Abstract
Previous studies have firmly demonstrated that wogonin, a naturally occurring monoflavonoid extracted from the root of the Chinese herb medicine Scutellaria baicalensis, could effectively inhibit the proliferation of several cancer cell lines. However, little is known about the effect of wogonin on differentiation induction of leukemic cells. Here we investigate the potential role of wogonin in the proliferation and differentiation of NB4, a human promyelocytic leukemia cell line derived from a patient with acute promyelocytic leukemia. Our results indicated that wogonin significantly suppressed the proliferation and efficiently induced the differentiation of NB4 cells. NB4 cell growth was inhibited by 55–60% after treatment with 50 µM wogonin for a period of 5 days. The results of the nitroblue tetrazolium (NBT) reduction test (with 67.13% positive cells by 50 µM wogonin for 5 days), Giemsa staining (with 67.24% positive cells by 50 µM wogonin for 5 days), and the expression of mature‐related cell‐surface differentiation antigens CD11b and CD14 (with 70.94% CD11b+ and 5.82% CD14+ cells by 50 µM wogonin for 5 days) demonstrated an increase in the differentiation‐inducing action of wogonin on the NB4 cells, which was accompanied by an increase in mRNA and protein expression of phospholipids scramblase 1 (PLSCR1). Meanwhile, the level of phosphorylated PKCδ (Ser643) was dramatically increased in wogonin treated NB4 cells. Interestingly, wogonin treatment displayed little effect on the apoptosis of NB4 cells. Taken together, the results reported here demonstrated that wogonin could promote the granulocytic differentiation of NB4 cells by up‐regulating the expression of PLSCR1 gene. (Cancer Sci 2008; 99: 689–695)
Wogonin (Fig. 1) is a naturally occurring monoflavonoid isolated from Scutellaria baicalensis radix, a traditional Chinese herbal medicine widely used in the treatment of inflammatory diseases including atopic dermatitis, hyperlipemia, and atherosclerosis.( 1 , 2 ) More recently, it has been reported that wogonin has diverse biological activities including antioxidant,( 3 ) anti‐inflammatory,( 4 ) antithrombotic,( 5 ) antiproliferative,( 6 ) and anticancer activities.( 7 , 8 , 9 ) However, the potential effect of wogonin on the differentiation of leukemia cells has not yet been explored. In this report, we investigated the differentiation‐inducing effect of wogonin using NB4 cells, a human promyelocytic leukemia cell line derived from a patient with acute promyelocytic leukemia (APL).
Figure 1.
Molecular structure of wogonin.
APL is characterized by the presence of a PML/RARα fusion protein in the transformed cells.( 10 , 11 ) The PML/RARα fusion protein blocks myeloid differentiation,( 12 ) and is the first model of a malignant disease treated with differentiation agents. The NB4 cell line carried the t(15;17) translocation resulting in the joining of the promyelocytic leukemia‐associated protein (PML) oncogene to the gene encoding the retinoic acid receptor‐α (RARα).( 13 , 14 ) It has been shown that the fusion gene plays a central role in leukemogenesis in APL and the presence of the PML/RARα fusion protein disrupts the normal location of the PML nuclear body and interferes with the function of PML for growth inhibition.( 14 ) Previous studies have also demonstrated that NB4 cell line could be differentiated along granulocytic or monocyte/macrophage pathway by various differentiation inducers, providing an excellent model for studying cell differentiation.( 13 , 15 , 16 )
Although many differentiation inducers have been identified, such as all‐trans retinoic acid (ATRA), 1,25(OH)2D3, phorbol 12‐myristate 13‐acetate (PMA), and dimethyl sulfoxide (DMSO),( 17 , 18 ) the exact mechanism for the differentiation of leukemic cells remains unclear. Furthermore, few of the known differentiation inducers have been conclusively proven to display clinical efficacy because of their in vivo instability or severe adverse reactions. Therefore, it is necessary to search for other safer alternatives, such as wogonin, that display similar or better efficacy but with reduced side‐effects.
The latest studies about differentiation induction provide strong evidence that phospholipids scramblase 1 (PLSCR1) plays a potential role in cell maturation.( 19 , 20 , 21 , 22 ) It has been shown that ATRA‐ and PMA‐induced APL cell maturation is accompanied by an elevation of PLSCR1 expression.( 23 )
In this study, we investigated the effects of wogonin‐induced differentiation of NB4 cells in order to find the potential therapeutic function of wogonin in APL. The results of Giemsa staining, nitroblue tetrazolium (NBT) reduction, and cell‐surface differentiation antigen CD11b/CD14 measurement indicated that wogonin could prime the differentiation of NB4 cells along the granulocytic pathway. Our results also demonstrated that wogonin effectively induced the differentiation of NB4 cells due to increased expression of PLSCR1 in this cell line.
Materials and Methods
Chemicals and antibodies. Wogonin was isolated from Scutellaria baicalensis radix according to the procedure reported previously.( 24 , 25 ) Preparations containing 90% or higher pure wogonin were used in all experiments. Stock solutions of wogonin were prepared by dissolving the compound in 95% ethanol at 30 mmol and were kept at –20°C until needed. Final concentrations of ethanol have been found to have no effect on differentiation or cell growth. All‐trans retinoic acid (ATRA, used as positive control), phorbol 12‐myristate 13‐acetate (PMA), NBT, Giemsa stain, and 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) were all purchased from Sigma‐Aldrich (St. Louis, MO, USA). Stock solutions of ATRA were prepared in DMSO at 10 mmol and stored at –20°C wrapped with aluminum foil to avoid direct light irradiation. RPMI 1640 medium and heat‐inactivated fetal bovine serum (FBS) were purchased from Gibco/invitrogen (Shanghai, China). Phycoerythrin (PE) antihuman CD11b (Cat. No.12–0118; Mouse IgG, K), and fluorescein‐isothiocyanate (FITC) antihuman CD14 (Cat. No.11‐0149; Mouse IgG, K) antibodies were obtained from eBioscience (San Diego, CA, USA). PLSCR1 siRNA (h) (sc‐44028) and antibodies of anti‐PLSCR1 (sc‐27779; goat polyclonal) and anti‐α‐actin (sc‐1616; goat polyclonal) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). IRDye 800 conjugated antigoat second antibody was obtained from Rockland (Gilbertsville, PA, USA).
Cell culture. All cell lines used in this study were maintained in RPMI 1640 medium supplemented with 10% heat‐inactivated FBS. The cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 till mid‐log phase. Cells were harvested by centrifugation at 250 × g for 5 min and resuspended in RPMI‐1640 to make a stock cell suspension containing 6.5 × 104 cells/mL. Aliquots of the cell stock were subsequently seeded into a six‐well plate or plastic incubating flask and treated with the various concentrations of wogonin and ATRA (during the treatment, cell viability was at least 90% by trypan‐blue exclusion assay). Stock solutions of wogonin and ATRA were added to the culture medium to the final concentration indicated in each experiment. The final concentration of DMSO in the culture medium was maintained below 0.01% and was found to have no effect on the differentiation or the rate of cell division.( 26 )
Cell growth and MTT assays. Cells (6.5 × 104) were suspended in 4 mL of culture medium and cultured with or without wogonin in the six‐well plate. Cell numbers were counted with a cell counting chamber (Qiujing, Shanghai, China) and microscope (YS100; Nikon, Ibaraki, Japan) after culturing for the indicated durations. The proliferation of NB4 cells was determined by MTT assay also. Briefly, the cells (6.5 × 104) were seeded in each well, which contained 80 µL of the RPMI 1640 medium supplemented with 10% FBS in a 96‐well flat bottom plate (Corning, New York, NY, USA) and then 20 µL of various concentrations of wogonin were added. After 5 days, 20 µL of MTT (5 mg/mL stock solution) was added and the cells were incubated for additional 4 h at 37°C. Then the plate was centrifuged at 420 g for 5 min at 4°C. The supernatant was discarded and the blue deposit‐formazan formed in the cells was dissolved with 100 µL DMSO. The optical density was measured at 570 nm.( 27 , 28 )
Giemsa staining. For morphological observation, cells were collected on the slides, dried and fixed in methanol for 10 min, and stained with Giemsa stain for 15 min at a pH of 7.0.( 29 , 30 ) The cells were then observed by light microscope (YS100; Nikon, Ibaraki, Japan). The images were captured with Nikon Coolpix 4500 digital camera.
NBT colorimetry differentiation assay. NBT colorimetry was performed for functional examination of the differentiated cells.( 31 , 32 , 33 , 34 ) Briefly, cells were incubated in a 96‐well flat bottom plate for 5 days. Then cells were resuspended in 100 µL RPMI‐1640 medium containing 2 mg/mL of NBT and 500 µg/mL of PMA. After incubation at 37°C for 2 h, the cells were pelleted and resolved in 100 µL DMSO, and their absorbance at 570 nm was determined. A parallel MTT plate was prepared as control at the same time.
Maturation ‐related cell‐surface differentiation antigens expression assay. Two differentiation antigen were measured by flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA) with a direct immunofluorescence analysis method as previously described.( 35 , 36 ) Briefly, cells were collected, washed, and incubated with monoclonal mouse antihuman PE‐labeled anti‐CD11b and FITC‐labeled anti‐CD14 for 30 min at 37°C. Fluorescence intensity was analyzed by flow cytometry. Data were based on examination of 10 000 cells/sample selected randomly from 5 × 105 cells.
Semiquantitative reverse transcription–polymerase chain reaction (RT‐PCR) for PLSCR1 mRNA. Total RNA was isolated by Tripure Isolation Reagent (Roche Diagnostic, Nutley, NJ, USA) and reverse transcription (RT) was performed by Promega M‐MLV Reverse Transcriptase with its suitable reaction system (Promega, Madison, WI, USA) following the manufacturer's instructions. PCR reactions to amplify PLSCR1 first described by Sims et al.( 37 ) and glyceraldehydes‐3‐phosphate dehydrogenase (GAPDH) cDNA were performed using the Eppendorf Mastercycler (Eppendorf, Hamburg, Germany) with specific primers for PLSCR1 (sense primer, 5′‐CAG CCT CCA TTA AAC TGT CC‐3′; antisense primer, 5′‐TCT TAG TGG TCT CTC CAG AG‐3′), and for GAPDH (sense primer, 5′‐TGA AGG TCG GAG TCA ACG GAT TTG G‐3′; antisense primer, 5′‐ATG TGG GCC ATG AGG TCC ACC AC‐3′).( 23 ) PCR consisted of 28 cycles with denaturing at 95°C for 45 s, annealing at 55°C for 45 s, and extension at 72°C for 60 s. Amplification cycles were preceded by a denaturation step (95°C for 5 min) followed by an elongation step (72°C for 10 min). After amplification, PCR products were analyzed with a 1% agarose gel, and the signal intensities of amplified PLSCR1 fragments were analyzed with the Syngene Bio Imaging System and normalized against 983‐bp GAPDH using the GeneGenius gel documentation and analysis system (Gene Tools Analysis version 3.03.03; SynGene, Cambridge, UK).
Western blot. Cells were harvested and washed with ice‐cold phosphate‐buffered saline (PBS), and lyzed with ice‐cold lysis buffer (50 mmol Tris[tris(hydroxymethyl) aminomethane]–HCl (pH 8.0), 150 mmol NaCl, 0.1% wt/vol sodium dodecyl sulfate (SDS), 1% non‐idet P‐40, 0.5% Sodium deoxycholate, and protease inhibitor aprotinin and PMSF) on ice for 30 min. Cell lysates were centrifuged at 10 600g for 10 min at 4°C and protein in the supernatants was quantified. Protein extracts were equally loaded on 15% SDS‐polyacrylamide gel and electrophoretically transferred to nitrocellulose membranes (BioTrace NT; PallCor, Ann Arbor, MI, USA). After blocking with 10%‐non‐fat milk in PBS for 1 h at 37°C, the membranes were incubated for 2 h with monoclonal antihuman PLSCR1 antibody (1 : 800), rabbit polyclonal antiphospho‐PKCδ (Ser643) (Cell Signaling, Beverly, MA, USA), and rabbit polyclonal anti‐PKCδ antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), in PBS‐T (PBS and 0.1% Tween 20) at 37°C followed by IRDye 800 conjugated secondary antibody for 1 h at 37°C. Detection was performed by the Odyssey Infrared Imaging System (LI‐COR, Lincoln, NE, USA). All blots were stripped and reprobed with polyclonal anti‐α‐actin antibody to ascertain equal loading of protein.
RNA Interference of PLSCR1. PLSCR1 siRNA (h) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and was used following the instructions provided by the vendor. This product contains a pool of three 19‐mer oligonucleotides with the following sequences specific to PLSCR1 mRNA target: P1, CCAAAUCAGCCAGUGUAUA (location 446); P2, CUGUCCACCUGGAUUAGAA (location 538); and P3, GUAGUGGAUUAGUGAAAGU (location 1210).
For transfection experiments, cells were treated with medium in the absence of serum for 4 h. Meanwhile, solution A (4.4 µL siRNA, 550 µL medium without serum) and solution B (11 µL liposome, 550 µL medium without serum) were prepared. After standing at room temperature for 5 min, solution A was added drop‐wise into solution B with gentle shaking after addition of each drop. The reaction mixture was allowed to stand at room temperature for 20 min; 1.3 mL of cell suspension and 500 µL mixture of solution A and solution B were added to each well in the six‐well plate. After gently shaking, the mixture was incubated at 37°C for 3 h before centrifugation at 153g for 6 min. After decanting the supernatant, cell medium with was were added to sediment and the mixture was incubated overnight (18–24 h). The mixture was then centrifuged at 153g for 6 min. The supernatant was discarded and 2 mL medium containing drugs was added to the cell pellet. This mixture was further incubated at 37°C for 3 days. The cells were then harvested and tested by flow cytometry.
Results
Effects of wogonin on the growth of human promyelocytic NB4 leukemia cells. Human promyelocytic leukemia cell line NB4 was cultured in the absence and presence of wogonin at various concentrations (0.1–100 µM). The effects of wogonin on cell growth were assessed by the commonly used MTT assay. As shown in Figure 2, wogonin exhibited potent cytotoxic activity against the growth of NB4 cells compared with control experiments at the same exposure time (P < 0.01, unpaired t‐test). The results in Figure 2 also demonstrated that the efficiency of cell growth inhibition increased dramatically as the concentration of wogonin was increased. For example, cell proliferation was almost completely inhibited in the presence of 100 µM wogonin, while almost no growth inhibition was observed when 0.1 µM wogonin was used. When NB4 cells were treated with 50 µM wogonin for a period of 5 days, approximately 60% growth inhibition was observed. This is comparable to the results observed with 1 µM ATRA, an agent commonly used for the treatment of APL.( 38 ) A linear regression of the data in Figure 2 allowed the prediction of the IC50 (39.3 ± 3.1 µM with 5 day treatment) of wogonin for NB4 cells. It was noted that the efficiency of growth inhibition was also correlated with exposure time at a given wogonin concentration. However, the dependence on exposure time was only significant when wogonin concentration was below IC50. When wogonin was applied at higher concentrations, the effect of contact time became less significant. Actually, compared with cells in the control experiments, significant growth inhibition was observed with as low as 1 µM wogonin from the third day of treatment (Fig. 2). It is worth mentioning that wogonin‐induced cell growth inhibition is not directly related to cell apoptosis as revealed by our flow cytometry measurement (see supplemental documents). The low apoptosis rates observed for NB4 cells treated with different concentrations of wognin during the entire observation period implicated that the growth inhibition effect of wogonin on the NB4 cell is mainly attributed to its ability to effectively induce the differentiation of this cell line as described below.
Figure 2.
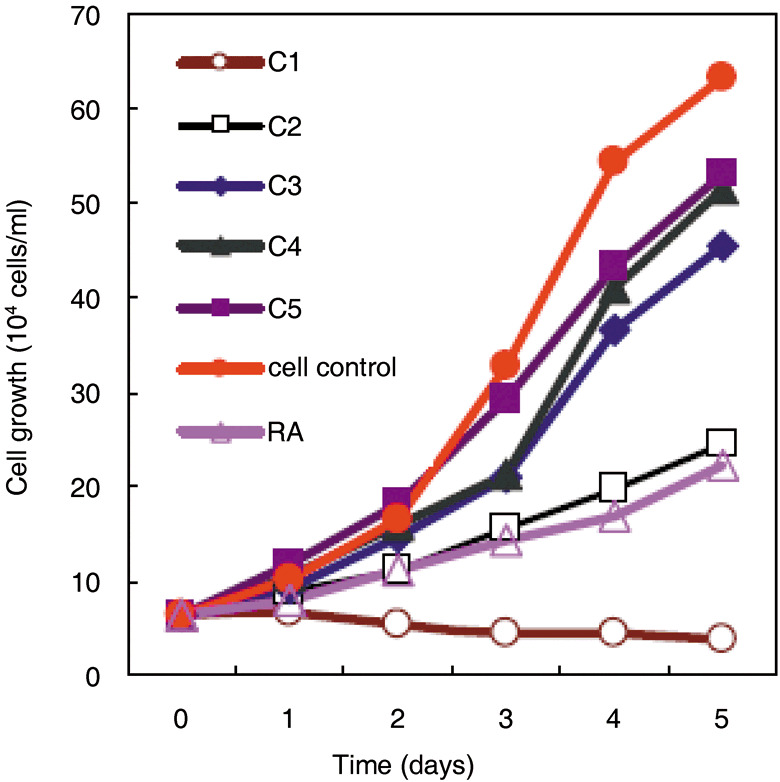
The effect of wogonin on the growth of NB4 cells compared with all‐trans retinoic acid (ATRA). Cells were treated with different concentrations of wogonin or 1 µM ATRA for 5 days. Values are means ± SD of three parallel experiments. C1–C5 are different concentrations of wogonin used and correspond to 100, 50, 10, 1.0, and 0.1 µM, respectively.
Effects of wogonin on the differentiation of human promyelocytic NB4 leukemia cells. To detect the differentiation‐inducing effect of wogonin on NB4 cells, the NBT reduction assay and Giemsa staining were carried out. As described in ‘Materials and Methods’, the ratio of differentiation of NB4 cells is calculated by comparing the absorbance of parallel NBT/MTT (of identical concentration). The amount of NBT reduction by NB4 cells is found to be directly proportional to the concentration of wogonin used in the experiments as shown in Figure 3b. Interestingly, more than 75% cell differentiation was observed when NB4 cells were treated with 100 µM wogonin although more than 80% cell growth was inhibited. This suggested that essentially all cells that survived after treatment with 100 µM wogonin were mature. On the other hand, only about 70% cells reached the mature stage after treatment with 50 µM wogonin. The differentiation‐inducing effect of 50 µM wogonin is similar to that of 1 µM ATRA, which can trigger the maturation of about 78% NB4 cells (data not shown). The results showed that 6–80 µM of wogonin was the most effective concentration.
Figure 3.
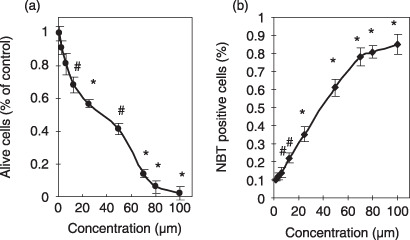
Growth inhibition (a) and nitroblue tetrazolium (NBT) reduction (b) of NB4 leukemia cells induced by wogonin. NB4 cells were cultured with wogonin for 5 days and cell viability was measured by the commonly used 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) assay as detailed in ‘Materials and Methods.’ Values are means ± SD of three separate experiments. (*P < 0.01, #P < 0.05).
At the same time, the morphologic maturation of NB4 cells induced by wogonin was investigated by Giemsa staining (Fig. 4a). Based on NBT reduction results, 70, 50, 25, 12.5, 6.2, and 3.1 µM wogonin‐treated cells were selected and stained with Giemsa. As shown in Figure 4a, NB4 cells treated with 25 µM wogonin were characterized by granulocyte (polymorphonuclear granulocytes, myelocytes, and metamyelocytes appeared to prevail; myeloblasts and promyelocytes were hardly observed), which is similar to the results after ATRA treatment (Fig. 4c). Similar results were observed for cells treated with other wogonin concentrations tested (data not shown). It was also noted that more mature cells were found after wogonin treatment (12.5, 6.2, and 3.1 µM, data not shown) as compared with the control experiment (Fig. 4b). These results also demonstrated that the number of mature NB4 cells increases as the concentration of wogonin is increased.
Figure 4.
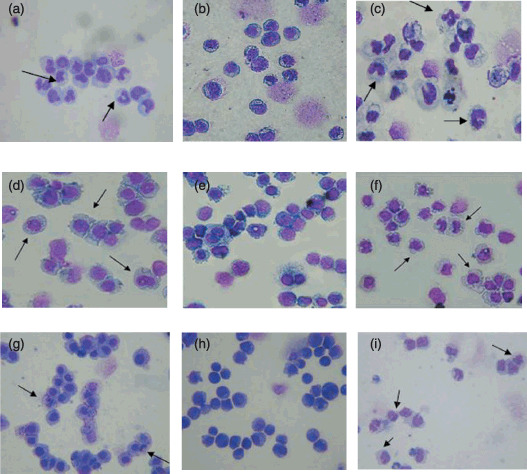
The differentiation‐inducing effect of wogonin on the mophology of NB4 cells. NB4 cells were treated with either 25 µM wogonin (a) or 1 µM all‐trans retinoic acid (ATRA) (c) for 5 days. The result of control experiment (no drugs applied) is included (b) for comparison purposes. The differentiation‐inducing effects of wagonin on U937 (d, 25 µM wogonin; e, control; and f, 1 µM ATRA) and HL60 cells (g, 25 µM wogonin; h, control; and i, 1 µM ATRA) were also studied to examine cell‐type specificity of wagonin as a differentiation inducer. The cells were fixed and stained with Giemsa after treatment. Cells indicated with arrows have been induced to mature at different phases (polymorphonuclear granulocytes, myelocytes, and metamyelocytes).
To evaluate cell‐type specificity of wagonin as a differentiation inducer, two additional leukemia cell lines, U937 and HL60, both non‐APL myeloid leukemia cells, were investigated under identical experimental conditions. Our results indicated that wogonin can induce differentiation in both U937 (Fig. 4d) and HL60 (Fig. 4g) cell lines, as with ATRA (Fig. 4f,h). Although this observation differed from our expectations (wogonin can induce differentiation of NB4 cells but not other leukemia cells), it does not compromise its potential use as a differentiation inducer for the treatment of acute promyelocytic leukemia (APL) because it has less adverse effects than ATRA. Since both ATRA and wogonin lack selectivity for APL cells, it is necessary to search for better and more selective agents for the treatment of APL.
Effect of wogonin on CD11b and CD14 expressions in NB4 cells. CD11b expression has been used as a marker of granulocytic differentiation, while CD14 expression has been used to monitor monocytic differentiation.( 39 , 40 ) In order to confirm the differentiation‐inducing effect of wogonin, we examined the expression of CD11b and CD14 in NB4 cells using flow cytometry. Figure 5 shows the flow cytometric diagrams of CD11b and CD14 expression in NB4 cells after treatment with 50, 12.5, and 3.1 µM wogonin. Compared with untreated cells (Fig. 5a 1), the number of CD11b positive cells increased significantly after treatment with 50 µM wogonin for 5 days (Fig. 5a 3). However, no significant change was observed for the expression of monocytic antigen CD14 (Fig. 5b3–b5 ). Similar results were found for ATRA‐treated NB4 cells (Fig. 5a 2 and b2). These results are in complete agreement with our MTT experiments, demonstrating that wogonin induced NB4 cells to differentiate toward granulocyte‐like cells and the degree of this effect is directly proportional to the concentration of wogonin employed.
Figure 5.
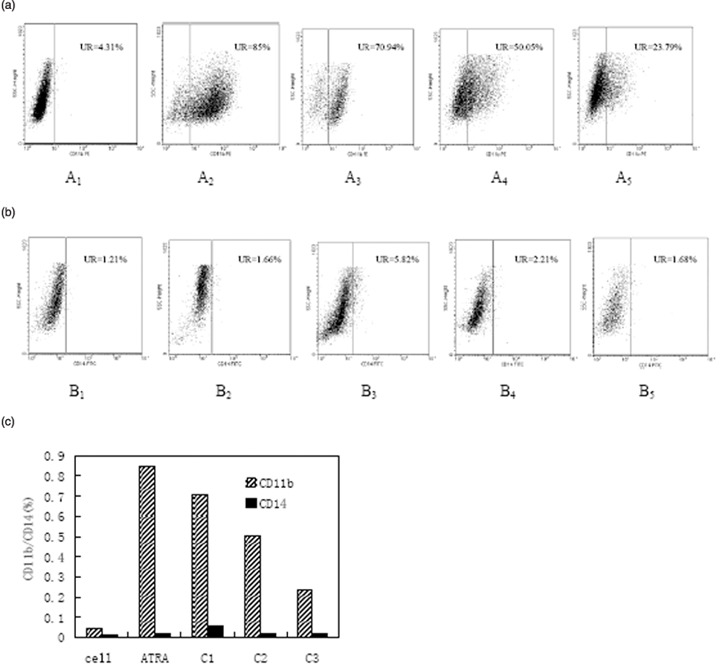
Flow cytometry measurement of cell‐surface differentiation antigen CD11b/CD14 expression in NB4 cells after treatment with different concentrations of wogonin. All‐trans retinoic acid (ATRA) (1 µM) was used as a positive control. The expression of CD11b (a) gradually increases while that of CD14 (b) remains unchanged after wogonin treatment. The subscripts 1–5 represent control, ATRA, 50, 12.5, and 3.1 µM of wogonin, respectively. A comparison of the expression level of CD11b and CD14 is given in (C), where cell stands for control experiment and C1, C2, and C3 represent 50, 12.5, and 3.1 µM of wogonin, respectively.
Wogonin induces PLSCR1 expression in NB4 cells. Although the results presented above have firmly established the role of wogonin in differentiation induction of NB4 cells, the mechanism or the molecular targets of wogonin remain to be defined. As PLSCR1 was required in the maturation of granulocytes by ATRA,( 23 , 41 ) we evaluated the expression of PLSCR1 during wogonin‐induced differentiation of NB4 cells. Our results (Fig. 6) indicated that low concentrations of wogonin (≤ 3.1 µM), which induced little cell differentiation, failed to modulate the expression of PLSCR1. However, higher concentrations (70, 50, 25, 12.5, and 6.2 µM) of wogonin, which induced significant cell differentiation, dramatically enhanced PLSCR1 expression (Fig. 6b). This observation was further supported by the increased PLSCR1 mRNA as proved by our semiquantitative RT‐PCR experiment (Fig. 6a). Although we could not compare the amount of PLSCR1 protein with the degree of wogonin‐induced differentiation of NB4 cells exactly, these results suggested that the induction of PLSCR1 gene expression is associated with wogonin‐induced granulocytic differentiation of NB4 cells. This implication is further supported by the dramatic increase in the phosphorylation of protein kinase Cδ (PKCδ) in NB4 cells treated with wogonin, although no significant change in the expression of PKCδ was detected (see supplemental document). This is in complete agreement with the mechanism proposed for ATRA‐induced differentiation of leukemic cells.( 23 )
Figure 6.
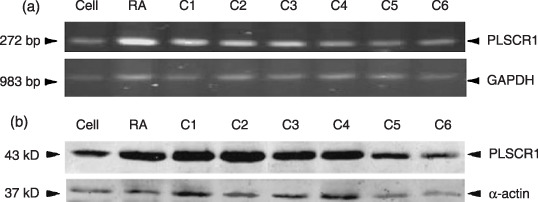
Effects of wogonin on PLSCR1 expression in NB4 cells, where C1–C6 represent wogonin concentrations of 70, 50, 25, 12.5, 6.2, and 3.1 µM, respectively. NB4 cells were treated with the indicated concentrations of wogonin or all‐trans retinoic acid (ATRA) for 5 days, semiquantitative reverse transcription–polymerase chain reaction (RT‐PCR) for PLSCR1 mRNA with G3PDH as loading control (a), and Western blot for PLSCR1 with α‐actin as loading control (b) were performed as detailed in ‘Materials and Methods’.
Effects of RNAi of PLSCR1 on wogonin‐induced differentiation in NB4 cells. The results presented above have firmly established the role and the mechanism or the molecular targets of wogonin in differentiation induction of NB4 cells. The effects of RNAi of PLSCR1 on wogonin‐induced differentiation in NB4 cells remain unclear from these experiments. Therefore, we performed the transfection experiment to test the interrelation between RNAi of PLSCR1 and wogonin‐induced differentiation. Our flow cytometry experiments (see supplemental document) indicated that wogonin (50 µM) treatment can significantly stimulate the expression of CD11b (56.1%) and CD14 (35.5%) compared to cells from control group. However, when PLSCR1 was nulled by RNA interference while keeping the concentration of wogonin at the same level (50 µM), the expression of CD11b was dramatically suppressed (15.5%), although the production of CD14 was only slightly decreased (9.8%). This observation suggested that proper expression of PLSCR1 and wogonin induced differentiation in NB4 cells are closely related. No experiments were carried out to test the effects of control siRNAs as they contain a scrambled sequence that will not lead to the specific degradation of any known cellular mRNA according to the manufacturer's instructions.
Discussion
Since the successful introduction of ATRA for the treatment of APL in 1986, a potentially less toxic cancer therapeutic strategy known as ‘differentiation therapy’ has been developed, which uses drugs to induce cancer cells to undergo terminal differentiation, thus preventing their further proliferation.( 38 , 42 , 43 ) However, a rapid increase in leukocytes is commonly observed during ATRA therapy, and the treatment is often accompanied by retinoic acid (RA) syndrome.( 44 ) Another drawback of ATRA therapy is the development of resistance, and the duration of remission is relatively short.( 45 , 46 ) Therefore, increasing efforts have been focused on developing novel and potent differentiation inducers with less adverse effects in recent years.
In the current work, we demonstrated that wogonin, one of the major constituents of Scutellaria baicalensis, is an effective differentiation inducer of NB4 cells. The exact mechanism of differentiation induction by wogonin on NB4 cells was unclear. However, the elevated expression of PLSCR1 in wogonin‐treated NB4 cells suggested that wogonin‐induced differentiation of NB4 cells is mediated by the modulation of PLSCR1 gene expression in this cell line. PLSCR1 is a possible adaptor protein, and the expression of PLSCR1 is specifically induced by special differentiation inducers in APL‐derived cell lines and primary APL cells that have the PML/RARa fusion protein. Therefore, the PLSCR1 gene could be a target of PML/RARα.( 41 ) PLSCR1 was originally identified based on its capability to promote transbilayer movement of membrane phospholipids,( 20 , 37 ) but recent studies have provided strong evidence for its role in cell signaling and in cell maturation.( 47 ) Proliferation and terminal differentiation of myeloid precursor cells in response to selective growth factors are impaired in PLSCR1− /– mice. It is also reported that in both monocytic and granulocytic lineages the expression of PLSCR1 markedly increases upon terminal differentiation into neutrophils and macrophages.( 21 ) Conversely, de novo expression of a mutant mRNA encoding a truncated form of murine PLSCR1 (also known as MmTRA1a, deleting the proline‐rich segment between codons 1–128) was identified in a monocytic leukemia cell line, and this mutation was found to correlate with the ability of these cells to proliferate in vivo.( 20 ) In contrast, the expression of full‐length PLSCR1 induced differentiation of these leukemic cells to macrophages.( 20 ) Furthermore, Nakamaki et al.( 41 ) reported that PLSCR1 mRNA was specifically induced during granulocytic and monocytic differentiation of APL cells by ATRA and PMA, respectively.( 22 ) Recent studies performed in patients with acute myelogenous leukemia (AML) and APL showed that higher levels of PLSCR1 mRNA were associated with significantly longer overall survival, independent of chromosomal aberrations,( 41 , 48 ) suggesting PLSCR1 mRNA level as a new prognostic factor for AML and APL.( 41 , 49 ) Here, we have demonstrated that the expression of PLSCR1 was markedly increased during wogonin‐induced granulocytic differentiation of NB4 cells. This finding is consistent with the results observed with ATRA. Therefore, it is suggested that PLSCR1 is one of the essential target‐genes in wogonin‐induced granulocytic differentiation of NB4 cells. This implication is further supported by our RNAi experiment that showed significant reduction in CD11b expression when PLSCR1 is nulled. Whether PLSCR1 expression is modulated directly by wogonin or indirectly via changes in the expression of PML/RARα after wognin treatment is under further investigation in our laboratories.
Interestingly, NB4 cells were inhibited almost 55–90% by 50–70 µM wogonin, while the differentiation ratio of the cells tested was 65–85%. This suggested that wogonin would not bring about serious adverse reactions (RA syndrome) observed during ATRA therapy that rapidly increased the number of leukocytes.( 11 ) Our results indicated that wogonin could serve as a new differentiation inducer, offering better management for human APL.
Supporting information
Fig. S1. Effects of wogonin concentration on early apoptosis of NB4 cells.
Fig. S2. Effects of treatment duration on early apoptosis of NB4 cells.
Fig. S3. Effects of wogonin on PKCδ and p‐PKCδ (Ser643) expression in NB4 cells.
Fig. S4. Flow cytometry measurement of the effect of RNAi on the expression of CD11b (a) and CD14 (b) in NB4 cells treated with or without wogonin (50 µM).
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (863 program, Nos. 2002AA2Z3112 and 2004AA2Z3A10), National Natural Science Foundation of China (No. 30472044), Ministry of Education of China under Special Award for Critical Research (No. 104099), the National Natural Science Foundation of China (Nos. 30472044 and 30701032), and National Institute of Health (SCORE S06GM‐008205).
References
- 1. Lee H, Kim YO, Kim H et al . Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J 2003; 17: 1943–4. [DOI] [PubMed] [Google Scholar]
- 2. Lim BO. Efficacy of wogonin in the production of immunoglobulins and cytokines by mesenteric lymph node lymphocytes in mouse colitis induced with dextran sulfate sodium. Biosci Biotechnol Biochem 2004; 68: 2505–11. [DOI] [PubMed] [Google Scholar]
- 3. Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta 1999; 1472: 643–50. [DOI] [PubMed] [Google Scholar]
- 4. You KM, Jong HG, Kim HP. Inhibition of cyclooxygenase/lipoxygenase from human platelets by polyhydroxylated/methoxylated flavonoids isolated from medicinal plants. Arch Pharm Res 1999; 22: 18–24. [DOI] [PubMed] [Google Scholar]
- 5. Kimura Y, Okuda H, Ogita Z. Effects of flavonoids isolated from scutellariae radix on fibrinolytic system induced by trypsin in human umbilical vein endothelial cells. J Nat Prod 1997; 60: 598–601. [DOI] [PubMed] [Google Scholar]
- 6. Huang HC, Wang HR, Hsieh LM. Antiproliferative effect of baicalein, a flavonoid from a Chinese herb, on vascular smooth muscle cell. Eur J Pharmacol 1994; 251: 91–3. [DOI] [PubMed] [Google Scholar]
- 7. Huang HC, Hsieh LM, Chen HW, Lin YS, Chen JS. Effects of baicalein and esculetin on transduction signals and growth factors expression in T‐lymphoid leukemia cells. Eur J Pharmacol 1994; 268: 73–8. [DOI] [PubMed] [Google Scholar]
- 8. Lee WR, Shen SC, Lin HY, Hou WC, Yang LL, Chen YC. Wogonin and fisetin induce apoptosis in human promyeloleukemic cells, accompanied by a decrease of reactive oxygen species, and activation of caspase 3 and Ca(2+)‐dependent endonuclease. Biochem Pharmacol 2002; 63: 225–36. [DOI] [PubMed] [Google Scholar]
- 9. Lim BO. Effects of wogonin, wogonoside, and 3,5,7,2′,6′‐pentahydroxyflavone on chemical mediator production in peritoneal exduate cells and immunoglobulin E of rat mesenteric lymph node lymphocytes. J Ethnopharmacol 2003; 84: 23–9. [DOI] [PubMed] [Google Scholar]
- 10. Borrow J, Goddard AD, Sheer D, Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science 1990; 249: 1577–80. [DOI] [PubMed] [Google Scholar]
- 11. Niitsu N, Ishii Y, Matsuda A, Honma Y. Induction of differentiation of acute promyelocytic leukemia cells by a cytidine deaminase‐resistant analogue of 1‐{beta}‐D‐arabinofuranosylcytosine, 1‐(2‐Deoxy‐2‐methylene‐{beta}‐D‐erythro‐pentofuranosyl) cytidine. Cancer Res 2001; 61: 178–85. [PubMed] [Google Scholar]
- 12. Benoit G, Roussel M, Pendino F, Segal‐Bendirdjian E, Lanotte M. Orchestration of multiple arrays of signal cross‐talk and combinatorial interactions for maturation and cell death: another vision of t(15;17) preleukemic blast and APL‐cell maturation. Oncogene 2001; 20: 7161–77. [DOI] [PubMed] [Google Scholar]
- 13. Lanotte M, Martin‐Thouvenin V, Najman S, Balerini P, Valensi F, Berger R. NB4, a maturation inducible cell line with t(15;17) marker isolated from a human acute promyelocytic leukemia (M3). Blood 1991; 77: 1080–6. [PubMed] [Google Scholar]
- 14. Melnick A, Licht JD. Deconstructing a disease: RAR{Alpha}, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood 1999; 93: 3167–215. [PubMed] [Google Scholar]
- 15. Clark CS, Konyer JE, Meckling KA. 1alpha,25‐dihydroxyvitamin D3 and bryostatin‐1 synergize to induce monocytic differentiation of NB4 acute promyelocytic leukemia cells by modulating cell cycle progression. Exp Cell Res 2004; 294: 301–11. [DOI] [PubMed] [Google Scholar]
- 16. Tohda S, Curtis JE, McCulloch EA, Minden MD. Comparison of the effects of all‐trans and cis‐retinoic acid on the blast stem cells of acute myeloblastic leukemia in culture. Leukemia 1992; 6: 656–61. [PubMed] [Google Scholar]
- 17. Scott RE. Differentiation, differentiation/gene therapy and cancer. Pharmacol Ther 1997; 73: 51–65. [DOI] [PubMed] [Google Scholar]
- 18. Tsiftsoglou AS, Pappas IS, Vizirianakis IS. Mechanisms involved in the induced differentiation of leukemia cells. Pharmacol Ther 2003; 100: 257–90. [DOI] [PubMed] [Google Scholar]
- 19. Ben‐Efraim I, Zhou Q, Wiedmer T, Gerace L, Sims PJ. Phospholipid scramblase 1 is imported into the nucleus by a receptor‐mediated pathway and interacts with DNA. Biochemistry (Mosc) 2004; 43: 3518–26. [DOI] [PubMed] [Google Scholar]
- 20. Kasukabe T, Kobayashi H, Kaneko Y, Okabe‐Kado J, Honma Y. Identity of human normal counterpart (MmTRA1b) of mouse leukemogenesis‐associated gene (MmTRA1a) product as plasma membrane phospholipid scramblase and chromosome mapping of the human MmTRA1b/phospholipid scramblase gene. Biochem Biophys Res Commun 1998; 249: 449–55. [DOI] [PubMed] [Google Scholar]
- 21. Zhou Q, Zhao J, Wiedmer T, Sims PJ. Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood 2002; 99: 4030–8. [DOI] [PubMed] [Google Scholar]
- 22. Freemantle SJ, Spinella MJ, Dmitrovsky E. Retinoids in cancer therapy and chemoprevention: promise meets resistance. Oncogene 2003; 22: 7305–15. [DOI] [PubMed] [Google Scholar]
- 23. Zhao KW, Li X, Zhao Q et al . Protein kinase Cdelta mediates retinoic acid and phorbol myristate acetate‐induced phospholipid scramblase 1 gene expression: its role in leukemic cell differentiation. Blood 2004; 104: 3731–8. [DOI] [PubMed] [Google Scholar]
- 24. Huang RL, Chen CC, Huang HL et al . Anti‐hepatitis B virus effects of wogonin isolated from Scutellaria baicalensis. Planta Med 2000; 66: 694–8. [DOI] [PubMed] [Google Scholar]
- 25. Li HB, Chen F. Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high‐speed counter‐current chromatography. J Chromatogr A 2005; 1074: 107–10. [DOI] [PubMed] [Google Scholar]
- 26. Matsuhisa T, Mori Y. Mode of differentiation of human promyelocytic leukemia cell line, HL‐60, by 1 alpha, 25‐dihydroxyvitamin D3. Blood Cells Mol Dis 1995; 21: 42–8. [DOI] [PubMed] [Google Scholar]
- 27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983; 65: 55–63. [DOI] [PubMed] [Google Scholar]
- 28. Seo B‐R, Lee K‐W, Ha J, Park H‐J, Choi J‐W, Lee K‐T. Saucernetin‐7 isolated from Saururus chinensis inhibits proliferation of human promyelocytic HL‐60 leukemia cells via G0/G1 phase arrest and induction of differentiation. Carcinogenesis 2004; 25: 1387–94. [DOI] [PubMed] [Google Scholar]
- 29. Goodman JL, Nelson C, Vitale B et al . Direct cultivation of the causative agent of human granulocytic ehrlichiosis. N Engl J Med 1996; 334: 209–15. [DOI] [PubMed] [Google Scholar]
- 30. Zimber A, Chedeville A, Abita J‐P, Barbu V, Gespach C. functional interactions between bile acids, all‐trans retinoic acid, and 1,25‐dihydroxy‐vitamin D3 on monocytic differentiation and myeloblastin gene down‐regulation in HL60 and THP‐1 human leukemia cells. Cancer Res 2000; 60: 672–8. [PubMed] [Google Scholar]
- 31. Idres N, Benoit G, Flexor MA, Lanotte M, Chabot GG. Granulocytic differentiation of human NB4 promyelocytic leukemia cells induced by all‐trans retinoic acid metabolites. Cancer Res 2001; 61: 700–5. [PubMed] [Google Scholar]
- 32. Kawaii S, Tomono Y, Katase E et al . Acridones as inducers of HL‐60 cell differentiation. Leuk Res 1999; 23: 263–9. [DOI] [PubMed] [Google Scholar]
- 33. Munshi CB, Graeff R, Lee HC. Evidence for a causal role of CD38 expression in granulocytic differentiation of human HL‐60 cells. J Biol Chem 2002; 277: 49 453–8. [DOI] [PubMed] [Google Scholar]
- 34. Pae HO, Seo WG, Kim NY et al . Induction of granulocytic differentiation in acute promyelocytic leukemia cells (HL‐60) by water‐soluble chitosan oligomer. Leuk Res 2001; 25: 339–46. [DOI] [PubMed] [Google Scholar]
- 35. Chen G‐Q, Shi X‐G, Tang W et al . Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I.As2O3 exerts dose–dependent dual effects on APL cells. Blood 1997; 89: 3345–53. [PubMed] [Google Scholar]
- 36. Drayson MT, Michell RH, Durham J, Brown G. Cell proliferation and CD11b expression are controlled independently during HL60 cell differentiation initiated by 1,25 alpha‐dihydroxyvitamin D(3) or all‐trans‐retinoic acid. Exp Cell Res 2001; 266: 126–34. [DOI] [PubMed] [Google Scholar]
- 37. Zhou Q, Zhao J, Stout JG, Luhm RA, Wiedmer T, Sims PJ. Molecular cloning of human plasma membrane phospholipid scramblase. A protein mediating transbilayer movement of plasma membrane phospholipids. J Biol Chem 1997; 272: 18 240–4. [DOI] [PubMed] [Google Scholar]
- 38. Huang ME, Ye YC, Chen SR et al . Use of all‐trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 1988; 72: 567–72. [DOI] [PubMed] [Google Scholar]
- 39. Brackman D, Lund‐Johansen F, Aarskog D. Expression of leukocyte differentiation antigens during the differentiation of HL‐60 cells induced by 1,25‐dihydroxyvitamin D3: comparison with the maturation of normal monocytic and granulocytic bone marrow cells. J Leukoc Biol 1995; 58: 547–55. [DOI] [PubMed] [Google Scholar]
- 40. Hmama Z, Nandan D, Sly L, Knutson KL, Herrera‐Velit P, Reiner NE. 1alpha,25‐dihydroxyvitamin D(3)‐induced myeloid cell differentiation is regulated by a vitamin D receptor‐phosphatidylinositol 3‐kinase signaling complex. J Exp Med 1999; 190: 1583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nakamaki T, Okabe‐Kado J, Yamamoto‐Yamaguchi Y et al . Role of MmTRA1b/phospholipid scramblase1 gene expression in the induction of differentiation of human myeloid leukemia cells into granulocytes. Exp Hematol 2002; 30: 421–9. [DOI] [PubMed] [Google Scholar]
- 42. Mistry AR, Pedersen EW, Solomon E, Grimwade D. The molecular pathogenesis of acute promyelocytic leukaemia. implications for the clinical management of the disease. Blood Rev 2003; 17: 71–97. [DOI] [PubMed] [Google Scholar]
- 43. Leszczyniecka M, Roberts T, Dent P, Grant S, Fisher PB. Differentiation therapy of human cancer: basic science and clinical applications. Pharmacol Ther 2001; 90: 105–56. [DOI] [PubMed] [Google Scholar]
- 44. Frankel SR, Eardley A, Lauwers G, Weiss M, Warrell RP Jr. The ‘retinoic acid syndrome’ in acute promyelocytic leukemia. Ann Intern Med 1992; 117: 292–6. [DOI] [PubMed] [Google Scholar]
- 45. Degos L, Chomienne C, Daniel MT et al . Treatment of first relapse in acute promyelocytic leukaemia with all‐trans retinoic acid. Lancet 1990; 336: 1440–1. [DOI] [PubMed] [Google Scholar]
- 46. Warrell RP Jr. Retinoid resistance in acute promyelocytic leukemia: new mechanisms, strategies, and implications. Blood 1993; 82: 1949–53. [PubMed] [Google Scholar]
- 47. Frasch SC, Henson PM, Kailey JM et al . Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cdelta. J Biol Chem 2000; 275: 23 065–73. [DOI] [PubMed] [Google Scholar]
- 48. Wiedmer T, Zhao J, Nanjundan M, Sims PJ. Palmitoylation of phospholipid scramblase 1 controls its distribution between nucleus and plasma membrane. Biochemistry (Mosc) 2003; 42: 1227–33. [DOI] [PubMed] [Google Scholar]
- 49. Yokoyama A, Yamashita T, Shiozawa E et al . MmTRA1b/phospholipid scramblase 1 gene expression is a new prognostic factor for acute myelogenous leukemia. Leuk Res 2004; 28: 149–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effects of wogonin concentration on early apoptosis of NB4 cells.
Fig. S2. Effects of treatment duration on early apoptosis of NB4 cells.
Fig. S3. Effects of wogonin on PKCδ and p‐PKCδ (Ser643) expression in NB4 cells.
Fig. S4. Flow cytometry measurement of the effect of RNAi on the expression of CD11b (a) and CD14 (b) in NB4 cells treated with or without wogonin (50 µM).
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


