Abstract
High atomic number material, such as gold, may be used in conjunction with radiation to provide dose enhancement in tumors. In the current study, we investigated the dose‐enhancing effect and apoptotic potential of gold nanoparticles in combination with single‐dose clinical electron beams on B16F10 melanoma tumor‐bearing mice. We revealed that the accumulation of gold nanoparticles was detected inside B16F10 culture cells after 18 h of incubation, and moreover, the gold nanoparticles were shown to be colocalized with endoplasmic reticulum and Golgi apparatus in cells. Furthermore, gold nanoparticles radiosensitized melanoma cells in the colony formation assay (P = 0.02). Using a B16F10 tumor‐bearing mouse model, we further demonstrated that gold nanoparticles in conjunction with ionizing radiation significantly retarded tumor growth and prolonged survival compared to the radiation alone controls (P < 0.05). Importantly, an increase of apoptotic signals was detected inside tumors in the combined treatment group (P < 0.05). Knowing that radiation‐induced apoptosis has been considered a determinant of tumor responses to radiation therapy, and the length of tumor regrowth delay correlated with the extent of apoptosis after single‐dose radiotherapy, these results may suggest the clinical potential of gold nanoparticles in improving the outcome of melanoma radiotherapy. (Cancer Sci 2008; 99: 1479–1484)
Radiation dose enhancement by high atomic number (Z) materials has long been investigated. In theory, loading high Z materials into the tumor could result in greater photoelectric absorption within the tumor than in surrounding tissues, and thereby enhance the dose delivered to a tumor during radiation therapy. At least 20 years ago, it was noted in vitro that this effect might be employed to enhance radiotherapy for cancer.( 1 ) Accumulating studies have demonstrated the dose enhancement caused by high Z materials in kilovoltage beams( 2 , 3 , 4 ) and in megavoltage beams.( 5 , 6 , 7 , 8 ) Moreover, enhanced cell killing was also observed when cells were irradiated adjacent to high Z materials by kilovoltage X‐rays.( 9 , 10 , 11 , 12 ) In clinical practice, electron beams from linear accelerators have increasingly taken the place of kilovoltage X‐ray beams for skin and subcutaneous tumors because they offer distinct advantages in terms of dose uniformity in the target volume and in minimizing the dosage to deeper tissues.( 13 ) Although kilovoltage beams could maximize tumor dose enhancement, it has technical restrictions. The use of kilovoltage X‐rays produces significant dose heterogeneity inside the target tumor.( 4 , 14 )
To be clinically useful, a radiosensitizer and/or dose enhancer should significantly increase the therapeutic ratio and should be readily available, easily utilized, and non‐toxic. Gold (Au; Z = 79) or nanogold (gold nanoparticles, AuNP) showed dose‐enhancing effects in cell experiments,( 15 ) the murine model,( 16 ) and through Monte Carlo calculations.( 17 ) Gold nanoparticles have been actively investigated in a wide variety of biomedical applications due to their biocompatibility and ease of conjugation to biomolecules.( 18 , 19 , 20 , 21 ) Besides, nanoparticles have the advantages of small size (1–100 nm) and ability to evade the immune system,( 22 , 23 ) and also have been shown to preferentially accumulate in tumors.( 24 , 25 , 26 , 27 , 28 )
While previous studies have primarily examined the dose enhancement factor by Au, it is also known that radiation‐induced apoptosis is a significant component of radiation‐induced cell death. Consequently, modulating the apoptotic response and thereby the radiosensitivity is of interest.( 29 , 30 , 31 , 32 , 33 , 34 ) Therefore, in the current study, we investigated the dose‐enhancing effect and apoptotic potential of gold nanoparticles in combination with single‐dose clinical electron beams on B16F10 melanoma tumor‐bearing mice.
Materials and Methods
Preparation of AuNP. AuNP were prepared as previously described with slight modifications.( 35 ) All glassware used in these preparations was thoroughly cleaned in aqua regia (3 parts HCl and 1 part HNO3), and all solutions were made using 18‐MΩ‐deionized, 0.22‐µm‐filtered water. Briefly, 50 mL of HAuCl4 (1 mM) was reduced with sodium citrate (38.8 mM, 5 mL) by boiling with vigorous stirring for 10 min. The resulting burgundy suspension was cooled, sterile‐filtered, and stored in glass bottles at room temperature or 4°C. The AuNP were spherical and well‐dispersed with an approximate diameter of 13 nm confirmed by a transmission electron microscopy. The particle concentration was approximately 10.72 nM (180 µg/mL) quantified by a maximum absorption at 520 nm. The prepared nanoparticle solution could be concentrated by centrifugation and was stable for at least 6 months.
Cells and mice. Murine B16F10 melanoma cells were cultured in Dulbecco's modified Eagle's medium supplemented with 50 µg/mL gentamicin, 2 mM L‐glutamine, and 10% cosmic calf serum (Hyclone, Logan, UT, USA) at 37°C in an atmosphere of 5% CO2. Female C57BL/6 mice (6–8‐week‐old) were obtained from the Laboratory Animal Center of the National Cheng Kung University. Animals were maintained in specific pathogen‐free animal care facilities under isothermal conditions with regular photoperiods. All animal experiments were performed following the guidelines approved by the Laboratory Animal Care and Use Committee of the National Cheng Kung University.
Visualization of AuNP. AuNP localization was visualized by silver enhancement or by fluorescence labeling of AuNP. B16F10 melanoma cells were seeded on cover glass at 37°C for 6 h, and the medium was replaced by culture medium with or without 10 nM AuNP or AuNP‐Alexa Fluor 594 conjugates (Alexa Fluor 594 was purchased from Invitrogen, Eugene, OR, USA). Eighteen hours post‐AuNP addition, cells were washed with phosphate‐buffered saline (PBS), the localization of AuNP was determined by silver enhancement of AuNP according to the manufacturer's instructions (Sigma, St Louis, MO, USA), and the localization of AuNP‐Alexa Fluor 594 conjugates was detected directly under a microscopy with an Olympus DP1T digital camera system (Olympus Optical, Tokyo, Japan).
Live‐cell endoplasmic reticulum (ER) and Golgi labeling. ER‐Tracker Red dye and BODIPY TR Ceramide Golgi Tracker (Molecular Probes, Carlsbad, CA, USA) were used, respectively, for live‐cell ER and Golgi labeling according to the manufacturer's instructions.
Animal studies.
Tumors. C57BL/6 mice were inoculated subcutaneously (s.c.) in the thigh with syngeneic mouse melanoma B16F10 cells (1 × 106) suspended in 0.1 mL of PBS at day 0, and at day 7, visible nodules developed at all injection sites with approximate tumor volumes of 50–90 mm3. Groups of four to seven tumor‐bearing mice were injected intravenously (i.v.) with 200 µL of 200 nM AuNP in phosphate buffer (PB), or with 200 µL of PBS via the lateral tail vein. All mice were monitored for tumor growth and survival as previously described.( 36 )
Irradiation. Approximately 24 h post‐AuNP injection, a 1‐inch diameter tumor region of the leg was irradiated with 6 MeV electrons using a Varian 2100C linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) under normoxic conditions. The delivered dose was 25 Gy per mice. Animals were anesthetized with pentobarbital intraperitoneally (90 µg/g body weight).
Biodistribution of AuNP. Groups of three tumor‐bearing mice were injected with AuNP or with PBS via the lateral tail vein. Twenty‐four hours postinjection, blood and tissues were excised. Samples were pooled and dried at 105°C until the weight remained constant, and then were homogenized into powder. For AuNP detection, 0.5 g of tissue samples was used. Six mililiters of concentrated HCO3 was added into each sample, and incubated at room temperature until the samples were completely nitrated. After nitration, 2 mL of H2O2 was added. Once the solution became clear, samples were then filtrated to remove cell debris. The resulting samples were then analyzed for Au concentration using atomic absorption detection (National Sun Yat‐Sen University, Kaohsiung, Taiwan).
Clonogenic survival. The effectiveness of the combination of AuNP and ionizing radiation was assessed by clonogenic assays. The B16F10 melanoma cell lines were treated with AuNP (10 nM) for 18 h and then exposed to different doses of ionizing radiation. Briefly, cells were irradiated with using a linear accelerator (Varian 2100C; Varian, Palo Alto, CA, USA) at room temperature in T‐25 flasks. After treatment, cells were trypsinized and counted. Known numbers were then replated and returned to the incubator to allow macroscopic colony development. Colonies were counted after 7 days, and the plating efficiency and surviving fraction for given treatments were calculated based on the survival of non‐irradiated cells treated with the vehicle or AuNP alone.
Detection of apoptosis. Apoptotic activity was analyzed on the basis of terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay. Tumors from groups of three tumor‐bearing mice were excised 6 h postradiotherapy,( 37 ) and embedded in OCT compound (Sakura Finetek USA, Torrance, CA, USA). Five‐micrometer thick cryostat sections from each representative specimen were obtained and fixed, and then processed with the DeadEnd Fluorometric TUNEL System (Promega, Madison, WI, USA) according to the manufacturer's protocol. The positive TUNEL signals were counted under a microscopy with an Olympus DP1T digital camera system (Olympus Optical). DAPI was used as the nuclear counter stain. Apoptotic cells were calculated by averaging the number of positive TUNEL signals from eight fields with highest density of TUNEL signals in each section.
Statistical analysis. Data were expressed as mean ± standard error of the mean (SEM). The survival analysis was done using the Kaplan–Meier survival curve and the log‐rank test. Other statistical differences were assessed with Student's t‐test. Statistical significance was set at P < 0.05.
Results
Visualization of AuNP in B16F10 cells. To detect the AuNP inside cultured cells, the localization of AuNP was visualized by silver enhancement (Fig. 1a) or by direct observation under a fluorescent microscopy (Fig. 1b). Our data demonstrated that after 18 h of incubation, AuNP could be detected inside the B16F10 cells. Moreover, we also found that the AuNP did not colocalize with the cell nucleus (Fig. 1b).
Figure 1.
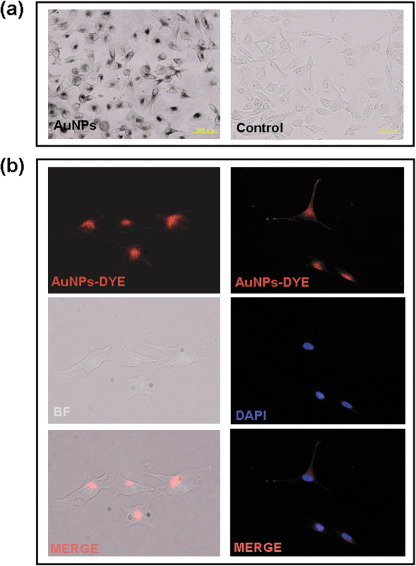
Visualization of gold nanoparticles (AuNP) inside B16F10 cells. AuNP localization in cells was visualized by silver enhancement or by fluorescence labeling of AuNP. (a) The localization of AuNP was determined by silver enhancement, and (b) the localization of AuNP‐. Alexa Fluor 594 conjugates were detected directly under a microscope (bar = 50 µm; original magnification 200×). BF, Bright field, DAPI, 4'‐6‐diamidino‐2‐phenylindole.
AuNP revealed colocalization with ER and Golgi apparatus in B16F10. As the AuNP revealed a non‐uniform distribution in the cytoplasm of B16F10 cells, to investigate the possibility that the subcellular localization of AuNP is in ER or Golgi, live‐cell ER or Golgi staining was used in addition to the silver enhancement of AuNP. Our results indicated that AuNP were localized in ER (Fig. 2a) and Golgi apparatuses (Fig. 2b) in B16F10 20 h after incubation.
Figure 2.
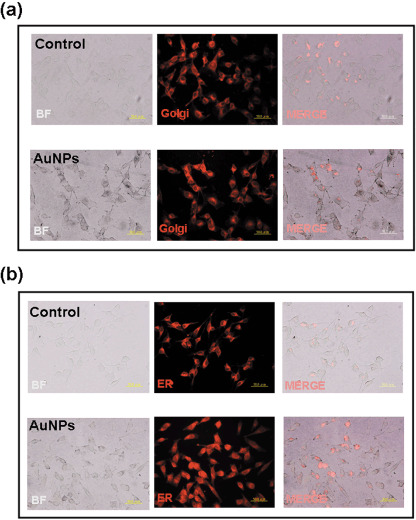
Gold nanoparticles (AuNP) colocalized with endoplasmic reticulum (ER) and Golgi in cells. B16F10 cells were cultured with AuNP for 20 h. The localization of AuNP was determined by silver enhancement, and the (a) ER‐Tracker Red dye and (b) BODIPY TR Ceramide Golgi Tracker were used for live‐cell ER and Golgi labeling (bar = 50 µm; oiginal magnification 200×; BF, bright field).
Biodistribution of Au 24 h postintravenous injection of AuNP in tumor‐bearing mice. To detect the biodistribution of AuNP 24 h postinjection, blood and tissues were excised and analyzed for Au concentration using the atomic absorption detection (Fig. 3a). The result showed that at 24 h following injection, a notable accumulation of AuNP inside tumor tissues was detected. The tumor‐to‐tumor surrounding muscle gold ratio was 6.4:1. Nevertheless, higher concentrations of AuNP were also found in spleen and liver, which indicated that AuNP were also uptaken by the reticuloendothelial system.
Figure 3.
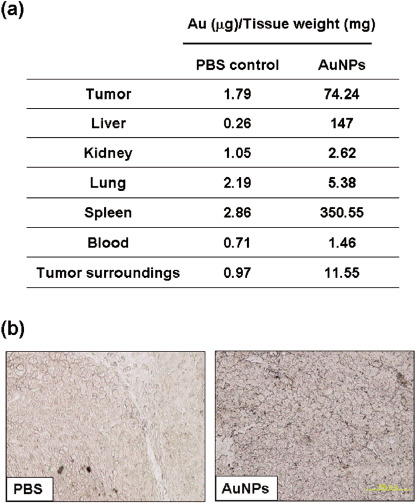
Biodistribution of gold nanoparticles (AuNP) in mice. (a) Twenty‐four hours after AuNP injection, tissues of tumor‐bearing mice were excised, processed, and used for AuNP detection using atomic absorption detection. (b) Silver staining of AuNP inside a tumor. Twenty‐four hours after AuNP injection, tumors were excised and paraffin‐embedded. Five‐micrometer thick sections from each representative specimen were obtained and then processed with the silver enhancement kit (bar = 200 µm). PBS, phosphate‐buffered saline.
In agreement with this biodistribution data, sliver enhancement of AuNP in the tumor biopsies also revealed the presence of AuNP inside tumor tissues (Fig. 3b).
AuNP radiosensitized melanoma cells. B16F10 cells were treated with AuNP and assessed for radiosensitization by clonogenic cell survival immediately after irradiation (Fig. 4). Our result revealed that AuNP radiosensitized B16F10 melanoma cells in the colony formation assay.
Figure 4.
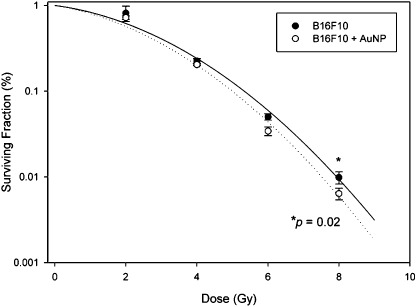
Gold nanoparticles (AuNP) radiosensitized melanoma cells. B16F10 cells were treated with AuNP (10 nM for 18 h) and assessed for radiosensitization by clonogenic cell survival immediately after irradiation. For the survival curves, each data point represents the average of three independent experiments each plated in triplicate ± SD (solid line, control; dotted line, 10 nM AuNP; *P = 0.02).
Antitumor effects of the combination of AuNP with radiotherapy in tumor‐bearing mice. To investigate whether the combination of AuNP and radiotherapy resulted in better antitumor effects in terms of tumor growth and survival than radiation alone, a syngeneic melanoma model was used in the animal study. Our result revealed that the tumor growth was both retarded in mice receiving either radiation alone or receiving AuNP followed by radiation (Fig. 5a) compared to the controls with no radiation. More importantly, tumor volume in the combination therapy group was significantly smaller compared with that in radiation alone group (P < 0.05), whereas administration of AuNP or PBS alone did not exert any antitumor effect on tumor‐bearing mice (Fig. 5a).
Figure 5.
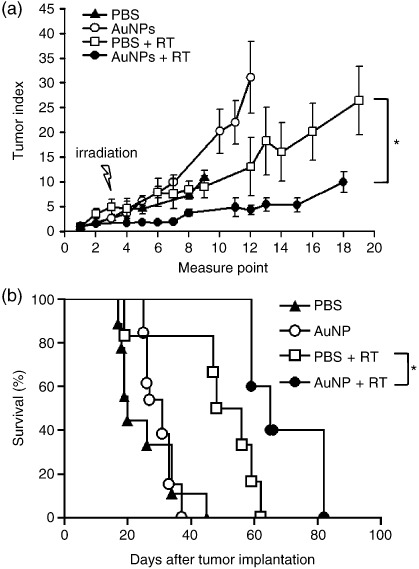
Antitumor effects of the combination treatment of gold nanoparticles (AuNP) and radiotherapy in tumor‐bearing mice. C57BL/6 mice were inoculated subcutaneously with B16F10 cells (1 × 106) at day 0. At day 7, tumor‐bearing mice were injected intravenously with 200 µL of 200 nM AuNP, or with 200 µL of phosphate‐buffered saline (PBS) 24 h before irradiation (25 Gy/mouse). Mice were monitored for (a) tumor growth and (b) survival (n = 4–7; *P < 0.05). RT, radiotherapy.
Furthermore, the Kaplan–Meier survival curves of the treated groups are illustrated in Figure 5b. The survival of mice with radiation and AuNP combination therapy was significantly longer than that of the radiation alone mice (P < 0.05; log‐rank test).
Apoptosis in tumors with AuNP and radiotherapy combination therapy. Increasing evidence has indicated the important contributing role of apoptosis in radiation‐induced cell death and for apoptosis as a determinant of radiosensitivity.( 30 , 31 , 32 ) In the present study, we examined whether apoptosis was associated with the antitumor effects of combination therapy. As shown in Figure 6a, the extent of apoptosis observed in TUNEL‐stained cryosections was found higher after a single‐dose radiotherapy compared to that in the no radiation controls. Noticeably, the number of apoptotic cells detected was significantly higher in the AuNP and radiation combination group than that in the radiation alone group (P < 0.05). The quantitative results are represented in Figure 6b.
Figure 6.
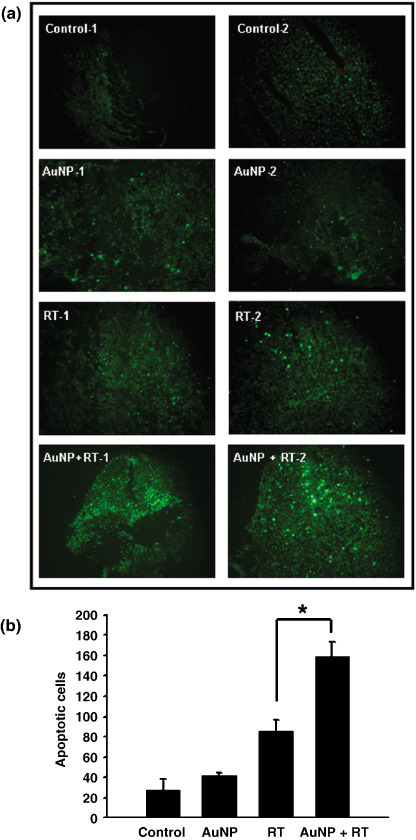
Apoptotic activity was analyzed by terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick end labeling (TUNEL) assay. Tumors were excised from mice 6 h postradiotherapy. Five‐micrometer thick representative cryostat sections were obtained and then processed with the TUNEL System. (a) Positive TUNEL straining was observed under a fluorescent microscopy. (b) Quantitative analysis. Apoptotic cells were calculated by averaging the number of positive TUNEL signals from eight fields with highest density of TUNEL signals in each section (n = 8; *P < 0.05; original magnification 40×). RT, radiotherapy.
Discussion
In the present study, our important finding was that in combination of AuNP with clinical electron beams in radiotherapy, an increase of apoptotic potential was observed in TUNEL‐stained cryosections of the tumors in mice. Knowing that radiation‐induced apoptosis has been considered a determinant of tumor responses to radiation therapy, and the length of tumor regrowth delay correlated with the extent of apoptosis after single‐dose radiotherapy,( 30 ) this result indicates the clinical potential of AuNP in improving the outcome of cancer radiotherapy.
Using sliver enhancement and fluorescent staining, we were able to determine the localization of AuNP inside B16F10 cells. We detected the presence of AuNP in cells after 18 h and 42 h of incubation, and furthermore, we found that AuNP were colocalized with ER and Golgi staining rather than with the nucleus of cells. It has been shown that continuous ER stress results in apoptotic cell death;( 38 ) therefore, the accumulation of AuNP in ER and Golgi may also contribute to the increase of the apoptotic potential of cells postirradiation.
Recently, considerable investigations have been made in exploring the role of apoptosis in cellular radiation responses. Although the contribution of cellular apoptotic potential to overall radiosensitivity and tumor responses to radiotherapy has been debated for several years, increasing evidence suggests that restoring the tumor apoptotic potential may have considerable therapeutic impact.
In the current study, we demonstrated the dose‐enhancing effect of AuNP in conjunction with single‐dose clinical electron beams in a B16F10 melanoma tumor‐bearing model, in which the tumor growth was retarded and the mice survival was prolonged. These effects were consistent with the results for a previous mammary tumor model;( 16 ) however, many less AuNP were injected intravenously into the mice in this study (1 g/kg compared to 2.7 g/kg). Besides, in the current study, the irradiation time‐point, 24 h post‐AuNP injection, was used instead of a time‐point of 2 min postinjection. Accumulation of unlabeled nanoparticles within tumor occurred through the enhanced permeability and retention effect, which takes advantage of the poorly formed tumor vasculature.( 24 , 25 , 26 , 27 , 28 ) Therefore, the time‐point we used for radiation after AuNP injection may be of benefit in producing high tumor to muscle gold ratio. (Fig. 3a and ( 39 )). In addition, by using electron beams produced by a linear accelerator in place of kilovoltage X‐rays, higher dose uniformity within the target tumor may be achieved.( 8 )
A recent study observed that the cellular uptake of AuNP peaked at diameters of 50 nm, in which spherical nanoparticles with diameters of 14, 30, 50, 74, and 100 nm were used.( 40 ) Since nanoparticle dose enhancement will be greatest for increased cellular uptake, 13 nm AuNP used in this study may have greater benefit than 1.9 nm AuNP used in the previous study,( 16 ) from a cellular uptake viewpoint.
In this study, radiation was given as a large single dose. However, in clinical radiotherapy, it is common practice to deliver the total dose as multiple small fractions in order to reduce normal tissue toxicity. Previous studies suggest that fractionated radiotherapy induces an accumulation of cell death by apoptosis proportional to the number of fractions. This may exceed the number of apoptotic cells induced by a high single dose.( 30 , 41 , 42 ) Therefore, using multiple fractions may be of significance in combination therapy of AuNP and radiation to induce more apoptotic cells and thereby improve the therapeutic ratio and survival of tumor‐bearing mice.
In conclusion, this study suggests that AuNP and radiotherapy combination therapy may have therapeutic potential for the treatment of melanoma. Our results demonstrate that intravenous injection of AuNP combined with clinical electron beams significantly retards the tumor growth and prolongs survival of mice. Increasing apoptotic potential in tumors may play an important role in this combination therapy.
Acknowledgments
This work was supported by grant H93‐A930 from the Center for Frontier Materials and Micro/Nano Science and Technology, National Cheng Kung University, Taiwan. We appreciated the expert technical support from Li‐Yao Chang (National Cheng Kung University Hospital, Taiwan).
References
- 1. Matsudaira H, Ueno AM, Furuno I. Iodine contrast medium sensitizes cultured mammalian cells to X rays but not to gamma rays. Radiat Res 1980; 84: 144–8. [PubMed] [Google Scholar]
- 2. Das IJ, Chopra KL. Backscatter dose perturbation in kilovoltage photon beams at high atomic number interfaces. Med Phys 1995; 22: 767–73. [DOI] [PubMed] [Google Scholar]
- 3. Das IJ. Forward dose perturbation at high atomic number interfaces in kilovoltage x‐ray beams. Med Phys 1997; 24: 1781–7. [DOI] [PubMed] [Google Scholar]
- 4. Verhaegen F, Reniers B, Deblois F et al . Dosimetric and microdosimetric study of contrast‐enhanced radiotherapy with kilovolt x‐rays. Phys Med Biol 2005; 50: 3555–69. [DOI] [PubMed] [Google Scholar]
- 5. Werner BL, Das IJ, Salk WN. Dose perturbations at interfaces in photon beams: secondary electron transport. Med Phys 1990; 17: 212–26. [DOI] [PubMed] [Google Scholar]
- 6. Li XA, Chu JC, Chen W et al . Dose enhancement by a thin foil of high‐Z material: a Monte Carlo study. Med Phys 1999; 26: 1245–51. [DOI] [PubMed] [Google Scholar]
- 7. Das IJ, Cheng CW, Mitra RK et al . Transmission and dose perturbations with high‐Z materials in clinical electron beams. Med Phys 2004; 31: 3213–21. [DOI] [PubMed] [Google Scholar]
- 8. Robar JL. Generation and modelling of megavoltage photon beams for contrast‐enhanced radiation therapy. Phys Med Biol 2006; 51: 5487–504. [DOI] [PubMed] [Google Scholar]
- 9. Rosengren B, Wulff L, Carlsson E et al . Backscatter radiation at tissue–titanium interfaces. Analyses of biological effects from 60Co and protons. Acta Oncol 1991; 30: 859–66. [DOI] [PubMed] [Google Scholar]
- 10. Rosengren B, Wulff L, Carlsson E et al . Backscatter radiation at tissue–titanium interfaces. Biological effects from diagnostic 65 kVp x‐rays. Acta Oncol 1993; 32: 73–7. [DOI] [PubMed] [Google Scholar]
- 11. Regulla DF, Hieber LB, Seidenbusch M. Physical and biological interface dose effects in tissue due to X‐ray‐induced release of secondary radiation from metallic gold surfaces. Radiat Res 1998; 150: 92–100. [PubMed] [Google Scholar]
- 12. Zellmer DL, Chapman JD, Stobbe CC et al . Radiation fields backscattered from material interfaces: I. Biological effectiveness. Radiat Res 1998; 150: 406–15. [PubMed] [Google Scholar]
- 13. Khan FM. Electron beam therapy. In: Khan FM, ed. The Physics of Radiation Therapy, 3rd edn. Philadelphia: Lippincott Willimas & Wilkins, 2003: 297–356. [Google Scholar]
- 14. Mesa AV, Norman A, Solberg TD et al . Dose distributions using kilovoltage x‐rays and dose enhancement from iodine contrast agents. Phys Med Biol 1999; 44: 1955–68. [DOI] [PubMed] [Google Scholar]
- 15. Herold DM, Das IJ, Stobbe CC et al . Gold microspheres: a selective technique for producing biologically effective dose enhancement. Int J Radiat Biol 2000; 76: 1357–64. [DOI] [PubMed] [Google Scholar]
- 16. Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol 2004; 49: N309–15. [DOI] [PubMed] [Google Scholar]
- 17. Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol 2005; 50: N163–73. [DOI] [PubMed] [Google Scholar]
- 18. Sokolov K, Follen M, Aaron J et al . Real‐time vital optical imaging of precancer using anti‐epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res 2003; 63: 1999–2004. [PubMed] [Google Scholar]
- 19. Mirkin CA, Letsinger RL, Mucic RC et al . A DNA‐based method for rationally assembling nanoparticles into macroscopic materials. Nature 1996; 382: 607–9. [DOI] [PubMed] [Google Scholar]
- 20. Tsai CY, Shiau AL, Cheng PC et al . A biological strategy for fabrication of Au/EGFP nanoparticle conjugates retaining bioactivity. Nano Lett 2004; 4: 1209–12. [Google Scholar]
- 21. Levy R, Thanh NT, Doty RC et al . Rational and combinatorial design of peptide capping ligands for gold nanoparticles. J Am Chem Soc 2004; 126: 10076–84. [DOI] [PubMed] [Google Scholar]
- 22. Moghimi SM, Hunter AC, Murray JC. Long‐circulating and target‐specific nanoparticles: theory to practice. Pharmacol Rev 2001; 53: 283–318. [PubMed] [Google Scholar]
- 23. Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. Faseb J 2005; 19: 311–30. [DOI] [PubMed] [Google Scholar]
- 24. Duncan R. The dawning era of polymer therapeutics. Nat Rev Drug Discov 2003; 2: 347–60. [DOI] [PubMed] [Google Scholar]
- 25. Jain RK. Transport of molecules, particles, and cells in solid tumors. Annu Rev Biomed Eng 1999; 1: 241–63. [DOI] [PubMed] [Google Scholar]
- 26. Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J Drug Target 2007; 15: 457–64. [DOI] [PubMed] [Google Scholar]
- 27. Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor‐selective macromolecular drug targeting. Adv Enzyme Regul 2001; 41: 189–207. [DOI] [PubMed] [Google Scholar]
- 28. Tanaka T, Shiramoto S, Miyashita M et al . Tumor targeting based on the effect of enhanced permeability and retention (EPR) and the mechanism of receptor‐mediated endocytosis (RME). Int J Pharm 2004; 277: 39–61. [DOI] [PubMed] [Google Scholar]
- 29. Chen CH, Zhang J, Ling CC. Transfected c‐myc and c‐Ha‐ras modulate radiation‐induced apoptosis in rat embryo cells. Radiat Res 1994; 139: 307–15. [PubMed] [Google Scholar]
- 30. Rupnow BA, Murtha AD, Alarcon RM et al . Direct evidence that apoptosis enhances tumor responses to fractionated radiotherapy. Cancer Res 1998; 58: 1779–84. [PubMed] [Google Scholar]
- 31. Rupnow BA, Knox SJ. The role of radiation‐induced apoptosis as a determinant of tumor responses to radiation therapy. Apoptosis 1999; 4: 115–43. [DOI] [PubMed] [Google Scholar]
- 32. Verheij M, Bartelink H. Radiation‐induced apoptosis. Cell Tissue Res 2000; 301: 133–42. [DOI] [PubMed] [Google Scholar]
- 33. Belka C, Jendrossek V, Pruschy M et al . Apoptosis‐modulating agents in combination with radiotherapy‐current status and outlook. Int J Radiat Oncol Biol Phys 2004; 58: 542–54. [DOI] [PubMed] [Google Scholar]
- 34. Nakajima T, Yukawa O, Tsuji H et al . Regulation of radiation‐induced protein kinase Cdelta activation in radiation‐induced apoptosis differs between radiosensitive and radioresistant mouse thymic lymphoma cell lines. Mutat Res 2006; 595: 29–36. [DOI] [PubMed] [Google Scholar]
- 35. Grabar KCFR, Hommer MB, Natan MJ. Preparation and characterization of Au colloid monolayers. Anal Chem 1995; 67: 735–43. [Google Scholar]
- 36. Shiau AL, Lin PR, Chang MY et al . Retrovirus‐mediated transfer of prothymosin gene inhibits tumor growth and prolongs survival in murine bladder cancer. Gene Ther 2001; 8: 1609–17. [DOI] [PubMed] [Google Scholar]
- 37. Garcia‐Barros M, Paris F, Cordon‐Cardo C et al . Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–9. [DOI] [PubMed] [Google Scholar]
- 38. Szegezdi E, Logue SE, Gorman AM et al . Mediators of endoplasmic reticulum stress‐induced apoptosis. EMBO Rep 2006; 7: 880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hainfeld JF, Slatkin DN, Focella TM et al . Gold nanoparticles: a new X‐ray contrast agent. Br J Radiol 2006; 79: 248–53. [DOI] [PubMed] [Google Scholar]
- 40. Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett 2006; 6: 662–8. [DOI] [PubMed] [Google Scholar]
- 41. Meyn RE, Stephens LC, Hunter NR et al . Reemergence of apoptotic cells between fractionated doses in irradiated murine tumors. Int J Radiat Oncol Biol Phys 1994; 30: 619–24. [DOI] [PubMed] [Google Scholar]
- 42. Ling CC, Guo M, Chen CH et al . Radiation‐induced apoptosis: effects of cell age and dose fractionation. Cancer Res 1995; 55: 5207–12. [PubMed] [Google Scholar]


