Abstract
Diffuse large B‐cell lymphomas are detected frequently in the oral cavity. Although tonsillar lymphomas have been rather well characterized, lymphomas originating from non‐tonsillar regions, such as the gingiva, palate, and tongue, have not been well studied. We examined the pathology of clinical samples obtained from 21 patients with localized primary non‐tonsillar oral diffuse large B‐cell lymphoma. Immunohistological examination of CD10, Bcl‐6, and MUM1 determined that 17 of 21 (81%) samples exhibited non‐germinal center B‐cell type, an increased proportion of non‐germinal center B‐cell type compared with previous reports in samples of tonsillar origin (P < 0.05). The four remaining samples exhibited germinal center B‐cell type, although one sample expressed MUM1. Follow‐up clinical survival data were obtained from the 17 patients over a range from 4 to 173 months (mean 52 months). All patients were treated with chemotherapies, irradiation, or surgical resection. Sixteen patients achieved complete remission and two patients relapsed, but no patient has died of disease. Extranodal diffuse large B‐cell lymphomas of non‐germinal center B‐cell type are generally characterized by poor prognosis, regardless of localized disease. Interestingly, our results indicate that, unlike similar lymphomas of tonsillar origin, localized primary non‐tonsillar oral diffuse large B‐cell lymphomas exhibit favorable prognosis, suggesting that these lymphomas may be clinicopathologically distinct. (Cancer Sci 2009; 100: 42–46)
Primary oral cavity lymphoma accounts for less than 5% of all oral malignancies,( 1 ) and only approximately 1% of all lymphomas and 2–12% of extranodal lymphomas.( 1 , 2 , 3 ) A cohort of 83 patients with oral cavity lymphoma represents 1.4 and 2.5% of all lymphoma and extranodal lymphoma cases, respectively, from our surgical pathology files. Diffuse large B‐cell lymphoma (DLBCL) is the most common kind of oral cavity lymphoma.( 4 ) Although heterogeneous by nature, DLBCL can be distinguished by genetic profiling as two distinct subtypes: germinal center B‐cell‐like (GCB) phenotype and non‐germinal center B‐cell‐like (non‐GCB) phenotype.( 5 , 6 , 7 ) Hans et al. reported that these two types of DLBCL can also be distinguished easily by immunohistological staining for CD10, BCL‐6, and MUM1 proteins.( 6 ) Notably, patients belonging to the former group present better prognosis than the latter group.
In recent studies, extranodal DLBCL were subclassified into two major prognostic categories: GCB type and non‐GCB type (Table 1).( 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 ) Whereas tonsillar DLBCL has been characterized extensively, to date there has been no clinicopathological characterization of non‐tonsillar oral DLBCL, to the best of our knowledge. We sought to examine and characterize GCB versus non‐GCB types of localized primary non‐tonsillar oral DLBCL, with particular focus on patient prognosis.
Table 1.
Immunophenotype of extranodal diffuse large B‐cell lymphomas (DLBCL)
| Extranodal DLBCL | Major immunophenotype |
|---|---|
| Central nervous system( 8 ) | Non‐GCB |
| Breast( 9 ) | Non‐GCB |
| Stomach( 10 ) | Non‐GCB |
| Leg‐type cutaneous( 11 ) | Non‐GCB |
| Testis( 12 ) | Non‐GCB |
| Intravascular type( 13 ) | Non‐GCB |
| Bone( 14 ) | GCB |
| Intestine( 10 ) | GCB |
| Tonsil( 15 ) | GCB |
| Thyroid( 16 ) | GCB |
| Non‐leg cutaneous( 11 ) | GCB |
GCB, germinal center B‐cell‐like.
Materials and Methods
Patients and materials. We selected 21 Japanese patients with localized primary non‐tonsillar oral DLBCL diagnosed between 1990 and 2007. We selected localized‐stage lymphomas only, because primary sites of advanced‐stage lymphomas were difficult to determine. All cases were retrieved from the surgical pathology files of the Department of Pathology, Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, Okayama University, Okayama, Japan.
Histological examination, immunohistochemistry, and in situ hybridization. Surgically resected or biopsied specimens of oral DLBCL were fixed in 10% formaldehyde and embedded in paraffin. Serial sections (4 µm) were cut from each paraffin‐embedded tissue block, and several sections were stained with hematoxylin.
Immunohistochemistry was carried out on paraffin sections using an automated Benchmark XT slide stainer (Ventana Medical Systems). The primary antibodies used were: CD20 (L26, 1:200; Novocastra), CD3 epsilon (PS‐1, 1:50; Novocastra), Bcl‐2 (3.1, 1:200; Novocastra), CD5 (4C7, 1:100; Novocastra), CD10 (56C6, 1:50; Novocastra), CD138 (MI15, 1:100; Dako), MUM1 (MUM1p, 1:50; Dako), Bcl‐6 (polyclonal, 1:50; Dako), and Ki‐67 (MIB‐1, 1:5000; Novocastra).
For each section, 10 high‐power fields were recorded, quantitated, and averaged for the estimated percentage of positively immunostained cells. The following categories were defined: negative (<30% positively stained tumor cells) or positive (>30% positively stained tumor cells).( 6 ) Ki‐67 immunoreactivity was evaluated semiquantitatively using the average estimated percentage of positive cells in 10 recorded high‐power fields.
In situ hybridization (ISH) with Epstein–Barr virus (EBV)‐encoded small RNA (EBER) oligonucleotides was carried out to test for the presence of EBV small RNA in formalin‐fixed paraffin‐embedded sections using the automated Benchmark XT slide stainer.
Statistical analysis. All statistical analyses were carried out with SPSS software (version 14.0; SPSS Inc., Japan). Actuarial overall survival curves were calculated using the Kaplan–Meier method,( 17 ) and differences were examined using the log‐rank test to detect significant prognostic factors.( 18 ) Overall survival was defined as the time from diagnosis to death from any cause or from last follow up.
Results
Characterization of patients with non‐tonsillar oral DLBCL. The median age of the 21 patients with localized primary non‐tonsillar oral DLBCL was 68 years (range 53–86 years) (Table 2). There were 11 men and 10 women. Eleven patients (52%) presented with disease in the gingival mucosa, six patients (28%) in the hard palate mucosa, two patients (9%) in the soft palate mucosa, one patient (4%) in the dorsum of the tongue, and one patient (4%) in the buccal mucosa. The anatomical presenting site was typically a painless oral mucosal mass, which increased gradually in size. According to the Ann Arbor classification, 17 patients were clinical stage IE, and four patients were stage IIE. Thirteen of 21 patients exhibited superficial ulceration of the tumor mass. Seven patients (33%) were at low–intermediate risk and the remaining 14 patients were at low risk according to the International Prognostic Index.( 19 ) Histologically, all cases were classified as diffuse large B‐cell lymphoma (Fig. 1). All patients were newly presenting, with no prior treatment history.
Table 2.
Clinical data of 21 primary non‐tonsillar oral diffuse large B‐cell lymphomas
| Patient no. | Age (years) | Sex | Primary site | IPI | LDH > normal values | Tumor size (maximum diameter; cm) | Ulcer | CS | Treatment | Relapse | Follow‐up period (months) | Follow‐up status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 69 | M | Hard palate | L | No | 2.5 | – | IE | CHOP + RT | + | 59 | Alive, FOD |
| 2 | 57 | F | Gingiva | L | No | 2.5 | + | IE | RT | – | 79 | Alive, FOD |
| 3 | 65 | F | Gingiva | LI | Yes | 3.5 | + | IE | R‐CHOP + RT | – | 13 | Alive, FOD |
| 4 | 68 | F | Gingiva | LI | No | 3.5 | + | IE | CHOP + RT | – | 22 | Alive, FOD |
| 5 | 60 | M | Gingiva | L | No | 2 | + | IE | R‐CHOP + RT | – | 16 | Alive, FOD |
| 6 | 68 | F | Gingiva | LI | Yes | 5< | – | IE | NA | NA | NA | NA |
| 7 | 62 | M | Gingiva | LI | No | 2, multiple | + | IE | R‐CHOP | – | 19 | Alive, FOD |
| 8 | 74 | F | Hard palate | L | No | 3 | – | IE | CHOP + RT | – | 129 | Alive, FOD |
| 9 | 85 | M | Soft palate | L | No | 3.5 | – | IE | NA | NA | NA | NA |
| 10 | 76 | M | Gingiva | L | No | 4 | + | IE | R‐THP‐COP + RT | – | 33 | Alive, FOD |
| 11 | 53 | M | Hard palate | L | No | 2 | – | IE | CHOP | NA | NA | NA |
| 12 | 72 | F | Gingiva | L | No | 3 | – | IE | NA | NA | NA | NA |
| 13 | 85 | M | Hard palate | L | No | 3 | – | IE | R‐CHOP | – | 37 | Alive, FOD |
| 14 | 86 | M | Soft palate | LI | Yes | 2 | + | IE | CHOP + RT | – | 126 | Alive, FOD |
| 15 | 71 | M | Buccal | L | No | 4.5 | + | IE | CHOP + RT | + | 67 | Alive, FOD |
| 16 | 63 | F | Hard palate | L | No | 3 | + | IE | CHOP + RT | NA | 4 | LTF |
| 17 | 62 | F | Gingiva | LI | Yes | 1.5 | + | IE | CHOP + RT | – | 177 | Alive, FOD |
| 18 | 77 | F | Gingiva | L | No | 3.5 | + | IIE | Partial resection + CHOP | – | 31 | Alive, FOD |
| 19 | 57 | M | Gingiva | L | No | 3 | – | IIE | R‐CHOP | – | 52 | Alive, FOD |
| 20 | 71 | F | Tongue | L | No | 2.5 | + | IIE | THP‐COP | – | 34 | Alive, FOD |
| 21 | 67 | M | Hard palate | LI | Yes | 5< | + | IIE | CHOP + RT | – | 29 | Alive, FOD |
CHOP, cyclophosphamide, adriamycin, vincristine, prednisolone; CS, clinical stage; F, female; FOD, free of disease; IPI, International Prognostic Index; L, low; LDH, lactate dehydrogenase; LI, low–intermediate; LTF, lost to follow up; M, male; NA, not available; R‐, with rituximab; RT, radiation; THP‐COP, cyclophosphamide, THP‐doxorubicin (pirarubicin), vincristine, prednisolone.
Figure 1.
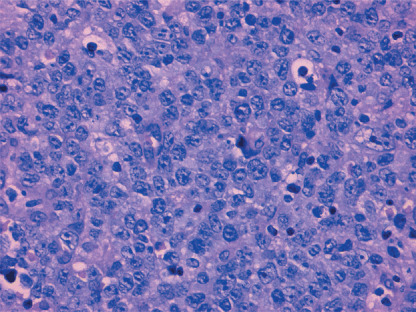
Diffuse infiltration and proliferation of large lymphoma cells in oral mucosa. Hematoxylin–eosin staining. Original magnification ×200.
Immunohistochemical and ISH analysis of non‐tonsillar oral DLBCL. The B‐cell immunophenotype of the lymphomas was confirmed by immunoreactivity with antibodies to CD20 in 21 cases (Table 3). Although no cases were positive for CD5 and CD138, four cases (19%) were positive for CD10, 12 cases (57%) were positive for Bcl‐2, and 10 cases (48%) were positive for BCL‐6. Ki‐67 ≥ 80% was observed in 10 cases (48%), and 18 cases (86%) displayed MUM1‐positive staining. As shown in 2, 3, the four CD10‐positive cases (19%) were classified as GCB type, and one of the four was positive for MUM1. Of the other 17 (81%), which were classified as non‐GCB type, 11 were CD10‐negative and BCL‐6‐negative, and six were CD10‐negative, BCL‐6‐positive, and MUM1‐positive. Of the 21 cases examined, EBER was detected in two cases (9%) by ISH.
Table 3.
Immunohistochemical findings of primary non‐tonsillar oral diffuse large B‐cell lymphoma
| Patient no. | EBER | CD3 | CD5 | CD10 | CD20 | CD138 | BCL‐6 | MUM1 | Bcl‐2 | Ki‐67 labeling (%) | Immunophenotype |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | – | – | – | – | + | – | – | + | + | 50 | Non‐GCB |
| 2 | – | – | – | – | + | – | – | + | – | 85 | Non‐GCB |
| 3 | + | – | – | – | + | – | – | + | + | 75 | Non‐GCB |
| 4 | – | – | – | – | + | – | – | + | – | 60 | Non‐GCB |
| 5 | – | – | – | – | + | – | + | + | + | 90 | Non‐GCB |
| 6 | – | – | – | + | + | – | + | – | + | 80 | GCB |
| 7 | + | – | – | – | + | – | + | + | + | 70 | Non‐GCB |
| 8 | – | – | – | – | + | – | – | + | + | 60 | Non‐GCB |
| 9 | – | – | – | – | + | – | – | + | + | 80 | Non‐GCB |
| 10 | – | – | – | – | + | – | + | + | + | 80 | Non‐GCB |
| 11 | – | – | – | – | + | – | – | + | – | 90 | Non‐GCB |
| 12 | – | – | – | + | + | – | + | + | – | 50 | GCB |
| 13 | – | – | – | + | + | – | + | – | + | 55 | GCB |
| 14 | – | – | – | – | + | – | – | + | – | 90 | Non‐GCB |
| 15 | – | – | – | – | + | – | – | + | + | 90 | Non‐GCB |
| 16 | – | – | – | + | + | – | + | – | – | 50 | GCB |
| 17 | – | – | – | – | + | – | + | + | – | 65 | Non‐GCB |
| 18 | – | – | – | – | + | – | + | + | – | 80 | Non‐GCB |
| 19 | – | – | – | – | + | – | – | + | – | 90 | Non‐GCB |
| 20 | – | – | – | – | + | – | + | + | + | 40 | Non‐GCB |
| 21 | – | – | – | – | + | – | – | + | + | 60 | Non‐GCB |
EBER, Epstein–Barr virus‐encoded small RNA; GCB, germinal center B‐cell.
Figure 2.
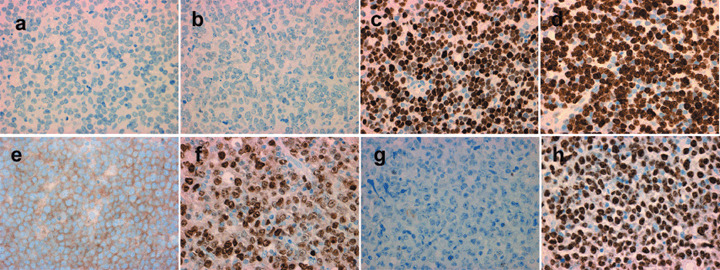
The immunohistochemical panel for case 2 (upper row), belonging to the non‐germinal center B‐cell‐like group, with (a) CD10‐negative, (b) Bcl‐6‐negative, and (c) MUM1‐positive staining, and (d) high proliferative activity as labeled by Ki‐67. The immunohistochemical panel for case 6 (lower row), belonging to the germinal center B‐cell‐like group, with (e) CD10‐positive, (f) Bcl‐6‐positive, and (g) MUM1‐negative staining, and (h) high proliferative activity as labeled by Ki‐67.
Figure 3.
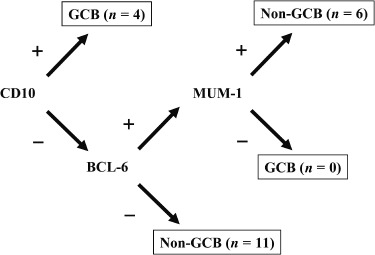
Distribution of germinal center B‐cell‐like (GCB) and non‐GCB type non‐tonsillar oral diffuse large B‐cell lymphomas according to Hans et al.( 6 )
Clinical data and statistics analysis in patients with non‐tonsillar oral DLBCL. Follow‐up clinical data were available for 17 patients. The duration of follow up ranged from 4 to 173 months (mean 52 months). Ten patients were treated initially with chemotherapy plus irradiation, five were treated with chemotherapy alone, one was treated with irradiation alone, and one was treated with chemotherapy plus surgery. Sixteen patients achieved complete remission. Although two patients relapsed, complete remission was achieved with alternative chemotherapy. Initial treatments for the two relapsed patients were cyclophosphamide, adriamycin, vincristine, and prednisolone plus radiation. To date, 16 patients are disease free and no patients died of disease (Table 2). These results differ from those of previous reports on localized non‐GCB type DLBCL (Fig. 4).( 9 , 12 )
Figure 4.
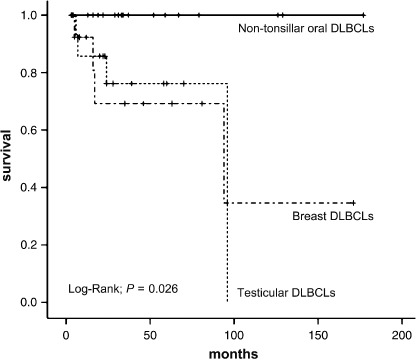
Comparsion of localized non‐tonsillar oral diffuse large B‐cell lymphomas (DLBCL) with localized testicular and breast DLBCL in overall survival.( 9 , 12 )
Discussion
Diffuse large B‐cell lymphoma is the most frequent and aggressive lymphoma, and represents a heterogeneous group that includes de novo large‐cell lymphomas, as well as transformed lymphomas from follicular or mucosa‐associated lymphoid tissue lymphomas.( 20 )
Recent studies have clarified that DLBCL can be further subclassified into two major prognostic categories: GCB type and non‐GCB type.( 5 , 6 ) Non‐GCB type DLBCL had a significantly poorer prognosis than GCB type DLBCL.
We carried out immunohistochemical analysis of 21 patients with localized primary non‐tonsillar oral DLBCL. Our results show that the majority (81%) of localized primary non‐tonsillar oral DLBCL can be identified as non‐GCB type, whereas Ree et al. reported that 51% of Asian tonsillar DLBCL are GCB type.( 15 ) Comparing our data, there was a statistically significant difference (P < 0.05, χ2‐test) in the ratio of GCB type to non‐GCB type between the subjects of the two studies. Moreover, one patient in our study was positive for CD10 and MUM‐1, which was categorized as GCB type according to Hans et al.( 6 ) However, Chang et al. reported that such double‐positive expression is characterized by poor prognosis similar to that of non‐GCB type.( 21 ) This outlying double expression pattern suggests that our patient cohort might in fact have more non‐GCB type. Our data indicated that localized primary non‐tonsillar oral DLBCL exhibit a rather uniform immunophenotype. Interestingly, patients with localized primary non‐tonsillar oral DLBCL presented with a good clinical course, despite having non‐GCB type.
Rituximab, a chimeric mouse–human monoclonal antibody against CD20, is used for monotherapy, as well as combination chemotherapy, against CD20‐positive B‐cell lymphoma.( 22 ) Recent application of rituximab improved DLBCL patient prognosis, but only six patients in our cohort were treated with rituximab.
The oral cavity is very sensitive and it is easy to notice abnormalities, which might lead to earlier diagnosis of disease. This could be associated with rather favorable patient prognoses. However, unexpectedly almost all patients had tumors that were ≥2 cm in diameter.
According to previous reports, DLBCL of the central nervous system,( 8 ) breast,( 9 ) stomach,( 10 ) leg type,( 11 ) testis,( 12 ) and intravascular type( 13 ) predominantly exhibit non‐GCB type, consistent with our study of localized primary non‐tonsillar oral DLBCL cases. However, patients with central nervous system, breast, and testicular DLBCL exhibit poor prognosis, regardless of localized disease.( 8 , 9 , 12 , 23 ) The patients with leg‐type cutaneous DLBCL presented multiple skin lesions on one or both legs, and had disease‐related 5‐year survival of 45 and 36%, respectively, which is significantly poorer than non‐leg‐type cutaneous DLBCL.( 11 , 24 ) In contrast, when comparing localized primary non‐tonsillar oral DLBCL with localized non‐GCB type DLBCL, there was a statistically significant difference in overall survival (Fig. 4). Localized‐stage gastric DLBCL have good prognosis,( 25 , 26 ) despite their non‐GCB type. Connor and Ashton‐Key have reported that gastric DLBCL might reflect a relationship with mucosa‐associated lymphoid tissue (MALT) lymphoma.( 10 )
Our data show that localized primary non‐tonsillar oral DLBCL have biologically similar behavior to DLBCL of gastric origin. The reasons for this remain unclear, but MALT lymphomas might show a histological transformation to high‐grade lymphoma of this region (e.g. the stomach), although the incidences of MALT lymphoma in these organs are not similar.( 27 , 28 )
High‐grade transformation might be recognized as such if a residual area of MALT lymphoma can be identified. In the absence of such areas, the tumors are morphologically indistinguishable from DLBCL.( 29 ) We checked the biopsied specimens thoroughly, and there were no low‐grade MALT lymphomatous components. As the biopsied specimens from the oral cavity were generally small in size, associated low‐grade components could not be entirely excluded. Interestingly, Kojima et al. reported that the high‐grade type of nodal marginal zone B‐cell lymphoma was categorized as an indolent tumor.( 30 ) They suggested that non‐GCB type DLBCL arising from MALT‐type nodal marginal zone B‐cell lymphoma may be separated from conventional non‐GCB type DLBCL.
The proliferating fraction of the lymphomas that we examined was generally high, with an average of 71%. Leval and Harris reported that a proliferating fraction of ≥80% is an adverse prognostic factor.( 31 ) Although 48% of our cases showed a proliferating fraction of ≥80%, no significant correlation was detected in the patients’ clinical courses and in the Ki‐67 labeling index.
Previous reports have shown that lymphomas associated with overexpression of Bcl‐2 behave aggressively and reduce overall survival.( 32 , 33 , 34 , 35 , 36 ) In our series, however, although Bcl‐2 expression was detected in 57% of the cases examined, there was no difference between the Bcl‐2‐positive and Bcl‐2‐negative groups.
In conclusion, we have demonstrated that localized primary non‐tonsillar oral DLBCL exhibited uniform clinicopathological characteristics, especially those with favorable prognoses in spite of the non‐GCB phenotype. A review of previous reports indicated that in most extranodal sites, each organ has a clear devision of GCB versus non‐GCB in DLBCL, and the patients’ prognoses depend on these lymphoma phenotypes, except for non‐tonsillar oral lymphoma.
Acknowledgments
We express our appreciation to Dr Masaru Kojima (Gunma prefectural cancer center) for helpful suggestions, and to the pathologists and clinicians involved in the diagnosis and treatment of the patients in this study at the hospitals that are affiliated with Okayama University.
References
- 1. Epstein JB, Epstein JD, Le ND, Gorsky M. Characteristics of oral and paraoral malignant lymphoma: a population‐based review of 361 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 92: 519–25. [DOI] [PubMed] [Google Scholar]
- 2. Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer 1972; 29: 252–60. [DOI] [PubMed] [Google Scholar]
- 3. Shima N, Kobashi Y, Tsutsui K et al . Extranodal non‐Hodgkin's lymphoma of the head and neck. A clinicopathologic study in the Kyoto‐Nara area of Japan. Cancer 1990; 66: 1190–7. [DOI] [PubMed] [Google Scholar]
- 4. Kolokotronis A, Konstantinou N, Christakis I et al . Localized B‐cell non‐Hodgkin's lymphoma of oral cavity and maxillofacial region: a clinical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99: 303–10. [DOI] [PubMed] [Google Scholar]
- 5. Alizadeh AA, Eisen MB, Davis RE et al . Distinct types of diffuse large B‐cell lymphoma identified by gene expression profiling. Nature 2000; 4052: 503–11. [DOI] [PubMed] [Google Scholar]
- 6. Hans CP, Weisenburger DD, Greiner TC et al . Confirmation of the molecular classification of diffuse large B‐cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103: 275–82. [DOI] [PubMed] [Google Scholar]
- 7. Rosenwald A, Wright G, Chan WC et al . The use of molecular profiling to predict survival after chemotherapy for diffuse large B‐cell lymphoma. N Engl J Med 2002; 346: 1937–47. [DOI] [PubMed] [Google Scholar]
- 8. Cmillerri‐Broet S, Criniere E, Broet P et al . A uniform activated B‐cell‐like immunophenotype might explain the poor prognosis of primary central nervous system lymphomas: analysis of 83 cases. Blood 2006; 107: 190–6. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida S, Nakamura N, Sasaki Y et al . Primary breast diffuse large B‐cell lymphoma shows a non‐germinal center B‐cell phenotype. Mod Pathol 2005; 18: 398–405. [DOI] [PubMed] [Google Scholar]
- 10. Connor J, Ashton‐Key M. Gastric and intestinal diffuse large B‐cell lymphomas are clinically and immunophenotypically different. An immunohistochemical and clinical study. Histopathology 2007; 51: 697–703. [DOI] [PubMed] [Google Scholar]
- 11. Campo E, Chott A, Kinney MC. Update on extranodal lymphomas. Conclusions of the workshop held by the EAHP and the SH in Thessaloniki. Greece Histopathol 2006; 48: 481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al‐Abbadi MA, Hattab EM, Tarawneh MS, Amr SS, Orazi A, Ulbright TM. Primary testicular diffuse large B‐cell lymphoma belongs to the nongerminal center B‐cell‐like subgroup: a study of 18 cases. Mod Pathol 2006; 19: 1521–7. [DOI] [PubMed] [Google Scholar]
- 13. Murase T, Yamaguchi M, Suzuki R et al . Intravascular large B‐cell lymphoma (IVLBCL): a clinicopathologic study of 96 cases with special reference to the immunophenotypic heterogeneity of CD5. Blood 2007; 109: 478–85. [DOI] [PubMed] [Google Scholar]
- 14. Kitsoulis P, Vlychou M, Papoudou‐Bai A et al . Primary lymphomas of bone. Anticancer Res 2006; 26: 325–38. [PubMed] [Google Scholar]
- 15. Ree HJ, Ohsima K, Aozasa K et al . Detection of germinal center B‐cell lymphoma in archival specimens: critical evaluation of Bcl‐6 protein expression in diffuse large B‐cell lymphoma of the tonsil. Hum Pathol 2003; 34: 610–16. [DOI] [PubMed] [Google Scholar]
- 16. Niitsu N, Okamoto M, Nakamura N et al . Clinicopathologic correlations of stage IE/IIE primary thyroid diffuse large B‐cell lymphoma. Ann Oncol 2007; 18: 1203–8. [DOI] [PubMed] [Google Scholar]
- 17. Kaplan EL, Meier P. Non‐parametric estimation from incomplete observation. Am J Stat Assoc 1958; 53: 457–8. [Google Scholar]
- 18. Peto PE, Pike MC, Armitage P et al . Design and analysis of randomized clinical trials requiring prolonged observation of each patients. Br J Cancer 1977; 35: 1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shipp MA. International non‐Hodgkin's lymphoma prognostic factors project. A predictive model for aggressive non‐Hodgkin's lymphoma. N Eng J Med 1993; 329: 987–94. [DOI] [PubMed] [Google Scholar]
- 20. Pileri SA, Dirnhofer S, Went PH et al . Diffuse large B‐cell lymphoma: one or more entities? Present controversies and possible tools for its subclassification. Histopathology 2002; 41: 482–509. [DOI] [PubMed] [Google Scholar]
- 21. Chang CC, McClintock S, Cleveland RP et al . Immunohistochemical expression patterns of germinal center and activation B‐cell makers correlate with prognosis in diffuse large B‐cell lymphoma. Am J Surg Pathol 2004; 28: 464–70. [DOI] [PubMed] [Google Scholar]
- 22. Coiffier B, Lepage E, Briere J et al . CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large B‐cell lymphoma. N Engl J Med 2002; 346: 235–42. [DOI] [PubMed] [Google Scholar]
- 23. Zucca E, Conconi A, Mughal TI et al . Patterns of outcome and prognostic factors in primary large‐cell lymphoma of the testis in a survey by the international extranodal lymphoma Study Group. J Clin Oncol 2003; 21: 20–7. [DOI] [PubMed] [Google Scholar]
- 24. Grange F, Bekkenk MW, Wechsler J et al . Prognostic factors in primary cutaneous large B‐cell lymphomas: a European multicenter study. J Clin Oncol 2001; 19: 3602–10. [DOI] [PubMed] [Google Scholar]
- 25. Ishikura S, Tobinai K, Ohtsu A et al . Japanese multicenter phase: study of CHOP followed by radiotherapy in stage I‐II1, diffuse large B‐cell lymphoma of the stomach. Cancer Sci 2005; 96: 349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferreri AJ, Montalbán C. Primary diffuse large B‐cell lymphoma of the stomach. Crit Rev Oncol Hematol 2007; 63: 65–71. [DOI] [PubMed] [Google Scholar]
- 27. Kemp S, Gallagher G, Kabani S et al . Oral non‐Hodgkin's lymphoma: review of the literature and World Health Organization classification with reference to 40 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008; 105: 194–201. [DOI] [PubMed] [Google Scholar]
- 28. Solomides CC, Miller AS, Christman RA et al . Lymphomas of the oral cavity: histology, immunologic type, and incidence of Epstein–Barr virus infection. Hum Pathol 2002; 33: 153–7. [DOI] [PubMed] [Google Scholar]
- 29. Kojima M, Nakamura N, Shimizu K et al . Marginal zone B‐cell lymphoma among primary B‐cell lymphoma of Waldeyer's ring: Histopathologic and immunohistochemical study of 16 tonsillectomy specimens. Int J Surg Pathol 2008; 16: 164–70. [DOI] [PubMed] [Google Scholar]
- 30. Kojima M, Inagaki H, Motoori T et al . Clinical implications of nodal marginal zone B‐cell lymphoma among Japanese: study of 65 cases. Cancer Sci 2007; 98: 44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leval LD, Harris NL. Variability in immunophenotype in diffuse large B‐cell lymphoma and its clinical relevance. Histopathology 2003; 43: 509–28. [DOI] [PubMed] [Google Scholar]
- 32. Gascoyne RD, Adomat SA, Krajewski S et al . Prognostic significance of Bcl‐2 protein expression and Bcl‐2 gene rearrangement in diffuse aggressive non‐Hodgkin's lymphoma. Blood 1997; 90: 244–51. [PubMed] [Google Scholar]
- 33. Hermine O, Haioun C, Lepage E et al . Prognostic significance of bcl‐2 protein expression in aggressive non‐Hodgkin's lymphoma. Groupe d’Etude des Lymphomes de l’Adulte (GELA). Blood 1996; 87: 265–72. [PubMed] [Google Scholar]
- 34. Hill ME, MacLennan KA, Cunningham DC et al . Prognostic significance of BCL‐2 expression and bcl‐2 major breakpoint region rearrangement in diffuse large cell non‐Hodgkin's lymphoma: a British National Lymphoma Investigation Study. Blood 1996; 88: 1046–51. [PubMed] [Google Scholar]
- 35. Iqbal J, Neppalli VT, Wright G et al . BCL2 expression is a prognostic marker for the activated B‐cell‐like type of diffuse large B‐cell lymphoma. J Clin Oncol 2006; 24: 961–8. [DOI] [PubMed] [Google Scholar]
- 36. Wilson WH, Teruya‐Feldstein J, Fest T et al . Relationship of p53, bcl‐2, and tumor proliferation to clinical drug resistance in non‐Hodgkin's lymphomas. Blood 1997; 89: 601–9. [PubMed] [Google Scholar]


