenesis share some molecular mechanisms. In mouse models of ectopic lymphoid neogenesis induced by chronic inflammation, the expression of LTα or LTαβ induces the formation of TLO that include blood vessels with the morphological characteristics of HEV and express several HEV‐associated adhesion molecules, such as PNAd and MAdCAM‐1,( 37 , 38 ) CXCL13, CCL19 and CCL21.( 38 , 39 ) Considering that LT/TNF‐signaling regulates lymphoid chemokine expression in stromal cells as described above,( 38 , 39 , 40 ) LT signaling is likely to up‐regulate chemokine expression by stromal cells and HEV‐like endothelial cells in the TLO, which would promote lymphoid cell accumulation in the tissue, leading to an increase in the local concentration of lymphoid‐cell‐produced growth factors and cytokines that further promote HEV‐like blood vessel development.
Other studies indicate that the expression of lymphoid chemokines is sufficient to induce TLO in a manner dependent on LTαβ signaling,( 41 , 42 ) suggesting that certain lymphoid chemokines function upstream of LTαβ signaling. For instance, CXCL13 and CCL19/CCL21 can up‐regulate the cell‐surface expression of LTαβ on B cells and naïve T cells, respectively.( 42 ) Here again, a positive feedback mechanism may be operating between certain chemokines and LTβR signaling, as proposed for lymphoid tissue neogenesis.( 43 ) The morphogenic differentiation of normal vessels into HEV appears to occur independently of the mature lymphocytes in TLO, because HEV are observed in the chronically inflamed pancreas of rat insulin promoter‐LT transgenic mice that are deficient in RAG‐2 expression; these mice are deficient in T and B cells and constitutively express LTα in the pancreas.( 37 )
Regulators of HEV Homeostasis
The specific properties of HEV‐EC, such as their cuboidal appearance and expression of specific molecules involved in lymphocyte trafficking, are maintained in lymphoid tissues under steady‐state conditions, but they lose these properties rapidly upon in vitro culture.( 44 , 45 ) Thus, the unique properties of HEV are modulated and maintained by the lymphoid tissue microenvironment. The molecular mechanisms of HEV maintenance recently began to be elucidated. Browning et al. ( 46 ) showed that the selective HEV differentiation status, such as having a “high endothelial” morphology and PNAd expression, are affected by blocking the LTαβ/LTβR signal. As described above, LTαβ/LTβR signaling can be triggered by cell–cell contact between LTαβ‐expressing cells (such as B cells) and LTβR‐expressing HEV‐EC.
In addition to LTαβ/LTβR signaling, soluble factors and/or cells released into the LN microenvironment may contribute to this process. Mebius et al. ( 47 ) and Drayson et al. ( 48 ) proposed that lymph‐borne factors are important, based on the observation that the mechanical ligation of afferent lymphatics resulted in the disappearance of the cuboidal morphology and the down‐regulation of several HEV‐associated molecules, such as GlyCAM‐1.( 4 ) Given that the loss of HEV properties is recovered by restoration of the afferent lymph flow, the conversion between HEV‐EC and flat EC occurs reversibly. In addition, Duijvestijn et al. ( 49 ) reported that whole‐body irradiation also substantially decreased the average height of HEV‐EC in several days and that subsequent intravenous injection of LN cells into the irradiated animals reversed this effect within a matter of hours, indicating that the HEV‐EC morphology is subject to change readily and that it is regulated by the cells entering the LN. A recent study by Liao et al. ( 6 ) indicates that LTβR signaling may be critically involved in the maintenance of HEV‐EC morphology.
Analyses of the gene expression patterns between differentiated and de‐differentiated HEV‐EC have provided some insight into the molecular mechanisms of HEV maintenance. For instance, Lacorre et al. ( 50 ) used a microarray approach to compare gene expression between freshly isolated HEV‐EC and their ex vivo cultured counterparts, and found that the expression of a cluster of genes encoding a variety of transcription factors, cell‐signaling molecules and intracellular effectors rapidly dropped off in ex vivo culture. Using the same approach, Baekkevold et al. ( 51 ) identified a nuclear factor, NF‐HEV, that was preferentially expressed in HEV‐EC. NF‐HEV was recently reported to be identical to IL‐33,( 52 ) an IL‐1‐like cytokine that induces T helper type 2 responses, although NF‐HEV’s function in HEV‐EC remains unknown.
As described above, our group and others have also identified several HEV‐EC‐associated genes by comparing the gene expression profiles between HEV‐EC and flat EC.( 21 , 22 , 23 , 25 , 53 ) We recently expanded this approach, and identified several transcription factors and key regulators of intracellular signaling that are abundantly expressed in HEV‐EC. These genes are highly expressed in mature HEV‐EC, but only weakly in flat or immature EC (H. Hayasaka et al., manuscript in preparation), indicating their possible contribution to HEV function. Further clarification is required to determine whether or not any of these HEV‐EC‐associated genes are involved in the homeostatic regulation of the HEV‐EC phenotype.
Potential Application to Tumor Immunotherapy
During the last decade, a lot of effort has been made to generate potent anti‐tumor immune responses as an effective immunotherapy. The basic strategies of cancer immunotherapy so far include vaccination with autologous tumor‐associated antigens, stimulation of immune responses by using immune modulators (such as recombinant interferons, cytokines), and the isolation and expansion of tumor‐reactive T cells for adoptive transfer. Although some of these strategies induce a detectable anti‐tumor response, they do not control established tumor growth because the primed T cells do not enter the tumor sites efficiently.
To improve the accessibility of primed T cells to a tumor or to the LN draining a tumor, one idea is to prime naïve T cells extranodally within a tumor mass and recruit them to the tumor and tumor‐draining LN. Schrama et al. ( 54 ) demonstrated that the treatment of melanoma with a tumor‐specific antibody‐LTα fusion protein induced PNAd+ HEV‐like blood vessels and TLO‐like lymphoid cell accumulations at the tumor site, where naïve T‐cell priming with tumor‐specific antigens and clonal expansion occurred. As a result, tumor‐reactive T cells were induced, which resulted in the eradication of pulmonary metastasis and subcutaneous tumors. Kirk et al. ( 55 ) also reported that anti‐tumor effector cells can be induced extranodally. They observed a modest but significant tumor regression by directly injecting dendritic cells (DC), which were genetically modified to secrete CCL21, into a growing tumor; the DC administration resulted in a substantial and sustained T‐cell infiltration into the tumor mass and subsequent tumor regression. Furthermore, the presence of TLO has been positively correlated with prolonged survival in human lung cancer patients.( 56 )
Thus, the induction of T‐cell infiltration and/or TLO generation at tumor sites appears to potentiate anti‐tumor responses, and in this regard, novel methods for manipulating HEV neogenesis are urgently needed. Understanding the transcription factors that initiate HEV development will help us construct novel strategies for inducing TLO at tumor sites. In addition, further elucidation of the signaling pathways that induce and regulate HEV neogenesis of and the factors involved in HEV maturation and maintenance will also improve our ability to induce TLO at tumor sites. These HEV‐oriented approaches may offer a powerful strategy for inducing effective cancer‐specific immunotherapies.
Abbreviations
- DARC
Duffy antigen receptor for chemokines
- ECM
extracellular matrix
- HEV
high endothelial venules
- LRHG
leucine‐rich HEV glycoprotein
References
- 1. Von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol 2003; 3: 867–78. [DOI] [PubMed] [Google Scholar]
- 2. Miyasaka M, Tanaka T. Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 2004; 4: 360–70. [DOI] [PubMed] [Google Scholar]
- 3. Bajenoff M, Egen JG, Koo LY et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006; 25: 989–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mebius RE, Dowbenko D, Williams A, Fennie C, Lasky LA, Watson SR. Expression of GlyCAM‐1, an endothelial ligand for L‐selectin, is affected by afferent lymphatic flow. J Immunol 1993; 151: 6769–76. [PubMed] [Google Scholar]
- 5. Mebius RE, Breve J, Duijvestijn AM, Kraal G. The function of high endothelial venules in mouse lymph nodes stimulated by oxazolone. Immunology 1990; 71: 423–7. [PMC free article] [PubMed] [Google Scholar]
- 6. Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol 2006; 177: 3369–79. [DOI] [PubMed] [Google Scholar]
- 7. Kumar V, Scandella E, Danuser R, et al. Global lymphoid tissue remodeling during a viral infection is orchestrated by a B cell‐lymphotoxin‐dependent pathway. Blood 2010; 115: 4624–6. [DOI] [PubMed] [Google Scholar]
- 8. Cupedo T, Jansen W, Kraal G, Mebius RE. Induction of secondary and tertiary lymphoid structures in the skin. Immunity 2004; 21: 655–67. [DOI] [PubMed] [Google Scholar]
- 9. Anderson ND, Anderson AO, Wyllie RG. Specialized structure and metabolic activities of high endothelial venules in rat lymphatic tissues. Immunology 1976; 31: 455–73. [PMC free article] [PubMed] [Google Scholar]
- 10. Lasky LA, Singer MS, Dowbenko D et al. An endothelial ligand for L‐selectin is a novel mucin‐like molecule. Cell 1992; 69: 927–38. [DOI] [PubMed] [Google Scholar]
- 11. Baumheter S, Singer MS, Henzel W et al. Binding of L‐selectin to the vascular sialomucin CD34. Science 1993; 262: 436–8. [DOI] [PubMed] [Google Scholar]
- 12. Kanda H, Tanaka T, Matsumoto M et al. Endomucin, a sialomucin expressed in high endothelial venules, supports L‐selectin‐mediated rolling. Int Immunol 2004; 16: 1265–74. [DOI] [PubMed] [Google Scholar]
- 13. Umemoto E, Tanaka T, Kanda H et al. Nepmucin, a novel HEV sialomucin, mediates L‐selectin‐dependent lymphocyte rolling and promotes lymphocyte adhesion under flow. J Exp Med 2006; 203: 1603–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maly P, Thall A, Petryniak B et al. The α(1,3)fucosyltransferase Fuc‐TVII controls leukocyte trafficking through an essential role in L‐, E‐, and P‐selectin ligand biosynthesis. Cell 1996; 86: 643–53. [DOI] [PubMed] [Google Scholar]
- 15. Yeh JC, Hiraoka N, Petryniak B et al. Novel sulfated lymphocyte homing receptors and their control by a Core1 extension β1,3‐N‐acetylglucosaminyltransferase. Cell 2001; 105: 957–69. [DOI] [PubMed] [Google Scholar]
- 16. Hiraoka N, Petryniak B, Nakayama J et al. A novel, high endothelial venule‐specific sulfotransferase expresses 6‐sulfo sialyl Lewisx, an L‐selectin ligand displayed by CD34. Immunity 1999; 11: 79–89. [DOI] [PubMed] [Google Scholar]
- 17. Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L‐selectin‐mediated lymphocyte rolling on MAdCAM‐1. Nature 1993; 366: 695–8. [DOI] [PubMed] [Google Scholar]
- 18. Yang BG, Tanaka T, Jang MH, Bai Z, Hayasaka H, Miyasaka M. Binding of lymphoid chemokines to collagen IV that accumulates in the basal lamina of high endothelial venules: its implications in lymphocyte trafficking. J Immunol 2007; 179: 4376–82. [DOI] [PubMed] [Google Scholar]
- 19. Nakasaki T, Tanaka T, Okudaira S et al. Involvement of the lysophosphatidic acid‐generating enzyme autotaxin in lymphocyte‐endothelial cell interactions. Am J Pathol 2008; 173: 1566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol 2008; 9: 415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kashiwazaki M, Tanaka T, Kanda H et al. A high endothelial venule‐expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int Immunol 2003; 15: 1219–27. [DOI] [PubMed] [Google Scholar]
- 22. Girard JP, Baekkevold ES, Yamanaka T, Haraldsen G, Brandtzaeg P, Amalric F. Heterogeneity of endothelial cells: the specialized phenotype of human high endothelial venules characterized by suppression subtractive hybridization. Am J Pathol 1999; 155: 2043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Usui T, Murai T, Tanaka T et al. Characterization of mac25/angiomodulin expression by high endothelial venule cells in lymphoid tissues and its identification as an inducible marker for activated endothelial cells. Int Immunol 2002; 14: 1273–82. [DOI] [PubMed] [Google Scholar]
- 24. Nagakubo D, Murai T, Tanaka T et al. A high endothelial venule secretory protein, mac25/angiomodulin, interacts with multiple high endothelial venule‐associated molecules including chemokines. J Immunol 2003; 171: 553–61. [DOI] [PubMed] [Google Scholar]
- 25. Saito K, Tanaka T, Kanda H et al. Gene expression profiling of mucosal addressin cell adhesion molecule‐1+ high endothelial venule cells (HEV) and identification of a leucine‐rich HEV glycoprotein as a HEV marker. J Immunol 2002; 168: 1050–9. [DOI] [PubMed] [Google Scholar]
- 26. Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol 2003; 3: 292–303. [DOI] [PubMed] [Google Scholar]
- 27. Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+ CD3− LTβ+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 1997; 7: 493–504. [DOI] [PubMed] [Google Scholar]
- 28. Van De Pavert SA, Olivier BJ, Goverse G et al. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol 2009; 10: 1193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adachi S, Yoshida H, Kataoka H, Nishikawa S. Three distinctive steps in Peyer’s patch formation of murine embryo. Int Immunol 1997; 9: 507–14. [DOI] [PubMed] [Google Scholar]
- 30. Hashi H, Yoshida H, Honda K et al. Compartmentalization of Peyer’s patch anlagen before lymphocyte entry. J Immunol 2001; 166: 3702–9. [DOI] [PubMed] [Google Scholar]
- 31. Cupedo T, Vondenhoff MF, Heeregrave EJ et al. Presumptive lymph node organizers are differentially represented in developing mesenteric and peripheral nodes. J Immunol 2004; 173: 2968–75. [DOI] [PubMed] [Google Scholar]
- 32. Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J Exp Med 2006; 203: 1903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Drayton DL, Bonizzi G, Ying X, Liao S, Karin M, Ruddle NH. IκB kinase complex α kinase activity controls chemokine and high endothelial venule gene expression in lymph nodes and nasal‐associated lymphoid tissue. J Immunol 2004; 173: 6161–8. [DOI] [PubMed] [Google Scholar]
- 34. Carragher D, Johal R, Button A et al. A stroma‐derived defect in NF‐κB2−/− mice causes impaired lymph node development and lymphocyte recruitment. J Immunol 2004; 173: 2271–9. [DOI] [PubMed] [Google Scholar]
- 35. Tumanov A, Kuprash D, Lagarkova M et al. Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity 2002; 17: 239–50. [DOI] [PubMed] [Google Scholar]
- 36. Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol 2006; 7: 344–53. [DOI] [PubMed] [Google Scholar]
- 37. Kratz A, Campos‐Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med 1996; 183: 1461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LTαβ directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV‐restricted sulfotransferase. J Exp Med 2003; 197: 1153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hjelmstrom P, Fjell J, Nakagawa T, Sacca R, Cuff CA, Ruddle NH. Lymphoid tissue homing chemokines are expressed in chronic inflammation. Am J Pathol 2000; 156: 1133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ngo VN, Korner H, Gunn MD et al. Lymphotoxin α/β and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J Exp Med 1999; 189: 403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luther SA, Lopez T, Bai W, Hanahan D, Cyster JG. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin‐dependent lymphoid neogenesis. Immunity 2000; 12: 471–81. [DOI] [PubMed] [Google Scholar]
- 42. Luther SA, Bidgol A, Hargreaves DC et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 2002; 169: 424–33. [DOI] [PubMed] [Google Scholar]
- 43. Ansel KM, Ngo VN, Hyman PL et al. A chemokine‐driven positive feedback loop organizes lymphoid follicles. Nature 2000; 406: 309–14. [DOI] [PubMed] [Google Scholar]
- 44. Mebius RE, Bauer J, Twisk AJ, Breve J, Kraal G. The functional activity of high endothelial venules: a role for the subcapsular sinus macrophages in the lymph node. Immunobiology 1991; 182: 277–91. [DOI] [PubMed] [Google Scholar]
- 45. Baekkevold ES, Jahnsen FL, Johansen FE et al. Culture characterization of differentiated high endothelial venule cells from human tonsils. Lab Invest 1999; 79: 327–36. [PubMed] [Google Scholar]
- 46. Browning JL, Allaire N, Ngam‐Ek A et al. Lymphotoxin‐beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 2005; 23: 539–50. [DOI] [PubMed] [Google Scholar]
- 47. Mebius RE, Streeter PR, Breve J et al. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J Cell Biol 1991; 115: 85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drayson MT, Ford WL. Afferent lymph and lymph borne cells: their influence on lymph node function. Immunobiology 1984; 168: 362–79. [DOI] [PubMed] [Google Scholar]
- 49. Duijvestijn AM, Rep M, Hendriks HR, Kraal G. Functional capacities of high endothelial venules appear not to be controlled by recirculating lymphocytes. Immunobiology 1990; 180: 295–307. [DOI] [PubMed] [Google Scholar]
- 50. Lacorre DA, Baekkevold ES, Garrido I et al. Plasticity of endothelial cells: rapid dedifferentiation of freshly isolated high endothelial venule endothelial cells outside the lymphoid tissue microenvironment. Blood 2004; 103: 4164–72. [DOI] [PubMed] [Google Scholar]
- 51. Baekkevold ES, Roussigne M, Yamanaka T et al. Molecular characterization of NF‐HEV, a nuclear factor preferentially expressed in human high endothelial venules. Am J Pathol 2003; 163: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carriere V, Roussel L, Ortega N et al. IL‐33, the IL‐1‐like cytokine ligand for ST2 receptor, is a chromatin‐associated nuclear factor in vivo . Proc Natl Acad Sci USA 2007; 104: 282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Izawa D, Tanaka T, Saito K et al. Expression profile of active genes in mouse lymph node high endothelial cells. Int Immunol 1999; 11: 1989–98. [DOI] [PubMed] [Google Scholar]
- 54. Schrama D, Thor Straten P, Fischer WH et al. Targeting of lymphotoxin‐α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid‐like tissue. Immunity 2001; 14: 111–21. [DOI] [PubMed] [Google Scholar]
- 55. Kirk CJ, Hartigan‐O’Connor D, Mule JJ. The dynamics of the T‐cell antitumor response: chemokine‐secreting dendritic cells can prime tumor‐reactive T cells extranodally. Cancer Res 2001; 61: 8794–802. [PubMed] [Google Scholar]
- 56. Dieu‐Nosjean MC, Antoine M, Danel C et al. Long‐term survival for patients with non‐small‐cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26: 4410–7. [DOI] [PubMed] [Google Scholar]


