Abstract
Drosophila tumor suppressor Scribble has been identified as an apical‐basolateral polarity determinant in epithelia. A human homolog of Drosophila Scribble, human Scribble (hScrib), has been identified as a protein targeted by human papillomavirus E6 for the ubiquitin‐mediated degradation dependent on E6AP, a cellular ubiquitin‐protein ligase. Human Scribble is classified as a LAP protein, having leucine‐rich repeats (LRRs) and PDZ domains. We investigated whether hScrib, which is thought to have a role in polarity determination based on the data of its Drosophila homolog, is involved in cell‐cycle regulation and proliferation control of epithelia. Transfection of hScrib inhibits cell‐cycle progression from G1 to S phase, and it up‐ and down‐regulates expression of adenomatous polyposis coli and cyclins A and D1, respectively. Knockdown of hScrib expression by siRNA leads to cell‐cycle progression from G1 to S phase. We explored functional domain mapping to reveal which domains of hScrib are critical for its cellular proliferation control and localization at the basolateral membrane. We found that LRRs and PDZ domain 1 are indispensable for hScrib to inhibit cell growth by blocking cell‐cycle progression and to keep its proper localization. These data indicate that basolateral membrane localization of hScrib is closely related to its proliferation control. Our findings suggest the possibility that hScrib is involved in signal transduction to negatively regulate cell proliferation by localizing at the basolateral membrane of epithelial cells through LRRs and PDZ domains. (Cancer Sci 2006; 97: 1217–1225)
Organized tissue architecture and cell polarity are hallmarks of epithelia. Construction of organized epithelial tissue architecture is based on proper localization of junctional proteins.( 1 ) Loss of cell polarity due to aberrant expression and localization of junctional proteins causes overgrowth of epithelia.( 2 ) Mutation of a tumor suppressor gene whose encoded protein regulates cell‐cycle progression is also characteristic of cancer tissues. Maintenance of tissue polarity and regulation of cell cycle are tightly linked to keep normal growth control and differentiation in epithelia.( 3 ) Loss of tissue architecture and disruption of the cell‐cycle control system leads to uncontrolled proliferation of epithelial cells and subsequent tumor formation.( 4 )
Drosophila tumor suppressor Scribble has recently been identified as an apical‐basal polarity determinant in epithelia.( 5 ) Drosophila Scribble localizes at the septate junction, which is functionally identical to the vertebrate tight junction, and loss of scribble mutation causes mislocalization of adherens junction to the basolateral membrane.( 5 ) Follicle cells homozygous for scribble mutation are rounded, rather than columnar, and are often multilayered, rather than sheet‐like.( 6 ) In Drosophila scribble mutant, disorganized epithelial tissue overproliferates and causes massive tumors in imaginal discs, brain, and follicles.( 6 ) Recently, scribble has been identified as a novel regulator of S‐phase entry in Drosophila by a genetic screen for dominant hypomorphic cyclin E mutation.( 7 )
The human homolog of Drosophila tumor suppressor Scribble, hScrib, has recently been identified as a target of the high‐risk human papillomavirus (HPV) E6 oncoprotein for ubiquitin‐mediated degradation dependent on the cellular ubiquitin‐protein ligase E6AP.( 8 ) The high‐risk E6s, but not low‐risk HPV E6s, have a conserved PDZ domain‐binding sequence (threonine‐X‐leucine/valine) at their C‐terminal region.( 8 , 9 ) Through this motif, high‐risk E6s bind to the PDZ (named for its occurrence in the PSD‐95, discs large and ZO‐1 proteins) domains of hScrib.( 10 ) The ternary complex formation of hScrib, E6 and E6AP leads to ubiquitin transfer to hScrib dependent on the E3 activity of E6AP.( 8 ) hScrib tagged with multi‐ubiquitin branches is recognized at the 26S proteosome and is degraded. We recently reported that the expression of hScrib is down‐regulated according to the progression of disease during cervical carcinogenesis.( 11 ) Decreased expression of hScrib without down‐regulation of hScrib mRNA transcription in the high‐grade cervical intraepithelial neoplasm, which is a precursor lesion of cervical cancer, supports the possibility that the ubiquitin‐mediated degradation of hScrib depending on HPV E6 and E6AP is involved in the development of cervical cancer.( 11 )
Human and Drosophila Scribbles have identical protein structures, consisting of 16 canonical leucine‐rich repeats (LRRs) at their N‐terminal region and four PDZ domains. Recently, proteins having 16 LRRs and one or four PDZ domains have been grouped as LAP (leucine‐rich and PDZ) proteins.( 12 ) LAP proteins are involved in the establishment of epithelial cell polarity, by localizing at the basolateral wall of epithelia.( 5 , 13 , 14 ) LAP proteins have no membrane‐localization signals by themselves and interaction with other transmembrane proteins through their LRRs or PDZ domains is necessary for them to localize at the basolateral wall of epithelia.( 13 , 15 , 16 ) The LRRs and PDZ domains are also thought to be critical in the control of cell polarity and inhibition of tumorous overproliferation of epithelial cells.
In this study, we explored a possible role of hScrib in the regulation of cell‐cycle progression and cell proliferation. Next we analyzed whether LRRs and/or PDZ domains of hScrib have a critical role in the proper functioning of cell growth control through the regulation of cell‐cycle progression.
Materials and Methods
Construction of green fluorescent protein (GFP)‐fusion hScrib expression plasmids . The full‐length cDNA of hScrib was subcloned into the multicloning site of the pEGFP‐C1 vector (BD Biosciences–Clontech, Palo Alto, CA, USA). To generate the deletion mutants of GFP‐fusion hScrib, the following cDNA sequences were amplified with polymerase chain reaction (PCR): LRR + PDZ 1–4 (amino acids 1–1191); LRR + PDZ 1–3 (amino acids 1–1096); LRR + PDZ 1–2 (amino acids 1–953); LRR + PDZ 1a (amino acids 1+818); LRR + PDZ 1b (amino acids 1+747); LRR (amino acids 1–495); LRRΔLAPSDb (amino acids 1–421); LRRΔLAPSDa‐b (amino acids 1–390); and PDZ 1–4 (amino acids 731–1191). The amplified PCR products were digested with restriction enzymes and subcloned into the pEGFP‐C1 vector. GFP‐PDZ 1–2 GRGI mutant, in which critical amino acid sequence GLGI to the structure of PDZ domain pocket in PDZ domain 1 is substituted by GRGI, was generated by Quick change site directed mutagenesis kit (Stratagene) using the following primers; 5′‐CAGACTGGGGGCCGGGGCATCAGCATTGCG‐3′, 5′‐CGCAATGCTGATGCCCCGGCCCCCAGTCTG‐3′.
Mammalian cell transfection and immunofluorescence analysis. NIH3T3 cells or Madin–Darby canine kidney (MDCK) cells were cultured on coverslips in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified incubator with 5% CO2. For transfection, 5 × 102 cells/cm2 were seeded onto glass coverslips the day before transfection in six‐well plates and subconfluent cells were transfected with 1.5 µg of various plasmids using Polyfect reagent (Qiagen, Valencia, CA, USA). Transfected subconfluent MDCK cells with various clones of GFP‐fusion hScrib expression plasmids containing various parts of hScrib cDNA subcloned into pEGFP‐C1 vector (BD Biosciences–Clontech) were grown on coverslips in the culture medium.
Forty‐eight hours later, cells were washed three times with phosphate‐buffered saline (PBS) and then fixed with 3.7% formaldehyde in PBS for 10 min. Cells were then washed three times with PBS, rinsed with distilled water, and made permeable with acetone at −20°C for 10 min. Cells were washed with PBS and incubated with the diluted the anti‐hScrib C‐terminus antibody (Scrib C‐20; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 30 min at room temperature. Cells were then washed three times with PBS and incubated with the rhodamine‐conjugated secondary antigoat antibody (Sigma, St Louis, MO, USA), and then washed three times with PBS.
Cells were mounted on a slide glass and examined under a confocal fluorescence microscopy (Carl Zeiss LSM 540 microscope). Images were captured with a CCD camera. For each GFP‐fusion hScrib expression clone, at least three independent experiments were performed.
Colony forming assay. NIH3T3, Hela, and MDCK cells (2 × 105 cells/cm2) were transfected with 4 µg of various GFP‐fusion hScrib expression plasmids using Polyfect reagent according to the manufacturer's instruction (Qiagen). After incubation for 30 h, cells were trypsinized and diluted 1:30, and cultured in DMEM supplemented with 10% FBS and G418 (1000 µg/mL) for 2 weeks. Cells were fixed with 100% methanol and stained with 0.4% Giemsa reagent (Gibco‐BRL) in PBS. Several independent colonies were isolated, and it was confirmed that they expressed appropriate GFP‐fusion hScrib proteins by immunoblotting assay. For each GFP‐hScrib construct, at least two independent experiments were performed.
Flow cytometry. NIH3T3 cells were grown at a density of 1 × 105 cells/mL in the culture medium. Cells were transfected with GFP expression vector pEGFP‐C1 vector (BD Biosciences–Clontech) or pEGFP‐C1‐hScrib full‐length plasmid. After 24 h, cells were further cultured in DMEM containing 0.4% FBS for 12 h, and then the medium was changed to fresh DMEM containing 10% FBS. After culturing for 12 h, cells were trypsinized and collected by centrifugation and then washed twice with PBS. Cells were resuspended in PBS containing 1% formaldehyde for 1 h at 4°C. Cells were washed twice again in PBS and resuspended in buffer containing ethanol and PBS at the ratio of 7:3 at −20°C overnight. After washing twice with PBS, cells were incubated in ribonuclease solution (0.25 mg/mL) (Qiagen) for 30 min at 37°C, followed by staining with propidium iodide (50 µg/mL) (Dojindo, Japan) on ice for 30 min in the dark. Gates were established for purification of the GFP expressing cells. Approximately 100 000 stained cells with propidium iodide were analyzed for GFP fluorescence and cell‐cycle distribution on EPICS XL Flow cytometry (Beckman Coulter), as described previously.
BrdU incorporation analysis in NIH3T3 cells transfected with pEGFP‐C1‐hScrib expression plasmids. NIH3T3 cells were transfected with various pEGFP‐C1‐hScrib expression plasmids. Twelve hours later, cells were cultured in DMEM containing 0.4% FBS for 12 h. The medium was changed to fresh medium containing 10% FBS and 50 µM bromodeoxyuridine (BrdU; Roche). After culturing for 16–18 h, cells were fixed with 3.7% formaldehyde in PBS for 30 min, treated with methanol at −20°C for 10 min and then treated with 1 M HCl for 10 min. Cells were washed with PBS and incubated with anti‐BrdU antibody (1:10 dilution, BrdU detection kit, Roche) for 1 h at room temperature. Cells were then washed six times with PBS and incubated with rhodamine‐conjugated antimouse antibody (Sigma) for 1 h and then washed three times with PBS.
Cells were mounted on a slide glass and examined under the confocal fluorescence microscope (Carl Zeiss LSM 540). For each pEGFP‐C1‐hScrib expression construct, at least three independent experiments were performed.
Western blotting. MDCK or NIH3T3 cells transfected with GFP‐fusion hScrib expression plasmids were grown in DMEM medium supplemented with 10% FBS. The transfection efficiency was determined to be over 80% for each cell line. After 24 h, cells cultured in DMEM containing 0.4% FBS for 12 h were then changed to fresh DMEM containing 10% FBS. After culturing for 12 h, cell extracts were made in the NP‐40 lysis buffer containing 100 mM Tris (pH 8.0), 100 mM NaCl, 1% NP‐40, and protease inhibitor cocktail (Sigma). Protein concentration was determined by standard Bradford assay. The cell extracts were fractionated by SDS‐PAGE and electrophoretically transferred onto the polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA).
The anti‐GFP antibody (Zymed, South San Francisco, CA, USA) was used at dilution of 1:1000 to detect the expression of GFP‐fusion hScrib. For adenomatous polyposis coli (APC), cyclin A, cyclin D1, and α‐tubulin proteins, proteins were visualized by Western blotting using a 1 : 1000 dilution of an anti‐APC (C‐20, Santa Cruz Biotechnology), and a 1:500 dilution of an anticyclin A, anticyclin D1 (Santa Cruz Biotechnology), and antitubulin (Calbiochem, San Diego, CA, USA). The level of protein expression was analyzed by the STORM 860 according to the manufacturer's recommendation (Molecular Dynamics, Sunnyvale, CA, USA).
siRNA transfection. All small interfering RNA (siRNA) were obtained from Qiagen as purified. A target sequence of hScrib siRNA was designed using HiPerformance‐2 for silencing siRNA duplexes from Qiagen. The sequence was submitted to a BLAST search against the human genome sequence to ensure that no gene of the human genome was targeted. An siRNA against human Discs large (hDlg) was used as a positive control, as described previously.( 17 )
The target sequence against hScrib was 5′‐CAG GAT GAA GTC ATT GGA ACA. Several cell‐lines, Caco2, HaCaT and 293T cells were transfected by the use of 5 µL of X‐treme GENE siRNA transfection reagent (Roche) per mL and a final siRNA concentration of 10 nM according to the manufacturer's instructions. Alexa 568‐labeled negative control siRNA (Qiagen) was used as a measure of transfection efficiency. The transfection efficiency was determined to be 80–90% for each cell line. The cells were fixed at 72 h post‐transfection for immunofluorescence or were lyzed in NP‐40 buffer for Western blot analysis.
Results
Protein structure of hScrib and expression of GFP‐fusion hScrib in the epithelial cell line MDCK. Human Scribble is classified as a LAP4 protein, containing 4 PDZ domains at its central region in addition to 16 canonical LRRs at its N‐terminal region. Between 16 LRRs and 4 PDZ domains, hScrib has two conserved regions of LAP proteins that are called LAP specific domains (LAPSD). LAPSDa consists of 38 amino acid residues related to LRR, while LAPSDb consists of 24 residues not related to LRR (Fig. 1A).
Figure 1.
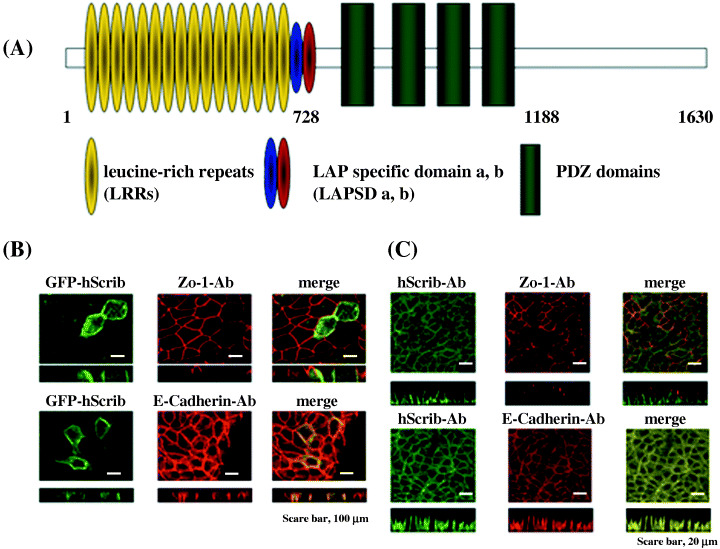
Protein structure and expression of GFP‐fusion of hScrib. (A) Schematic representation of hScrib. Leucine‐rich repeats (yellow ovals), LAP (leucine‐rich and PDZ domain) specific domain a (LAPSD a) (blue oval), LAPSD b (red oval), and PDZ domains (green boxes) are indicated. (B,C) Immunofluorescence images: a. expression of GFP‐fusion hScrib in MDCK cells; b. endogenous expression of hScrib analyzed by the anti‐hScrib polyclonal antibody; c. merged image of a and b indicates that GFP‐fused hScrib colocalizes with the endogenous hScrib. (Original magnification × 200; bar = 20 µm).
We constructed a GFP‐fusion hScrib expression plasmid and examined its localization in the MDCK epithelial cell line. GFP‐fusion hScrib localized broadly at the basolateral membrane of MDCK epithelial cells (Fig. 1B). Localization and expression of GFP‐fusion hScrib was identical to that of an adherens junction marker, E‐cadherin, but not to that of a tight junction marker, ZO‐1 (Fig. 1B). Localization and expression of GFP‐fusion hScrib was identical to that of endogenous hScrib revealed by immunofluorescence analysis with the anti‐hScrib polyclonal antibody, which indicated that the endogenous hScrib localizes at the adherens junction (Fig. 1B,C).
Expression and localization of GFP‐fusion hScrib mutants in MDCK cells. We constructed GFP‐fusion hScrib deletion mutants lacking C‐terminal region, PDZ domains, LAPSDa, and LAPSDb (Fig. 2), and examined their expression by Western blotting (Fig. 3). We also subcloned hScrib cDNA sequence encoding PDZ domains 1–4 into pEGFP expression vector and tested its expression by Western blotting (2, 3). These pEGFP‐hScrib expression plasmids and GFP expression vector were transfected into MDCK cells and confirmed to express the proteins with expected size (Fig. 3).
Figure 2.
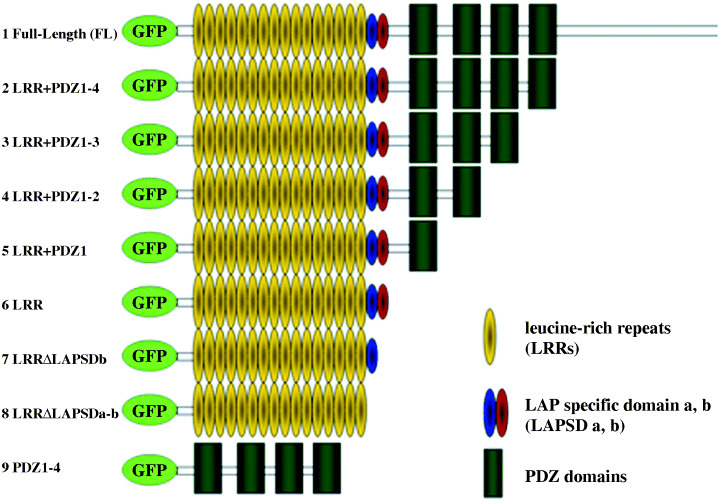
Generation of GFP‐fused hScrib domain and deletion constructs. The hScrib cDNA encoding these hScrib domains were subcloned into the pEGFP C‐1 vector.
Figure 3.
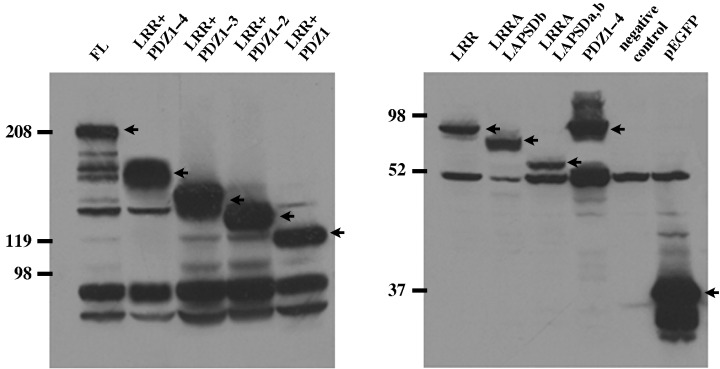
Characterization of hScrib domain and deletion constructs. The hScrib cDNA encoding the hScrib domains subcloned into the pEGFP C‐1 vector were transfected and expressed in MDCK cells. All the pEGFP hScrib domain constructs expressed the protein with expected size. Arrowheads indicate the bands corresponding to the expressed GFP‐fused hScrib domain constructs.
Minimal domains of hScrib necessary for its basolateral membrane localization in MDCK cells. We transfected GFP‐fused hScrib LRR + PDZ 1–4 lacking the C‐terminal region into MDCK cells. GFP‐fused LRR + PDZ 1–4 localized at the basolateral membrane of MDCK cells (Fig. 4A). Colocalization of GFP‐tagged signal of hScrib LRR + PDZ 1–4 and the immunofluorescence signal of endogenous hScrib analyzed with the anti‐hScrib polyclonal antibody indicated that the C‐terminal region of hScrib is dispensable for the proper localization of hScrib (Fig. 4A). We further analyzed whether PDZ domains of hScrib are critical for its proper basolateral membrane localization. GFP‐fused hScrib LRR + PDZ 1–4 lacking PDZ domain 4 and the C‐terminal region localized at the basolateral membrane, showing an identical localization pattern to the endogenous hScrib (data not shown). Likewise, GFP‐fused hScrib LRR + PDZ 1–2 lacking PDZ domains 3 and 4 and the C‐terminal region also showed colocalization with the endogenous hScrib (data not shown). Furthermore, GFP‐fused LRR + PDZ 1 lacking PDZ domains 2–4 and the C‐terminal region showed identical localization to the endogenous hScrib (Fig. 4B). In contrast, loss of PDZ domain 1 had a great impact on the localization of hScrib. GFP‐fused LRR lacking PDZ domains 1–4 and the C‐terminal region showed a totally different localization pattern from that of GFP‐fused LRR + PDZ 1–4, LRR + PDZ 1–3, LRR + PDZ 1–2, and LRR + PDZ 1.
Figure 4.
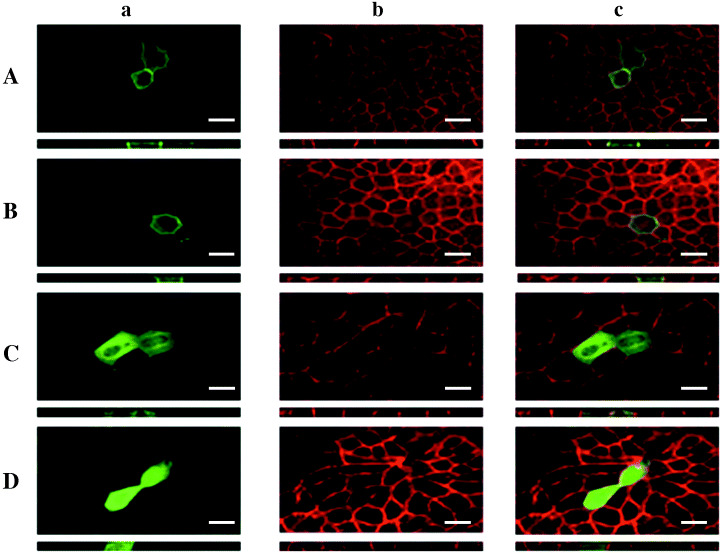
Analysis of critical domain of hScrib essential for its proper basolateral membrane localization (original magnification × 200; bar = 20 µm). (A) GFP‐fused hScrib LRR + PDZ 1–4. (B) GFP‐fused hScrib LRR + PDZ domain 1. (C) GFP‐fused hScrib LRR. (D) GFP‐fused hScrib PDZ domains 1–4. All the GFP‐fused hScrib domain constructs were transfected and analyzed in MDCK cells. Under the immunofluorescence image of X‐Y section, the image of Z‐section is shown. a. Immunofluorescence analysis of the expression of GFP‐fused hScrib domain construct. b. Immunofluorescence analysis of the expression of endogenous hScrib. c. Merged image of a. and b.
GFP‐fused LRR of hScrib was faintly expressed at the basolateral membrane. It localizes mainly in the cytoplasm, but not in the nucleus (Fig. 4C). GFP‐fused PDZ domains 1–4 of hScrib showed no membrane‐associated localization and were expressed in the cytoplasm and nucleus (Fig. 4D).
hScrib inhibits proliferation of cells through its LRRs and PDZ domains. We analyzed whether hScrib negatively regulates proliferation of mammalian cells. We transfected pEGFP‐full length hScrib, which encodes GFP‐fused hScrib, and pEGFP vector into mouse NIH3T3 cells. Two weeks after the transfection, G418‐resistant colonies were stained with Giemsa reagent, and the number of stained colonies was counted. NIH3T3 cells transfected with pEGFP‐full‐length hScrib had a quarter of the drug‐resistant colony count of the cells transfected with pEGFP vector alone (control) (Fig. 5A,B). The relative number of G418‐resistant colonies of cells transfected with pEGFP‐full‐length hScrib was calculated as the percentage of the number of drug‐resistant colonies of cells transfected with pEGFP vector (Fig. 5B). The cells transfected with pEGFP‐hScrib LRR + PDZ 1–4, LRR + PDZ 1–3, LRR + PDZ 1–2, and LRR + PDZ 1 formed approximately a quarter of the drug‐resistant colony count of the cells transfected with pEGFP vector alone (Fig. 5B). In contrast, the cells transfected with pEGFP hScrib‐LRR, LRRΔLAPSDb, LRRΔLAPSDa‐b, or PDZ 1–4 showed no obvious differences in relative number of the drug‐resistant colonies (Fig. 5B).
Figure 5.
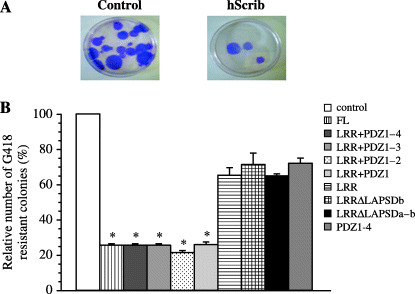
Inhibition of proliferation of mouse NIH3T3 cells by overexpression of hScrib. (A) Representative G418 resistant colony formation in NIH3T3 cells transfected with pEGFP vector alone (control) or pEGFP‐hScrib expression plasmid. The drug‐resistant colonies were stained with Giemsa reagent. (B) Colony formation assay using the pEGFP‐hScrib cDNA encoding hScrib domain constructs in NIH3T3 cells. Results are shown as the mean percentage of the control ± SEM. *P < 0.05 vs control.
Next, we analyzed whether hScrib has a role in the inhibition of proliferation of epithelial cells in Hela cells and MDCK cells. The transfection of hScrib inhibited the formation of the drug‐resistant colonies to 25% of the control in these cell lines (Fig. 6).
Figure 6.
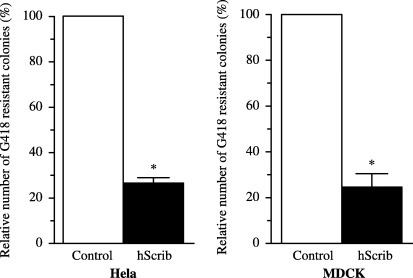
Inhibition of the proliferation of Hela and MDCK cells by overexpression of hScrib. Colony formation assays were done by transfection of pEGFP vector (control) or pEGFP‐hScrib in Hela cells and MDCK cells. Results are shown as the mean percentage of the control ± SEM. *P < 0.05 vs control.
hScrib negatively regulates cell‐cycle progression from G0/G1 to S phase depending on its LRRs and PDZ domains. We investigated whether hScrib inhibits proliferation of epithelial cells through cell‐cycle regulation. NIH3T3 cells were transfected with pEGFP‐full‐length hScrib or pEGFP vector. We compared cell‐cycle profiles of the cells transfected with pEGFP‐full‐length hScrib or pEGFP vector by flow cytometry (Fig. 7). The percentage of cells in S phase in NIH3T3 cells transfected with pEGFP‐full‐length hScrib was significantly lower than that of cells transfected with pEGFP vector alone (25.6 ± 1.9%vs 39.6 ± 0.3%; P < 0.05). The percentage of cells in G0/G1 phase in NIH3T3 cells transfected with pEGFP‐full‐length hScrib was high (62.1 ± 4.7%), compared with that in the cells transfected with pEGFP vector alone (48.3 ± 1.1%; P < 0.05).
Figure 7.
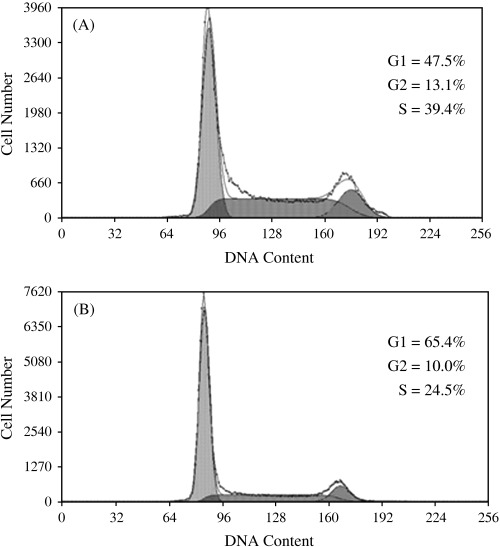
Human Scribble negatively regulates cell‐cycle progression from G1 to S phase. The cell‐cycle profiles of NIH3T3 cells transfected with (A) pEGFP vector or (B) pEGFP‐hScrib were analyzed by flow cytometry. The result is representative of three independent experiments. The percentage of cells in S phase was lower in the cells transfected with pEGFP‐hScrib than the cells transfected with pEGFP vector.
Next, we analyzed which domains of hScrib are critical for its cell‐cycle regulatory function. We transfected pEGFP hScrib expression mutants into serum‐starved NIH3T3 cells. Cell‐cycle progression of quiescent NIH3T3 cells transfected with pEGFP hScrib expression mutants was induced by addition of serum to the medium. Progression of the cell cycle was analyzed by the incorporation of BrdU into DNA in the cells transfected with pEGFP hScrib expression mutants or pEGFP vector. Approximately one half of the cells transfected with pEGFP vector alone (control) incorporated BrdU by the induction of cell‐cycle progression in the presence of serum (8, 9). In contrast, the cells transfected with pEGFP full‐length hScrib showed lower rate of BrdU incorporation (P < 0.05 vs control) (8, 9). The cells transfected with pEGFP hScrib LRR + PDZ 1–4, LRR + PDZ 1–3, LRR + PDZ 1–2 (8, 9), or LRR + PDZ 1 showed lower rates of BrdU incorporation than the cells transfected with pEGFP vector (P < 0.05).
Figure 8.
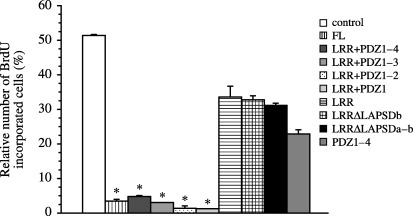
BrdU incorporation analysis in NIH3T3 cells transfected with pEGFP‐hScrib expression plasmid clones. Results are shown as the mean percentage of the control ± SEM. *P < 0.05 vs control, LRR, LRRΔLAPSDb, LRRΔLAPSDa‐b, and PDZ 1–4.
Figure 9.
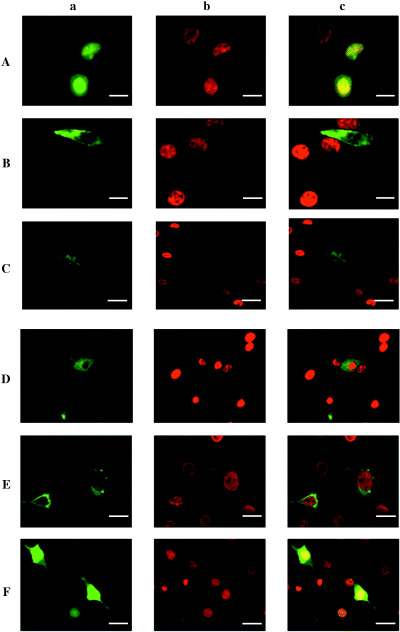
BrdU incorporation analysis in NIH3T3 cells transfected with pEGFP‐hScrib expression clones. Cell‐cycle regulatory function of hScrib was analyzed by the rate of BrdU incorporation in serum‐starved NIH3T3 cells transfected with pEGFP‐hScrib expression clones. a. Immunofluorescence image of GFP‐fused hScrib domain construct. b. Immunofluorescence image of BrdU‐incorporated cells. c. Merged image of a. and b. NIH3T3 cells were transfected with (A) pEGFP vector, (B) pEGFP‐hScrib expression plasmid, (C) pEGFP‐hScrib LRR + PDZ 1–2 expression plasmid, (D) pEGFP‐hScrib LRR + PDZ 1–2 GRGI, which was a mutant in which the conserved Gly‐Leu‐Gly‐Ile pocket motif in PDZ domain 1 was replaced with Gly‐Arg‐Gly‐Ile expression plasmid, (E) pEGFP‐hScrib LRR expression plasmid, and (F) pEGFP‐hScrib PDZ 1–4 expression plasmid.
To determine the importance of PDZ domain 1 in the control of cell‐cycle progression, we constructed a GFP‐PDZ 1–2 GRGI mutant, in which the amino acid sequence GLGI critical to the structure of PDZ domain pocket in PDZ domain 1 is substituted by GRGI. Approximately half of the cells transfected with pEGFP in this GFP‐PDZ 1–2 GRGI mutant incorporated BrdU by the induction of cell‐cycle progression in the presence of serum (Fig. 9D). The cells transfected with pEGFP‐hScrib LRR (8, 9), LRRΔLAPSDb, LRRΔLAPSDa‐b, or PDZ 1–4 (8, 9) showed higher rates of BrdU incorporation than the cells transfected with pEGFP full‐length hScrib (P < 0.05). These cells showed relatively lower BrdU incorporation compared with the cells transfected with pEGFP vector, although not reaching statistical significance.
Our previous study revealed that hScrib interacts with tumor suppressor APC through PDZ domain 1 and 4 of hScrib and the C‐terminal PDZ domain‐binding motif of APC.( 18 ) We investigated whether cell‐cycle arrest from G1 to S phase induced by the transfection of hScrib up‐regulates APC levels in the transfected cells. We also analyzed whether hScrib transfection reduces cyclin D1. Cells transfected with GFP‐hScrib(FL) showed up‐regulation of APC (Fig. 10). In those cells, levels of cyclin A and cyclin D1 were reduced (Fig. 10). In cells transfected with GFP‐hScrib LRR + PDZ 1(1–818aa) and GFP‐hScrib LRR + PDZ 2, APC was up‐regulated and cyclins A and D1 were reduced, as for cells transfected with GFP‐hScrib(FL) (Fig. 10). In contract, cells transfected with GFP‐hScrib PDZ 1–4, GFP‐hScrib LRR + PDZ 1(1–747aa), and GFP‐hScrib LRR showed no up regulation of APC and nor reduction of cyclins A and D1.
Figure 10.
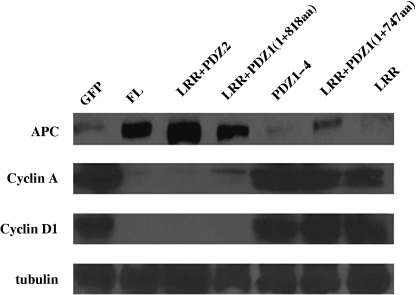
Effect of hScrib on the activity of the APC‐cyclin D pathway. Cell lysates generated from NIH3T3 cells transfected with GFP or each GFP‐fusion hScrib expression clones were examined by Western blotting with an anti‐APC antibody, anticyclin A antibody, anticyclin D1 antibody. An antitubulin antibody was used to equilibrate loading for each lane. Cell lysates from GFP‐fusion hScrib expression clones that have its LRRs and at least one complete PDZ domain expressed 5–7‐fold less cyclin A and D1 than lysates from GFP‐transfected cells. In contrast, LRR + PDZ 1b1+747 in which the conserved hydrophobic pocket was partially deleted had no effect on the regulation of cyclins A and D1.
BrdU incorporation assay revealed that LRR + PDZ 1 is the minimal domain for the negative cell‐cycle regulatory function of hScrib. These results were confirmed by flow cytometry. The percentage of cells in S phase in NIH3T3 cells transfected with GFP‐hScrib(FL), LRR + PDZ 1–2, and LRR + PDZ 1 was significantly lower than the cells transfected with GFP alone (Fig. 11). In contrast, the percentage of S phase in cells transfected with GFP‐hScrib LRR and LRR + PDZ 1–2 GRGI mutant was the same as cells transfected with GFP alone (Fig. 11).
Figure 11.
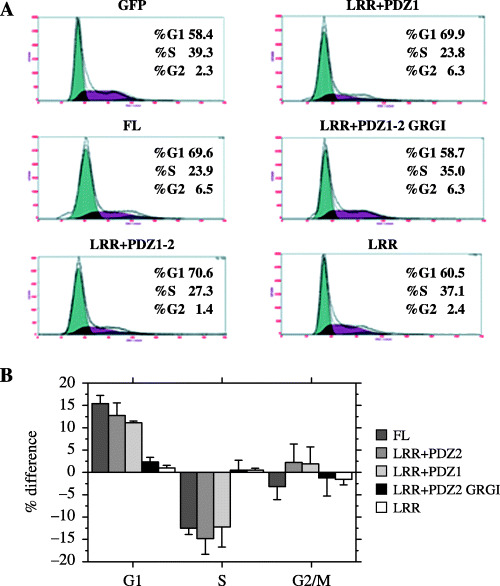
(A) NIH3T3 cells transfected with GFP only, GFP‐hScrib(FL), mutant hScrib were analyzed by flow cytometry to examine the effects of hScrib on cell‐cycle progression. Gates were established such that only cells with high GFP fluoresce. Note that LRR + PDZ 1–2 GRGI, a mutant in which the conserved Gly‐Leu‐Gly‐Ile pocket motif in PDZ domain 1 was replaced with Gly‐Arg‐Gly‐Ile, has no effect on cell‐cycle regulation of G1/S transition. (B) The approximate percentages of cells in each of the major phases of the cell cycle were calculated using the Multicycle software. The average difference in the percentage of cells (Y‐axis) in each phase of the cell cycle (X‐axis) among GFP‐hScrib mutants from three different experiments is shown. Percentages of cells transfected with GFP as a control were set to zero in the three phases (G1, S, and G2/M), and changes of cell‐cycle distribution were shown for cells transfected with GFP‐hScrib full‐length and mutants.
Knockdown of hScrib leads to progression of cell cycle from G1 to S phase. We investigated the involvement of hScrib in cell‐cycle regulation by a knockdown technique with siRNA against hScrib. The siRNA against hScrib successfully down‐ regulated the expression of hScrib in 293T, Caco‐2, and HaCaT cells (Fig. 12A). BrdU incorporation assay showed that BrdU was incorporated in Caco‐2 cells transfected with hScrib siRNA, but it was not incorporated into non‐transfected cells showing normal expression by immunofluorescence analysis with anti‐hScrib antibody (Fig. 12A). Cell profiles of Caco‐2 cells transfected with hScrib siRNA or control siRNA were analyzed by the flow cytometry. Knockdown of hScrib by siRNA led to cell‐cycle progression of G1 to S phase (Fig. 12B).
Figure 12.
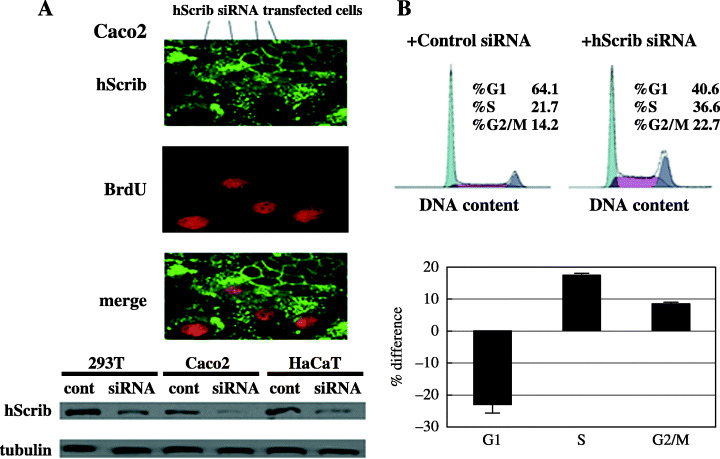
(A) Effect of hScrib on the G1 to S transition of the cell cycle. Caco‐2 cells transfected with hScrib siRNA were double stained for anti‐hScrib antibody (green) and BrdU (red). The knockdown effects of hScrib siRNA were evaluated by Western blotting. (B) hScrib siRNA transfection increases the S phase population as determined by flow cytometry. Caco‐2 cells transfected with negative control siRNA or hScrib siRNA were labeled with propidium iodide and analyzed by flow cytometry. Percentages of cells in the three phases from control siRNA transfection were set to zero, and the bars represent the changes for hScrib siRNA transfection.
Discussion
Loss of scribble mutation leads to disruption of apical‐basolateral polarity and tumorous overgrowth of imaginal discs, follicles, and brain in Drosophila epithelia.( 6 , 19 ) The Drosophila Scribble is thought to exert its inhibitory effect on the proliferation of epithelia through negative regulation of the cell cycle. Recently, scribble has been identified to be a novel negative regulator of S phase entry by a genetic screen for dominant modifier of a cyclin E hypomorphic mutation, DmcycE jp . ( 7 ) Cell‐cycle progression from G1 to S phase by extracellular signals is critical for epithelial cells to proliferate properly according to their environments. Progression of cell cycle is regulated by the activity of cyclin‐dependent kinases (CDK) and their cyclin partners.( 20 ) Phosphorylation of tumor suppressor Rb by cyclin D/Cdk4 and cyclin E/Cdk2 inactivates pRb and results in dissociation of Rb from its binding partner E2F. E2F then acts as a transcription factor of S phase genes, including cyclin E, its own.( 21 ) hScrib has been reported to have a role in the rescue of apical‐basolateral polarity defect and overgrowth of Drosophila epithelia with scribble mutation.( 22 ) Expression of hScrib in Drosophila scribble mutant effectively suppressed the giant larvae phenotype and neoplastic overgrowth of Drosophila scribble neural tissues. These results suggest that hScrib can substitute the functions of Drosophila Scribble and that it has a role in the inhibition of proliferation of epithelial cells through regulation of cell‐cycle progression from G1 to S phase.
In this study, we found that hScrib suppresses the proliferation of mammalian epithelial cells by regulating cell‐cycle entry from G1 to S phase. Knockdown of hScrib expression leads to cell‐cycle progression from G1 to S phase (11, 12). These data clearly indicate that the human homolog of Drosophila tumor suppressor Scribble is involved in cell‐cycle regulation through intracellular signal transductions as is the Drosophila homolog.
How is the junctional protein hScrib connected to the cell‐cycle regulation or signal transduction pathway? Our analysis of critical domains of hScrib for inhibition of cell proliferation, cell‐cycle regulation and intracellular localization gave us some clues to solve this problem. hScrib requires at least LRRs and PDZ domain 1 to exert its inhibitory effect on cellular proliferation and cell‐cycle regulation. Furthermore, it needs LRRs and at least one PDZ domain to localize at the original location, the basolateral membrane of epithelia, more specifically at the adherens junction.( 11 )
PDZ domains are approximately 90 residue repeats found in scaffolding proteins implicated in ion‐channel and hormone receptor clustering. The class 1 PDZ domains recognize the conserved binding motif, T/S‐X‐L/V motif, of the C‐terminal region of their binding partner.( 23 ) Our previous work revealed that the PDZ domain 1 of hScrib is essential for interaction with the C‐terminal region of APC.( 18 ) Disruption of the PDZ domain pocket motif in PDZ domain 1 of GFP‐hScrib LRR + PDZ 1–2 (GRGI mutant) caused the loss of the inhibitory role of hScrib in cell‐cycle regulation, which strongly indicates that the interaction of hScrib with APC is critical for its inhibition of cell growth and negative regulation of cell‐cycle progression. Western blotting of lysates of cells transfected with GFP‐hScrib mutants clearly showed that its inhibition of cell‐cycle entry from G1 to S phase (i.e. reduction of cyclins A and D1), depends on the up‐regulation of APC (Fig. 6).( 24 )
Leucine‐rich repeats are generally 20–29 residues long and contain a conserved 11‐residue consensus sequence L‐X‐X‐L‐X‐L‐X‐X‐N/C‐X‐L. Crystal structure analysis revealed that LRRs correspond to structural units, each consisting of a β strand and an α helix.( 25 ) The structural units are allied so that all β strands and α helices are parallel to a common axis forming a horseshoe‐shaped structure with parallel β sheets lining the inner concave part and α helices flanking the outer curved structure.( 25 ) The architecture of the LRRs provides the versatile framework for the interaction with the binding partner.( 25 , 26 ) hScrib could interact with two different binding partners independently through its LRRs and PDZ domain 1, resulting in localization at the adherens junction.
Analysis of domain mapping of Drosophila Scribble revealed that both LRRs and PDZ domains are required for Scribble to localize efficiently at the septate junction of epithelial cells.( 27 ) Loss of LRRs and PDZ domains of Drosophila Scribble leads to mislocalization of Scribble in nucleus and cytoplasm, respectively, of epithelia.( 27 ) Furthermore, only the Scribble proteins containing both LRRs and PDZ domains have the ability to target Mira, the cell fate scaffolding protein, to the basolateral cortex of mitotic neuroblasts and to establish mitotic spindle and cell size asymmetry in neuroblasts.( 27 ) The LRRs are essential to the organization of the polarized epithelial monolayer, while loss of PDZ domains leads to disruption of septate junctions and overproliferation in the absence of epithelial disorganization.( 28 ) These data suggest a two‐step model for the localization of Drosophila Scribble, in which LRRs bring Scribble to the plasma membrane and PDZ domains enhance membrane‐associated Scribble to the septate junction.( 28 ) Our data showed that the PDZ domain of hScrib alone, which is its binding domain with APC, is not enough to exert its proper localization at the adherens junction and inhibitory activity of cell growth and cell‐cycle progression.
Taken together, the Drosophila findings and our data suggest that hScrib targets the adherens junctions of epithelia by a two‐step mechanism. In the first step, LRRs bring hScrib to the plasma membrane. In the second step, PDZ domains restrict the localization of hScrib at the adherens junction, resulting in its involvement in the transduction of extracellular signals towards the cytoplasm and nucleus of epithelial cells. hDlg is a human homolog of Drosophila neoplastic tumor suppressor, in which mutation causes disruption of tissue architecture and overgrowth of epithelia. Human Dlg, which is targeted by high‐risk HPV E6 for the ubiquitin‐mediated degradation like hScrib, interacts with tumor suppressor APC through its PDZ domain and is involved in cell‐cycle regulation cooperatively with APC.( 10 , 29 ) Our previous study showed that hScrib interact with APC through its PDZ domains and thereby join the Wnt signaling pathway.( 18 ) It is also reported that hScrib interacts with βPIX exchange factor and that the interaction between them is involved in neural transmission.( 15 ) Drosophila scribble has been shown to exhibit genetic interaction with scad (αPS3‐integrin‐cell adhesion), phyllopod (signaling), dEB1 (microbubule‐binding protein‐cytoskeletal), and moira (chromatin remodeling).( 7 ) Human scribble could interact with the human homolog of these cell adhesion, signaling, cytoskeletal, and chromatin remodeling genes, and thereby could be involved in the common pathway to negatively regulate the entry of cell cycle from G1 to S phase.
Our data on the functional domain mapping of hScrib indicates that hScrib is a tumor suppressor in mammalian epithelia, having a role in the inhibition of cell proliferation through cell‐cycle regulation. Localization at the adherens junction is required for hScrib to exert its proper inhibitory role in the proliferation of epithelia, depending on its LRRs and at least one PDZ domain. Further work on the identification of its binding partner through LRRs or PDZ domains will reveal the mechanisms of how hScrib controls cell growth and cell‐cycle progression.
Acknowledgments
This work was supported by a grant from the Mitsui Life Social Welfare Foundation (to S. N.) and by a Grant‐in‐Aid for Scientific Research (no. 16591645, to S. N.) from the Ministry of Education, Science and Culture, Japan.
References
- 1. Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev 2004; 18: 1909–25. [DOI] [PubMed] [Google Scholar]
- 2. Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer 2001; 1: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greaves S. Growth and polarity: the case for scribble. Nat Cell Biol 2000; 2: E140. [DOI] [PubMed] [Google Scholar]
- 4. Muller HA, Bossinger O. Molecular networks controlling epithelial cell polarity in development. Mech Dev 2003; 120: 1231–56. [DOI] [PubMed] [Google Scholar]
- 5. Bilder D, Perrimon N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 2000; 403: 676–80. [DOI] [PubMed] [Google Scholar]
- 6. Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 2000; 289: 113–6. [DOI] [PubMed] [Google Scholar]
- 7. Brumby A, Secombe J, Horsfield J et al. A genetic screen for dominant modifiers of a cyclin E hypomorphic mutation identifies novel regulators of S‐phase entry in Drosophila . Genetics 2004; 168: 227–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin‐mediated degradation by the high‐risk papillomavirus E6 proteins and the E6AP ubiquitin‐protein ligase. Mol Cell Biol 2000; 20: 8244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high‐risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA 1997; 94: 11612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsumine A, Ogai A, Senda T et al. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science 1996; 272: 1020–3. [DOI] [PubMed] [Google Scholar]
- 11. Nakagawa S, Yano T, Nakagawa K et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer 2004; 90: 194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bilder D, Birnbaum D, Borg JP et al. Collective nomenclature for LAP proteins. Nat Cell Biol 2000; 2: E114. [DOI] [PubMed] [Google Scholar]
- 13. Borg JP, Marchetto S, Le Bivic A et al. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol 2000; 2: 407–14. [DOI] [PubMed] [Google Scholar]
- 14. Bryant PJ, Huwe A. LAP proteins: what's up with epithelia? Nat Cell Biol 2000; 2: E141–3. [DOI] [PubMed] [Google Scholar]
- 15. Audebert S, Navarro C, Nourry C et al. Mammalian Scribble forms a tight complex with the betaPIX exchange factor. Curr Biol 2004; 14: 987–95. [DOI] [PubMed] [Google Scholar]
- 16. Jaulin‐Bastard F, Saito H, Le Bivic A et al. The ERBB2/HER2 receptor differentially interacts with ERBIN and PICK1 PSD‐95/DLG/ZO‐1 domain proteins. J Biol Chem 2001; 276: 15256–63. [DOI] [PubMed] [Google Scholar]
- 17. Laprise P, Viel A, Rivard N. Human homolog of disc‐large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J Biol Chem 2004; 279: 10157–66. [DOI] [PubMed] [Google Scholar]
- 18. Takizawa S, Nagasaka K, Nakagawa S et al. Human scribble, a novel tumor suppressor identified as a target of high‐risk HPV E6 for ubiquitin‐mediated degradation, interacts with adenomatous polyposis coli. Genes Cells 2006; 11: 453–64. [DOI] [PubMed] [Google Scholar]
- 19. Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol 2003; 5: 53–8. [DOI] [PubMed] [Google Scholar]
- 20. Ekholm SV, Reed SI. Regulation of G (1) cyclin‐dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol 2000; 12: 676–84. [DOI] [PubMed] [Google Scholar]
- 21. Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1‐phase progression. Genes Dev 1999; 13: 1501–12. [DOI] [PubMed] [Google Scholar]
- 22. Dow LE, Brumby AM, Muratore R et al. hScrib is a functional homologue of the Drosophila tumour suppressor Scribble. Oncogene 2003; 22: 9225–30. [DOI] [PubMed] [Google Scholar]
- 23. Morais Cabral JH, Petosa C, Sutcliffe MJ et al. Crystal structure of a PDZ domain. Nature 1996; 382: 649–52. [DOI] [PubMed] [Google Scholar]
- 24. Heinen CD, Goss KH, Cornelius JR et al. The APC tumor suppressor controls entry into S‐phase through its ability to regulate the cyclin D/RB pathway. Gastroenterology 2002; 123: 751–63. [DOI] [PubMed] [Google Scholar]
- 25. Kobe B, Deisenhofer J. A structural basis of the interactions between leucine‐rich repeats and protein ligands. Nature 1995; 374: 183–6. [DOI] [PubMed] [Google Scholar]
- 26. Legouis R, Jaulin‐Bastard F, Schott S, Navarro C, Borg JP, Labouesse M. Basolateral targeting by leucine‐rich repeat domains in epithelial cells. EMBO Rep 2003; 4: 1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Albertson R, Chabu C, Sheehan A, Doe CQ. Scribble protein domain mapping reveals a multistep localization mechanism and domains necessary for establishing cortical polarity. J Cell Sci 2004; 117: 6061–70. [DOI] [PubMed] [Google Scholar]
- 28. Zeitler J, Hsu CP, Dionne H, Bilder D. Domains controlling cell polarity and proliferation in the Drosophila tumor suppressor Scribble. J Cell Biol 2004; 167: 1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC‐hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene 2000; 19: 365–72. [DOI] [PubMed] [Google Scholar]


