Abstract
Numerous lines of evidence have shown that angiogenesis plays a pivotal role in the development of tumors. Therefore anti‐angiogenesis therapy represents a potentially promising approach to cancer therapy. Recently, a new inhibitor called vasohibin was discovered to operate as an intrinsic and highly specific feedback inhibitor in the process of angiogenesis. However, to date, reports on the antitumor and anti‐angiogenic properties of vasohibin have been very limited. To explore the potential of vasohibin as an anti‐angiogenesis therapeutic, we constructed a recombinant adenovirus encoding vasohibin. Our data showed that the recombinant adenovirus encoding vasohibin could prevent tumor angiogenesis and tumor growth. Notably, angiogenesis in the tumors was prevented without any apparent side‐effects. Therefore, the findings suggested that the recombinant adenovirus encoding vasohibin might be valuable as a potential strategy for antitumor angiogenesis therapy in the clinic. (Cancer Sci 2009)
Traditional strategies for cancer therapy usually target tumor cells and aim at eradicating tumor cells.( 1 , 2 , 3 ) However, due to the heterogeneity and genetic instability of tumor cells, these strategies currently have limited clinical utility for human cancers.( 4 , 5 , 6 , 7 ) One alternative to overcome these obstacles of traditional therapy is to target the tumor vasculatures rather than the tumor cells themselves.( 8 , 9 , 10 , 11 )
Angiogenesis is regulated in large part by the balance of various pro‐angiogenic stimulators, such as vascular endothelial growth factor (VEGF), and a diverse group of endogenous inhibitors of angiogenesis.( 12 , 13 , 14 ) Recently, a new inhibitor, called vasohibin, has been identified to be selectively induced in endothelial cells by pro‐angiogenic stimulatory growth factors such as VEGF, and it appears to operate as an intrinsic and highly specific feedback inhibitor of activated endothelial cells engaged in the process of angiogenesis.( 15 , 16 )
In addition, several reports have shown that vasohibin is associated with neovascularization and might play an important role in the regulation of intratumoral angiogenesis.( 17 , 18 ) However, until recently, the therapeutic effect of vasohibin in antitumor angiogenesis has not been well characterized. Therefore, to explore the potential of vasohibin in inhibiting tumor angiogenesis, we constructed a recombinant adenovirus encoding vasohibin to study the effect.
Materials and Methods
Cell culture and RT‐PCR.
Human umbilical vein endothelial cells were cultured on type‐I collagen‐coated dishes in endothelial basal medium containing endothelial cell growth supplements and 10% FCS. Total RNA was extracted and the first‐strand cDNAs were generated. PCR was carried out in a DNA thermal cycler with 100 μL reaction mixture containing 5 U Taq DNA polymerase. The following primer pairs were synthesized and used for amplification: sense, 5′‐AGATCCCCATACCGA GTGTG‐3′; antisense, 5′‐GGGCCTCTTTGGTCATTTCC‐3′.
Construction of recombinant adenovirurs encoding vasohibin.
The recombinant adenovirurs vector encoding vasohibin was constructed using the Adeno‐XTM Expression System (Clontech, Palo Alto, CA, USA) according to the manufacturer’s instructions. Briefly, the vasohibin cDNA was cloned into the shuttle vector pDC315 and sequenced. The desired replication‐deficient adenovirus containing the full‐length cDNA of vasohibin was generated by homologous recombination through co‐transfection of plasmids pDC315‐vasohibin and pBHG1oXE1, 3Cre in HEK 293 cells using the DOTAP liposome reagent (Roche, Mannheim, Germany). After several rounds of plaque purification, the adenovirus containing the vasohibin gene was amplified and purified from cell lysates by banding twice in CsCl density gradients. Viral products were desalted and stored at −80°C in PBS containing 10% glycerol (v/v). The infectious titer was determined by a standard plaque assay. A second recombinant, E1,E3‐deleted adenovirus carrying the LacZ protein under the control of CMV promoter (Ad‐LacZ), was used as a control vector.
Adenovirus‐mediated gene transfer. Transduction of Cos‐7 cells with Ad‐vasohibin was done in 6‐well plates with 1 × 106 Cos‐7 cells/well in 3 mL RPMI‐1640 medium containing 10% FBS. Virus was added to the wells at an MOI of 200 and the Cos‐7 cells were harvested after 24 h of incubation.
Western blot assay. For Western blot assay, proteins of the cell extracts were separated SDS‐PAGE and transferred onto a nitrocellulose membrane. The membrane was incubated with 5% non‐fat milk in PBS and then with anti‐vasohibin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h at room temperature. After washing, the membranes were incubated with an alkaline phosphatase‐conjugated goat antimouse IgG antibody (Amersham Biosciences, Little Chalfont, UK) for 1 h at room temperature. Immunoreactive bands were detected using the ECL Western blot analysis system (Amersham Biosciences).
Animals and cell lines. All the experiments were carried out using 6–8‐week‐old female BALB/c mice purchased from the Experimental Animal Center of Chinese Academy of Medical Science (Beijing, China). All animals were housed under specific pathogen‐free conditions. Mice were allowed to acclimatize for at least 1 week before experiments commenced. All experimental procedures were carried out following approval of the Institutional Animal Care Committee. The mouse hepatocellular carcinoma cell line, H22, was maintained in our laboratory. Cells were cultured in flasks with DMEM supplemented with 10% (v/v) FBS and 1% penicillin–streptomycin at 37°C in a humidified atmosphere of 5% CO2.
Inhibitory effect of vasohibin on H22 cells. Cell proliferation was measured by a colorimetric assay using MTT. In brief, the H22 cells were seeded in 96‐well plates in triplicate at 5 × 103 cells/well and incubated in culture medium overnight. Then the cells were treated with Ad‐vasohibin (1 × 109 pfu) or controls in a total volume of 0.2 mL each well for 24 h. Thereafter, 20 μL of the indicator dye MTT solution (5 mg/mL) was added to each well and cultures were continued for 48 h at 37°C, 5% CO2. After centrifugation, the supernatant was removed from each well. The colored formazan crystal produced from MTT was dissolved with 0.15 mL DMSO, then the optical density (OD) value A490 was measured by the multiscanner autoreader (Dynatech MR 5000; Dynatech Laboratories, Chantilly, VA, USA). The following formula was used: cell proliferation inhibited (%) = [1 − (OD of the experimental samples/OD of the control) × 100%].
Systemic delivery of vasohibin and tumor cell challenge. Mice received an i.v. tail vein injection of Ad‐vasohibin (1 × 109 pfu) or Ad‐LacZ (1 × 109 pfu). The control mice received 100 μL PBS. Three days before the injection, the mice were challenged with an s.c. injection of 1 × 104 H22 cells into the left flank to induce a primary tumor model. The tumor volumes and mean lifespans of the mice were observed. Tumor volume was measured in two dimensions and calculated as: length/2 × width2.
Evaluation of anti‐angiogenic effect. Twenty‐one days after the tumor challenge, the mice were killed. The tumor tissues were fixed in acetone and stained with an antibody reactive to CD31 as described for microvessel density analysis. The sections were then stained with labeled streptavidin–biotin reagents. Vessel density was determined by counting the number of microvessels per high‐power field in the sections.
Evaluation of side‐effects. Seven days after injection, a full thickness wound was excised from the dorsum of the mice. The defect was created by elevating the skin and panniculus carnosus in the center of the outlined defect using forceps, followed by excision of the outlined area using scissors. Wound area was measured twice weekly. Fifteen days after this excision, mice were killed and scar tissues were removed for histological examination. We evaluated vaccinated and control mice by both the wire hang test and the footprint test, as well as by overall behavior and determination of body weight. To test hematopoiesis, animals were subjected to complete peripheral blood counts.
Statistical analysis. The statistical significance of differential findings between experimental groups and controls was determined by Student’s t‐test and considered significant if two‐tailed P‐values were <0.05.
Results
Gene transfer and vasohibin protein expression. Protein expression of vasohibin was shown by transient transfection of adenovirus into Cos‐7 cells and was detected by Western blot assay. In accordance with protocols mentioned above, Cos‐7 cells were transfected with Ad‐vasohibin or Ad‐LacZ at MOI 200 for 24 h. As seen in Fig. 1, the expression of the vasohibin protein was detected after Ad‐vasohibin transfection. However, there was no expression of vasohibin protein after Ad‐LacZ transfection and non‐treated Cos‐7 cells.
Figure 1.
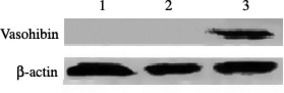
Expression of vasohibin protein in Cos‐7 cells by Western blot analysis. Cos‐7 cells were transfected with Ad‐vasohibin or Ad‐LacZ at an MOI of 200 for 24 h. The expression of the vasohibin protein was detected after Ad‐vasohibin transfection. However, there was no expression of vasohibin protein after Ad‐LacZ transfection or in non‐treated Cos‐7 cells. Lane 1, non‐treated Cos‐7 cells; lane 2, Cos‐7 cells transfectd with Ad‐LacZ; lane 3, Cos‐7 cells transfected with Ad‐vasohibin.
Inhibition of tumor growth in vitro. Cell proliferation was measured by a colorimetric assay using MTT, and the inhibition rate was calculated. The H22 cells were incubated with Ad‐vasohibin or controls. The result showed that Ad‐vasohibin could not inhibit the proliferation of H22 cells compared with control groups (Fig. 2).
Figure 2.
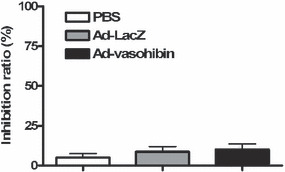
Inhibitory effect of vasohibin on H22 hepatocellular carcinoma cells. Cell proliferation was measured by a colorimetric assay using MTT. Cells were seeded in 96‐well plates in triplicate at 5 × 103 cells/well and incubated in culture medium overnight. The cells were treated with Ad‐vasohibin (1 × 109 pfu) or controls in a total volume of 0.2 mL each well for 24 h. Then 20 μL of the indicator dye MTT solution (5 mg/mL) was added to each well and cultures were continued for 48 h at 37°C, 5% CO2. After centrifugation, the supernatant was removed from each well. The colored formazan crystal produced from MTT was dissolved with 0.15 mL DMSO then the optical density (OD) value A490 was measured by a multiscanner autoreader. The following formula was used: cell proliferation inhibited (%) = [1 − (OD of the experimental samples/OD of the control) × 100%].
Inhibition of tumor growth prolongs lifespan of mice. To explore whether the Ad‐vasohibin has antitumor function in vivo, 3 days before the injection, the mice (n = 10) were challenged with an s.c. injection of 1 × 104 H22 cells into the left flank to induce a primary tumor model. After tumor challenge, mice received an i.v. tail vein injection of Ad‐vasohibin (1 × 109 pfu) or Ad‐LacZ (1 × 109 pfu). The control mice received 100 μL PBS. Tumor volume and lifespan of the mice were observed. We found the tumor volume expanded rapidly after 20 days of tumor challenge in the PBS and Ad‐LacZ groups. However, in the Ad‐vasohibin group, the tumor volume expanded steadily (Fig. 3). In addition, deaths started to occur at approximately 40 days of tumor challenge in the Ad‐vasohibin group, compared to 20–25 days in the other treatment groups. Moreover, the mean lifespan of mice in the Ad‐vasohibin group was prolonged significantly (Fig. 4). These data show that the Ad‐vasohibin had superior antitumor efficiency.
Figure 3.
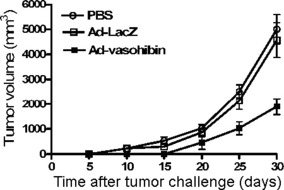
Effect of Ad‐vasohibin on primary tumor growth in BALB/c mice. Three days before injection, 10 mice were challenged with an s.c. injection of 1 × 104 H22 hepatocellular carcinoma cells into the left flank to induce a primary tumor model. Mice then received an i.v. tail vein injection of Ad‐vasohibin (1 × 109 pfu) or Ad‐LacZ (1 × 109 pfu). Control mice received 100 μL PBS. The average tumor volume of mice is depicted (mean ± SEM from three experiments). Twenty days after injection, Ad‐vasohibin significantly suppressed tumor growth. There were significant differences between the Ad‐vasohibin group and control groups (P < 0.05).
Figure 4.
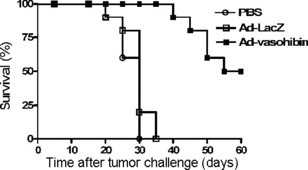
Effect of Ad‐vasohibin on the average lifespan of BALB/c mice (n = 10) challenged with an s.c. injection of 1 × 104 H22 hepatocellular carcinoma cells into the left flank to induce a primary tumor model. Three days later, mice received an i.v. tail vein injection of Ad‐vasohibin (1 × 109 pfu) or Ad‐LacZ (1 × 109 pfu). The control mice received 100 μL PBS. The average lifespan depicted in the histogram shows mean ± SEM from three experiments. There were significant differences between the Ad‐vasohibin group and control groups (P < 0.05).
Inhibition of tumor angiogenesis. To assess whether injection with Ad‐vasohibin could inhibit tumor angiogenesis, angiogenesis of tumor tissue was evaluated by counting the number of microvessels on the sections stained with anti‐CD31 antibody. The results showed that the average number and vessels per high‐power field were both lower in the Ad‐vasohibin group compared with control groups (Fig. 5). These data suggested injection with Ad‐vasohibin could potentially inhibit tumor angiogenesis.
Figure 5.
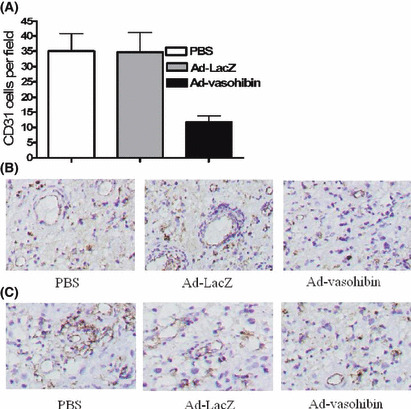
Inhibition of angiogenesis in tumor tissue. Mice were killed 21 days after the tumor challenge. tumor tissues were fixed in acetone and stained with an antibody reactive to CD31. (A) Vessel density was determined by counting the number of the microvessels per high‐power field in tumor sections stained with antibody reactive to CD31. Compared with control groups, the vessel density in the Ad‐vasohibin group was much lower than that of control groups (P < 0.05). (B) Representative immunohistochemical analysis of CD31 expression in the tumor of Ad‐vasohibin group and control groups. (C) Representative immunohistochemical analysis of vasohibin expression in the tumor. Magnification, ×200. The number in histogram as mean ± SEM in each experiment. Three repeated experiments showed consistent results. There were significant differences between the Ad‐vasohibin group and control groups (P < 0.05).
Analysis of the side‐effects of Ad‐vasohibin. To further examine whether injection with Ad‐vasohibin had an effect on normal physiological angiogenesis, we analyzed wound healing in immunized mice using a cutaneous excision wound model. A full thickness wound was created on the dorsum of immunized and control mice 10 days after the injection. Wound areas were measured until completely healed. No significant difference in wound healing was observed between experimental and control mice (Fig. 6). Furthermore, neuromuscular performance, as determined by both wire hang and footprint tests, body weight, overall behavior, balancing tests, and peripheral blood examination did not indicate any impairment attributable to vaccination.
Figure 6.
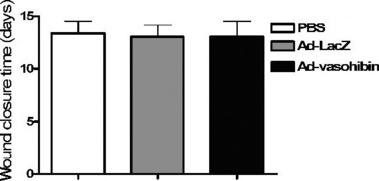
Effect of Ad‐vasohibin on wound healing. Seven days after injection of Ad‐vasohibin, Ad‐LacZ, or PBS, a full thickness wound was excised from the dorsum of the mice (n = 10). The defect was created by elevating the skin and panniculus carnosus in the center of the outlined defect using forceps, followed by excision of the outlined area using scissors. Wound areas were measured until completely healed and the average wound closure time observed. There were no significant differences between the Ad‐vasohibin group and control groups (P > 0.05).
Discussion
Targeting genetically stable endothelial cells reduces the likelihood of developing resistance and can be applied to a wide variety of tumor types. Anti‐angiogenic therapy is widely used and is attractive in the antitumor therapy field.( 19 , 20 , 21 ) A previous study showed that a switch to the actively angiogenic phenotype is dependent upon the local balance between angiogenic factors and inhibitors.( 22 ) Angiogenic factors include VEGF and its family members fibroblast growth factor‐2 and hepatocyte growth factor, and inflammatory cytokines such as tumor necrosis factor‐α and interleukin‐8.( 23 ) VEGF is the principal angiogenic factor, and it is induced by hypoxia and stimulates the migration and proliferation of endothelial cells (ECs).( 24 ) In addition, negative feedback regulation is one of the most important physiological mechanisms of biological organisms and has been shown to control a wide range of phenomena.( 25 ) However, to date, no such regulators have been established for the regulation of angiogenesis.
Recently, a novel angiogenesis inhibitor that is induced in ECs by angiogenic factors and inhibits angiogenesis in an autocrine manner was identified and designated as vasohibin. Further study indicated that the recombinant vasohibin protein inhibited migration, proliferation, and network formation by ECs as well as angiogenesis in vivo.( 15 ) Therefore, vasohibin might act as a potential target for antitumor angiogenesis therapy. Although a variety of vectors are available for gene transfer, recombinant adenovirus is the most efficient. The adenoviral vector is a highly efficient and reproducible method of gene transfer.( 26 , 27 , 28 ) Consequently, in this study, the recombinant adenovirus encoding vasohibin was constructed and the antitumor angiogenesis efficiency of this vector was assessed.
In this study, we used an E1,E3‐deleted adenoviral vector containing the full‐length vasohibin gene under the CMV promoter. The results show that this is an adenovirus vector strategy that provides a highly efficient and reproducible method of gene transfer. Western blot analysis showed that expression of the vasohibin protein in Ad‐vasohibin transduced Cos‐7 cells was significantly increased compared with that of untreated Cos‐7 cells or Ad‐LacZ transfected Cos‐7 cells. In addition, the vector has in vivo antitumor function.
We next assessed whether the Ad‐vasohibin could inhibit tumor growth in vitro by measuring H22 cell proliferation by the MTT assay. The results showed the Ad‐vasohibin could not inhibit the proliferation of H22 cells compared with control groups. Additionally, we injected H22 cells into BALB/c mice and gave them an i.v. tail vein injection of Ad‐vasohibin or a control vector. We found that the tumor volume increased at a slower rate, and the mean lifespan of mice was prolonged in the Ad‐vasohibin group. These data show that the Ad‐vasohibin suppressed tumor angiogenesis but not the tumor cells.
To further explore the anti‐angiogenic potential of this vector, angiogenesis of tumor tissue was evaluated by counting the number of microvessels in sections that were stained with an anti‐CD31 antibody. The results showed that both the average number and the number of vessels per high‐power field were lower in the Ad‐vasohibin group compared to the control groups. These data suggest that injection with Ad‐vasohibin could potentially inhibit tumor angiogenesis.
To examine whether injection with Ad‐vasohibin had any side‐effects on normal physiological angiogenesis, we analyzed wound healing time, neuromuscular performance, body weight, overall behavior, balancing tests, and peripheral blood counts. The results showed there were no significant differences between the Ad‐vasohibin and control groups. A potential reason for the different responses between cancer angiogenesis and wound healing might be explained by a recent study that showed that, even in highly angiogenic tumors, cancer angiogenesis is 4–20 times less extensive as compared with the physiological wound angiogenesis.( 29 ) Similar differences might exist between wound angiogenesis and tumor angiogenesis. Thus, the effect of vasohibin on endothelial cells might be sufficient to inhibit tumor angiogenesis but not the much stronger angiogenesis process that occurs under physiological conditions.
Many anti‐angiogenesis therapies have been used, such as thromobospondin‐1 (TSP‐1), pigment epithelium‐derived factor (PEDF) and endostatin, however, their physiological roles in the regulation of angiogenesis have not been fully established.( 30 , 31 , 32 ) In addition, a diverse group of endogenous inhibitors of angiogenesis, most of which are extrinsic to endothelial cells, exist. Until recently, none appeared to be induced as a consequence of a specific, self‐regulating, feedback inhibition response. Vasohibin is selectively induced and appears to operate as an intrinsic and highly specific feedback inhibitor in the process of angiogenesis. Therefore, a therapeutic strategy based on vasohibin represents a promising approach.
In summary, results from this study suggest that i.v. injections of Ad‐vasohibin effectively reduced tumor growth and significantly increased the lifespan of experimental animals through the inhibition of tumor angiogenesis. Furthermore, angiogenesis in the tumors was suppressed in experimental mice without apparent side‐effects. Accordingly, our results provide evidence that recombinant adenovirus encoding vasohibin might be an ideal strategy for antitumor angiogenesis therapy in the clinic.
Acknowledgments
This work was supported by the “973” 2005CB522605 program of China.
References
- 1. Izuishi K, Kato K, Ogura T, Kinoshita T, Esumi H. Remarkable tolerance of tumor cells to nutrient deprivation: possible new biochemical target for cancer therapy. Cancer Res 2000; 60: 6201–7. [PubMed] [Google Scholar]
- 2. Hu H, Chen D, Liu Y et al. Target ability and therapy efficacy of immunoliposomes using a humanized antihepatoma disulfide‐stabilized Fv fragment on tumor cells. J Pharm Sci 2006; 95: 192–9. [DOI] [PubMed] [Google Scholar]
- 3. Casas A, Perotti C, Ortel B et al. Tumor cell lines resistant to ALA‐mediated photodynamic therapy and possible tools to target surviving cells. Int J Oncol 2006; 29: 397–405. [PubMed] [Google Scholar]
- 4. Benouchan M, Do Nascimento F, Sebbah‐Louriki M et al. Bystander cell killing spreading from endothelial to tumor cells in a three‐dimensional multicellular nodule model after Escherichia coli nitroreductase gene delivery. Biochem Biophys Res Commun 2003; 311: 822–8. [DOI] [PubMed] [Google Scholar]
- 5. Loeper S, Romeike BF, Heckmann N et al. Frequent mitotic errors in tumor cells of genetically micro‐heterogeneous glioblastomas. Cytogenet Cell Genet 2001; 94: 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Dietmaier W, Gansbauer S, Beyser K et al. Microsatellite instability in tumor and nonneoplastic colorectal cells from hereditary non‐polyposis colorectal cancer and sporadic high microsatellite‐instable tumor patients. Pathobiology 2000; 68: 227–31. [DOI] [PubMed] [Google Scholar]
- 7. Jouanneau J, Moens G, Bourgeois Y, Poupon MF, Thiery JP. A minority of carcinoma cells producing acidic fibroblast growth factor induces a community effect for tumor progression. Proc Natl Acad Sci U S A 1994; 91: 286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato M, Arap W, Pasqualini R. Molecular targets on blood vessels for cancer therapies in clinical trials. Oncology 2007; 21: 1346–52; discussion 54‐5, 67, 70 passim. [PubMed] [Google Scholar]
- 9. Pramanik D, Majeti BK, Mondal G et al. Lipopeptide with a RGDK tetrapeptide sequence can selectively target genes to proangiogenic alpha5beta1 integrin receptor and mouse tumor vasculature. J Med Chem 2008; 51: 7298–302. [DOI] [PubMed] [Google Scholar]
- 10. Dome B, Magyar M. [Tumor vasculature as a therapeutic target in non‐small cell lung cancer]. Magy Onkol 2008; 52: 247–59. [DOI] [PubMed] [Google Scholar]
- 11. Xiang R, Luo Y, Niethammer AG, Reisfeld RA. Oral DNA vaccines target the tumor vasculature and microenvironment and suppress tumor growth and metastasis. Immunol Rev 2008; 222: 117–28. [DOI] [PubMed] [Google Scholar]
- 12. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti‐VEGF antibody for treating cancer. Nat Rev 2004; 3: 391–400. [DOI] [PubMed] [Google Scholar]
- 13. Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin‐1. Science 1994; 265: 1582–4. [DOI] [PubMed] [Google Scholar]
- 14. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe K, Hasegawa Y, Yamashita H et al. Vasohibin as an endothelium‐derived negative feedback regulator of angiogenesis. J Clin Invest 2004; 114: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kerbel RS. Vasohibin: the feedback on a new inhibitor of angiogenesis. J Clin Invest 2004; 114: 884–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosaka T, Kimura H, Heishi T et al. Vasohibin‐1 expression in endothelium of tumor blood vessels regulates angiogenesis. Am J Pathol 2009; 175: 430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tamaki K, Moriya T, Sato Y et al. Vasohibin‐1 in human breast carcinoma: a potential negative feedback regulator of angiogenesis. Cancer Sci 2009; 100: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev 2002; 2: 727–39. [DOI] [PubMed] [Google Scholar]
- 20. Kerbel RS. Inhibition of tumor angiogenesis as a strategy to circumvent acquired resistance to anti‐cancer therapeutic agents. BioEssays 1991; 13: 31–6. [DOI] [PubMed] [Google Scholar]
- 21. McCarthy M. Antiangiogenesis drug promising for metastatic colorectal cancer. Lancet 2003; 361: 1959. [DOI] [PubMed] [Google Scholar]
- 22. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31. [DOI] [PubMed] [Google Scholar]
- 23. Ribatti D, Vacca A, Presta M. The discovery of angiogenic factors: a historical review. Gen Pharmacol 2000; 35: 227–31. [DOI] [PubMed] [Google Scholar]
- 24. Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev 2002; 2: 795–803. [DOI] [PubMed] [Google Scholar]
- 25. Lord BI. Feedback regulators in normal and tumour tissues. J Cell Sci 1988; 10: 231–42. [DOI] [PubMed] [Google Scholar]
- 26. Van Maerken T, Sarkar D, Speleman F, Dent P, Weiss WA, Fisher PB. Adenovirus‐mediated hPNPase(old‐35) gene transfer as a therapeutic strategy for neuroblastoma. J Cell Physiol 2009; 219: 707–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sato N, Wang S, Li L et al. A novel strategy for introducing exogenous bcl‐2 into neuronal cells: the Cre/loxP system‐mediated activation of bcl‐2 for preventing programmed cell death using recombinant adenoviruses. Mol Cell Neurosci 1998; 12: 65–78. [DOI] [PubMed] [Google Scholar]
- 28. Magnusson MK, Hong SS, Boulanger P, Lindholm L. Genetic retargeting of adenovirus: novel strategy employing “deknobbing” of the fiber. J Virol 2001; 75: 7280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res 2000; 60: 1388–93. [PubMed] [Google Scholar]
- 30. Sargiannidou I, Zhou J, Tuszynski GP. The role of thrombospondin‐1 in tumor progression. Exp Biol Med (Maywood) 2001; 226: 726–33. [DOI] [PubMed] [Google Scholar]
- 31. Doll JA, Stellmach VM, Bouck NP et al. Pigment epithelium‐derived factor regulates the vasculature and mass of the prostate and pancreas. Nat Med 2003; 9: 774–80. [DOI] [PubMed] [Google Scholar]
- 32. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29: 15–8. [DOI] [PubMed] [Google Scholar]


