Abstract
Recent molecular biological studies have identified podoplanin as a candidate cancer stem cell (CSC) marker in squamous cell carcinoma (SqCC). The purpose of this study was to examine the expression pattern of podoplanin, and the other stem cell markers CD44 and p63, and their relationship to clinico‐pathological features including survival in pulmonary SqCC. We examined histologically the expression of podoplanin, CD44, and p63 in 162 consecutive SqCC by immunostaining. Podoplanin expression was observed in 107 (66%) tumors, CD44 in 145 (89.5%), and p63 in 151 (93.2%), respectively. In 95.3% of the podoplanin‐positive tumors, tumor cells showing strong expression were localized in the periphery of the tumor nests. However, this peripheral localization was observed in only 55.9% of the CD44‐positive and 43% of p63‐positive tumors. In 88.8% of the podoplanin‐positive tumors, positive cells were localized more peripherally in the tumor nests than CD44‐ or p63‐positive cells and when CD44 and p63 expressions were compared in these podoplanin‐positive tumors, p63‐positive layers in the periphery of the tumor nests were broader compared to CD44‐positive layers. These findings suggest tumor cells are aligned in the “hierarchical distribution pattern” according to the expression of these three markers. Patients who had podoplanin‐positive tumors with the “hierarchical pattern” resulted in significantly better overall survival than those who had podoplanin‐negative tumors (P = 0.043). These results suggest that podoplanin expression would reflect the most immature status in the differentiation process of SqCC, and SqCC with hierarchical expression would be a well‐organized tumor group with lower biological aggressiveness based on the CSC concept. (Cancer Sci 2009)
Lung cancer is the leading cause of cancer mortality worldwide, and two main types of non‐small‐cell lung carcinoma (NSCLC), adenocarcinoma and squamous cell carcinoma (SqCC), account for over half the cases of lung cancer. There have been recent advances in molecularly targeted agents for the treatment of pulmonary adenocarcinoma, but not much progress has been made in the treatment of SqCC,( 1 , 2 , 3 ) and the molecular mechanisms of SqCC are not completely understood.
Considering that the components of SqCC are heterogeneous and that its histology and marker expression are similar to those of normal epithelium, it suggests a “developmental hierarchy”. Based on the concept that stem cells sit at the top of the developmental hierarchy, the cells at the basal (peripheral) region of SqCC nests may possess stem‐cell‐like properties. The notion that within established tumor, the great majority of the cancer cells cannot sustain the lesion and only a few cells, cancer stem cells (CSCs), are tumorigenic and possess the metastatic phenotype is CSC hypothesis. CSCs, a very small population of specialized cells, have self‐renewal and extensively proliferative characteristics to sustain tumor formation.( 4 , 5 ) Recent molecular biological studies have identified podoplanin, CD44, and p63 as candidate stem cell markers in normal squamous epithelium and SqCC.( 6 , 7 , 8 )
Podoplanin is a mucin‐like transmembrane glycoprotein that is highly and specifically expressed in lymphatic endothelial cells.( 9 ) Podoplanin on cancer cells has been shown to act as a platelet‐aggregation factor and cell–cell adhesion promoter,( 10 ) and induction of expression of PA2.26, a homologue of human podoplanin, in mouse epidermal cells and tumor cells has been shown to be related to increased cell migration and malignant transformation.( 11 ) In addition, we have previously reported that podoplanin is a novel marker to enrich tumor‐initiating cells with stem‐cell‐like properties in SqCC in vivo and in vitro. Using the human SqCC cell line A431, sorted podoplanin‐positive cells have higher colony formation and tumorigenicity than podoplanin‐negative cells, and xenografted tumors derived from podoplanin‐positive cells are similar to those in human oral SqCC tissue and normal epithelium.( 6 )
The cell surface glycoprotein CD44 is involved in cell migration and cell adhesion. CD44 has been found to support anchorage‐independent growth in vitro and tumor growth and metastasis in experimental models of solid cancer.( 12 ) CD44+ cells in breast and lung carcinoma, and head and neck SqCC, have been shown to possess the CSC properties of self‐renewal and differentiation.( 8 , 13 , 14 , 15 )
p63, a homologue of the tumor suppressor p53, plays a crucial role in initiating epithelial stratification during development and in maintaining epidermal structures, including in the oral mucosa, skin, teeth, and other sites, and p63 has been shown to be a specific marker of human corneal and squamous epithelial stem cells.( 7 , 16 , 17 , 18 ) The human p63 gene codes for at least six protein isoforms as a result of initiation of transcription at two different promoter sites that contain (TA) or lack (ΔN) a transactivation domain. The isoforms have different functions and form complicated networks in different systems.( 7 , 15 , 19 )
The purpose of this study was to examine the expression pattern of podoplanin, CD44, and p63, and their implication for clinico‐pathological features in pulmonary SqCC.
Materials and Methods
Patients.
During the period from January 1998 to December 2003, a total of 1279 patients underwent surgical resection for primary lung cancer at the National Cancer Center Hospital East, Chiba, Japan, and we reviewed the cases of the 167 consecutive patients in whom complete resection of pulmonary SqCC had been possible. All patients signed the Institutional Review Board–approved informed consent form. Staging was performed according to the International Union Against Cancer’s tumor‐node‐metastasis (TNM) classification. The tumors were histologically subtyped and graded according to the third edition of the World Health Organization (WHO) guidelines. Five of the 167 patients were not included in this study; three because they received preoperative chemotherapy and two because of the poor quality of the specimens obtained. Thus, 162 patients were ultimately eligible for inclusion in this study, and their median follow‐up period was 5.0 years.
Clinical characteristics were retrieved from the clinical records available. The following clinico‐pathological factors were assessed retrospectively in relation to immunohistochemical analysis: age (<70 years vs≥70 years), tumor size (≤3 cm vs >3 cm), pathological nodal involvement (positive vs negative), grade of differentiation (well or moderately differentiated vs poorly differentiated), location of the tumor (central vs peripheral), vascular invasion (absent vs present), lymphatic invasion (absent vs present), and pleural invasion (absent vs present). Central location of a tumor was defined as a tumor location limited to the trachea, bronchi, or segmental bronchi, and peripheral location as location more peripheral than the subsegmental bronchi. Cumulative smoking was presented by a smoking index, defined as a product of the numbers of cigarette per day and the duration (years).
Pathological studies and tissue microarray (TMA) construction.
After fixing the specimens with cold methanol and embedding in paraffin, serial 4‐μm sections were stained with hematoxylin–eosin (H&E) and by the alcian blue‐periodic acid‐Schiff method to visualize cytoplasmic mucin and by the Verhoeff‐van‐Gieson (VvG) method to visualize elastic fibers. Sections stained by the VvG method were examined for the presence of vascular invasion and pleural invasion. The sections were reviewed by two pathologists (Y.S. and G.I.) and the histological diagnoses were based on the revised WHO histological classification.
For microarray construction, the above two pathologists marked morphologically representative tumor areas, avoiding necrotic areas and the area in which cancer cells and stromal cells are intermingled, and definitely containing interfaces between the tumor nests and stroma, on an H&E‐stained slide of donor tissue. The TMAs were constructed with a manual tissue‐arraying instrument (Azumaya, Tokyo, Japan). The microarray instrument is used to remove a tissue core from the donor block with a thin‐walled needle having an inner diameter of approximately 2.0 mm. Core samples were precisely placed in an empty paraffin block (the recipient block) at a specifically assigned location. Two core samples of each tumor were routinely corrected from two different areas. Normal lung tissue from the some patient’s specimen was used as a positive control for each staining. Specimens from the 162 cases were punched, and core samples were mounted in the same recipient blocks.
Immunohistochemical analysis.
TMA recipient blocks were cut into 4‐μm sections and mounted on silane‐coated slides. After deparaffinizing the sections in xylene and dehydrating them in a graded ethanol series, the slides were washed three times in phosphate‐buffered saline (PBS) and immersed in a 0.3% hydrogen peroxide solution in methanol for 15 min. to inhibit endogenous peroxidase activity. The slides were then washed three times in PBS, and nonspecific binding was blocked by preincubation with 2% normal swine serum in PBS (blocking buffer) for 30 min at room temperature. Individual slides were then incubated overnight at 4°C with anti‐podoplanin (clone D2‐40; Signet, Dedham, MA, USA) at a 1:50 dilution, and anti‐CD44 (clone DF1485; Novocastra, Newcastle, UK) at a 1:40 dilution, and anti‐p63 (clone 4A4; Dako Cytomation, Carpinteria, CA, USA) at a 1:200 dilution. Finally, the slides were washed three times with PBS and incubated with the EnVision+ System HRP (Dako, Glostrup, Denmark), and the reaction products were stained with diaminobenzidine and counterstained with hematoxylin.
When more than 10% of the tumor cells showed an unequivocally strong reaction with an antibody, the tumor was classified as positive. Cytoplasm and/or membrane immunoreactivity was considered to indicate podoplanin and CD44 expression. p63 expression was considered positive if distinct nuclear staining was present. Moreover, we discriminated between “peripheral expression pattern” and “diffuse expression pattern” in positive immunoreactivity by the tumor nests with these antibodies. Peripheral expression pattern was defined as cells showing strong expression localized to the periphery of the tumor nests with no or weak expression in the central area. Diffuse expression pattern was defined as a less clear staining intensity between the peripheral and central areas of the tumor nests.
Statistical analysis.
The associations between immunohistochemical expression status and clinico‐pathological parameters were analyzed by using the χ2‐test or Fisher’s exact test. Overall survival was measured from the date of surgery to the date of death from any cause or the date on which the patient was last known to be alive. Survival curves were plotted according to the Kaplan–Meier method and compared using the log‐rank test. All tests were two‐sided, and P‐values <0.05 were considered statistically significant. The Stat‐view 5.0 software package was used to perform the statistical analysis (SAS Institute, Cary, NC, USA).
Results
Characteristics of the patients.
The clinico‐pathological characteristics of the 162 patients are summarized in Table 1. Male predominance and a high smoking index were outstanding characteristics, and there were only three never smokers. The 5‐year overall survival rate of the 162 patients as a whole was 67.7%.
Table 1.
Clinico‐pathological features of squamous cell carcinoma cases (n = 162)
| Gender | |
| Male | 147 |
| Female | 15 |
| Age | |
| Median (range) | 67 (31–84) |
| Smoking index | |
| Median (range) | 960 (0–2760) |
| Tumor size (cm) | |
| Median (range) | 3.8 (1.0–9.4) |
| N Stage | |
| pN0 | 117 |
| pN1/pN2 | 45 |
| Tumor location | |
| Central | 37 |
| Peripheral | 125 |
| Differentiation | |
| Well/moderate | 3/104 |
| Poor | 55 |
| Vascular invasion | |
| Absent | 113 |
| Present | 49 |
| Lymphatic invasion | |
| Absent | 105 |
| Present | 57 |
| Pleural invasion | |
| Absent | 116 |
| Present | 46 |
| Pathological stage | |
| IA | 50 |
| IB | 62 |
| IIA | 13 |
| IIB | 34 |
| IIIA | 3 |
Expression of podoplanin, CD44, and p63 by cancer cells and characteristic immunostaining.
We examined the expression of podoplanin, CD44, and p63 by immunohistochemical staining in a series of 162 specimens of SqCC of the lung. Podoplanin and CD44 were expressed mainly in the cytoplasm and membrane of the tumor cells, and p63 was expressed in the nuclei. The results of the immunohistochemical analysis are shown in Figure 1. In normal lung tissue, podoplanin expression was consistently detected in the endothelium of the lymphatic vessels and basal cells in the bronchial epithelium (Fig. 1a). CD44 expression was observed in the basal layer of the bronchial epithelium and peribronchial mesenchymal cells (Fig. 1c), and p63 was expressed in the nuclei of basal cells in the bronchial epithelium (Fig. 1e). The tumor cells in 107 (66%) of the 162 specimens were positive for podoplanin. The tumor cells in 145 (89.5%) were positive for CD44, and the tumor cells in 151 (93.2%) were positive for p63 (Table 2). As discussed, there were two patterns of expression in the cases that immunohistochemically stained podoplanin positive. A total of 102 (95.3%) of the 107 podoplanin‐positive cases showed peripheral expression patterns, (Fig. 1b) whereas only five cases (4.7%) showed diffuse expression patterns in the tumor nests. Figure 1(d,f) shows the results for CD44 and p63 staining of the same specimen as in Figure 1b. The CD44 staining pattern was similar to that of podoplanin, but the area of intense CD44 staining was broader than that of podoplanin. Eighty‐one (55.9%) of the CD44‐positive cases showed peripheral expression patterns (Fig. 1d), whereas 64 cases (44.1%) showed diffuse expression patterns. In 65 (43%) of the p63‐positive cases there were peripheral expression patterns, but the difference in the staining intensity of p63 between the peripheral and central areas was less clear compared with podoplanin and CD44 staining (Fig. 1f).
Figure 1.
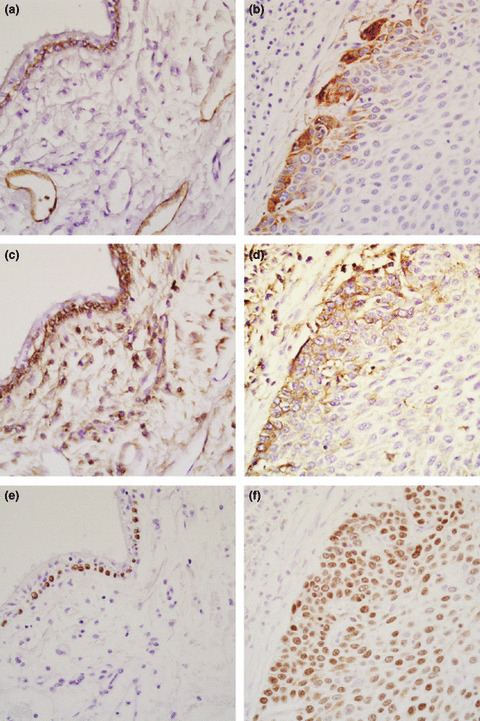
Immunohistochemical analysis of podoplanin, CD44, and p63 expression in squamous cell carcinomas of the lung and a normal part of the specimen from one patient. (a) Podoplanin expression was detected in the endothelium of lymphatic vessels and in bronchial basal cells. (b) Podoplanin expression is mainly localized at the periphery of invading tumor nests. (c) CD44 expression was expressed in the bronchial basal cells. (d) CD44 expression was predominantly found in the peripheral areas of the tumor nests, but its distribution was broader than that of podoplanin. (e) p63 expression was observed in the nuclei of the bronchial basal cells. (f) Difference in p63 expression between the peripheral area and central area was less clear.
Table 2.
Expression of podoplanin, CD44, and p63 by cancer cells
| Molecular marker | Negative (%) | Positive (%) |
|---|---|---|
| Podplanin | 55 (34.0) | 107 (66.0) |
| CD44 | 17 (10.5) | 145 (89.5) |
| p63 | 11 (6.8) | 151 (93.2) |
As shown in Figure 1(b,d,f), the distribution of podoplanin‐positive cells appeared to be localized more peripherally within the tumor nests than the distribution of CD44‐ and p63‐positive cells. When CD44 and p63 expressions were compared, p63‐positive cell layers were broader compared to CD44‐positive cell layers in the tumor nest periphery. We named this expression the “hierarchical distribution pattern” (Fig. 2).
Figure 2.
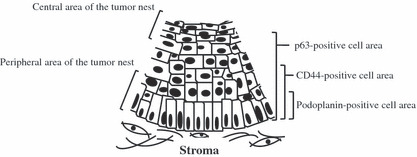
Schema of the hierarchical distribution pattern of podoplanin, CD44, and p63 within tumor nests. The distribution of podoplanin‐positive cells appeared to be more localized to the peripheral area of the tumor nests than the distribution of CD44‐ and p63‐positive cells. The distribution of p63‐positive cells was broader than that of the CD44‐positive cells.
The hierarchical distribution pattern was observed in 95 (88.8%) of the 107 podoplanin‐positive cases (Table 3). Of the 12 remaining cases, there were diffuse extensive expression cases of all three markers in five. Further, there were CD44‐negative/p63 peripheral staining patterns in two, negative staining cases for both CD44 and p63 in two, negative staining for CD44/diffuse staining pattern for p63 in one, p63‐positive cells more restricted to the periphery of the tumor nests than in the CD44‐positive cases in one, and CD44‐positive cells expressed in only the central area in one.
Table 3.
Characteristic distribution of podoplanin‐, CD44‐, and p63‐positive cells in podoplanin‐positive squamous cell carcinoma
| Podoplanin positive cases (n = 107) | ||
|---|---|---|
| Hierarchical distribution pattern | Diffuse expression pattern | Others |
| 95 (88.8%) | 5 (4.7%) | 7 (6.5%) |
Correlation between clinico‐pathological features and the hierarchical distribution‐positive cases.
Correlations between the hierarchical distribution‐positive cases and the clinico‐pathological features of the patients are shown in Table 4. The hierarchical distribution‐positive cases was significantly associated with the absence of lymphatic invasion (P = 0.035). No other clinico‐pathological factors were correlated with them.
Table 4.
Correlation between clinico‐pathological features and the hierarchical distribution–positive cases
| Variables | Hierarchical distribution cases (n = 95) | Podoplanin‐negative cases (n = 55) | P‐values |
|---|---|---|---|
| Gender | |||
| Male | 85 | 50 | 0.778 |
| Female | 10 | 5 | |
| Age | |||
| <70 | 48 | 33 | 0.262 |
| ≧70 | 47 | 22 | |
| Tumor size | |||
| ≦3 cm | 42 | 21 | 0.471 |
| >3 cm | 53 | 34 | |
| N Stage | |||
| pN0 | 74 | 36 | 0.097 |
| pN1 or pN2 | 21 | 19 | |
| Differentiation | |||
| Well or moderate | 64 | 34 | 0.491 |
| Poor | 31 | 21 | |
| Location | |||
| Central | 16 | 15 | 0.128 |
| Peripheral | 79 | 40 | |
| Vascular invasion | |||
| Absent | 67 | 38 | 0.853 |
| Present | 28 | 17 | |
| Lymphatic invasion | |||
| Absent | 68 | 30 | 0.035 |
| Present | 27 | 25 | |
| Pleural invasion | |||
| Absent | 69 | 38 | 0.644 |
| Present | 26 | 17 | |
The hierarchical distribution cases showed better overall survival.
The 5‐year overall survival rate of patients with the podoplanin‐positive cases and the podoplanin‐negative cases was 74.4% and 54.8%, respectively. Patients with the podoplanin‐positive cases had a longer overall survival time than those with the podoplanin‐negative cases (P = 0.018; Fig. 3a), whereas staining with CD44 and p63 had no prognostic significance (P = 0.941 and 0.640, respectively; data not shown). Moreover, we examined the prognostic value of the hierarchical distribution–positive cases and podoplanin‐negative cases. The 5‐year overall survival rate of the hierarchical distribution–positive cases was 71.7% and the hierarchical distribution‐positive cases had a more favorable outcome than podoplanin‐negative cases (P = 0.043; Fig. 3b).
Figure 3.
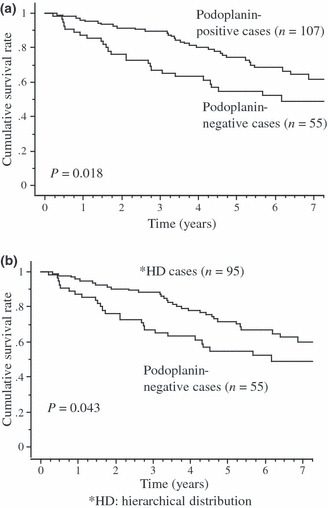
Kaplan–Meier curves for overall survival. Overall survival curves of patients according to whether their tumor was podoplanin‐positive or podoplanin‐negative. The 5‐year overall survival rates of the former and the latter were 74.4% and 54.8%, respectively. Overall survival curves of patients stratified according to whether their tumor was podoplanin‐positive with the hierarchical distribution pattern or podoplanin‐negative. The 5‐year overall survival rates of the former and the latter were 71.7% and 54.8%, respectively.
Discussion
In this study, we analyzed immunohistochemically the expression of podoplanin, CD44, and p63 in 162 pulmonary SqCCs. Focusing on the positive expression patterns, in 102 (95.3%) of the 107 podoplanin‐positive tumors, tumor cells showing strong expression were localized in the periphery of the tumor nests. A similar pattern of podoplanin expression has been demonstrated immunohistochemically in head and neck, skin, and uterine cervix.( 20 , 21 , 22 , 23 ) Meanwhile, this peripheral localization pattern was observed in only 55.9% (81/145) of the CD44‐positive tumors and 43% (65/151) of p63‐positive tumors. As shown in Figure 1(b,d), both the podoplanin‐positive cells and CD44‐positive cells resided at the periphery of tumor nests; however, the podoplanin‐positive cells were more specifically restricted to the peripheral layers than the CD44‐positive cells. Furthermore, when CD44 and p63 expressions were compared, p63‐positive cell layers in the periphery of the tumor nests were broader compared to CD44‐positive cell layers in the majority of cases (141/145; 97.2%; data not shown). The hierarchical distribution pattern was observed in almost 90% of the podoplanin‐positive cases (Table 3). In non‐cancerous squamous epithelium also, this hierarchical distribution pattern could be found (data not shown). Furthermore, we have previously reported that in human squamous SqCC cell line A431, almost all cultured A431 cells were positive for CD44 whereas the frequency of podoplanin‐positive cells was approximately 35% in flow cytometric analysis.( 6 ) This finding implies that podoplanin‐positive A431 cells are a limited subpopulation of CD44‐positive cells under in vitro condition, and is compatible with our immunohistochemical results. Considering a morphological representative for the developmental hierarchy based on the CSC hypothesis, podoplanin expression would reflect the most immature CSC status in its differentiation process, and podoplanin may be a marker of cells with a capacity for further maturation of SqCC. On the other hand, CD44 expression reflects even more differentiated cells of SqCC, and p63 expression may broadly reflect the CSC differentiation process ranging from immature CSC status to mature cells.
In the current study, podoplanin immunoreactivity had a prognostic significance, and this result is compatible with our previous study limited to pathological stage IB SqCC.( 20 ) Furthermore it is notable that patients who had podoplanin‐positive tumors with the hierarchical distribution pattern had significantly better overall survival than those who had podoplanin‐negative tumors. Additionally these tumors showed a significant correlation of the absence of lymphatic invasion and had a certain tendency to no lymph node metastasis. These results suggest that SqCC with the hierarchical distribution pattern may indicate lower biological aggressiveness. It seems possible that SqCC showing the hierarchical distribution pattern is a well‐organized tumor group based on the CSC concept, whereas SqCC with an unclear hierarchy is a disordered tumor group in terms of the developmental hierarchy.
In this study, 55 cases (34%) of SqCC were podoplanin‐negative. This type of SqCC may fall into the following categories. First, CSCs of podoplanin‐negative SqCC are enriched in the subpopulation expressing molecular markers other than podoplanin. Second, such cases may be a kind of tumor with no hierarchical structure based on the CSC concept. Some cancers contain small subpopulations of cancer‐initiating cells, whereas others contain common tumorigenic cells with little evidence of hierarchical organization.( 24 ) Podoplanin‐negative SqCC might be the latter type of carcinoma. It will be important to analyze the biological features of podoplanin‐negative pulmonary SqCCs in order to understand SqCC biology.
We examined the human SqCC cell lines, TE3, TE4, and TE10 other than A431, and the frequency of podoplanin expressing cells was 100%, 100%, and 0.5%, respectively (Atsumi et al, unpublished data). Given the expression frequencies in these SqCC cell lines, whether the podoplanin‐positive cells can always represent a CSC subpopulation in a variety of SqCCs will remain a matter of debate.
CD44 is transmembrane hyaluronan receptor, and its cytoplasmic region, comprising 72 amino acid residues, has been shown to associate with actin filaments in various cells, a process mediated by ERM (ezrin/radixin/moesin) proteins.( 25 ) Villar et al. demonstrated that the cytoplasmic domain of podoplanin also binds ERM proteins to promote epithelial–mesenchymal transition.( 25 ) Considering the similar localization of podoplanin‐ and CD44‐positive cells within the tumor nests and their common signaling via ERM proteins, it might be possible to think that signaling via Podoplanin and CD44 collectively mediate to express the biological properties of CSC .
Targeting CSCs has been proposed as an effective approach to cancer treatment, because CSCs are thought to be insensitive to conventional treatments and to be responsible for relapses. From this standpoint, the realization of CSCs due to a specific molecular marker, podoplanin, may lead to a new treatment strategy for SqCC.
Disclosure Statement
No potential conflicts of interest are disclosed.
Acknowledgments
The technical support of Hiroko Hashimoto, Manabu Yamazaki, and Shinya Yanagi is gratefully acknowledged. This study was supported in part by a Grant‐in‐Aid for Cancer Research (19‐10) from the Ministry of Health, Labour, and Welfare of Japan, and a Grant‐in‐Aid for the Third Term Comprehensive 10‐year Strategy for Cancer Control from the Ministry of Health, Labour, and Welfare of Japan.
References
- 1. Fukuoka M, Yano S, Giaccone G et al. Multi‐institutional randomized phase II trial of gefitinib for previously treated patients with advanced non‐small‐cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol 2003; 21: 2237–46. [DOI] [PubMed] [Google Scholar]
- 2. Chang A, Parikh P, Thongprasert S et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non‐small cell lung cancer: subset analysis from the ISEL study. J Thorac Oncol 2006; 1: 847–55. [PubMed] [Google Scholar]
- 3. Shepherd FA, Rodrigues Pereira J, Ciuleanu T et al. Erlotinib in previously treated non‐small‐cell lung cancer. N Engl J Med 2005; 353: 123–32. [DOI] [PubMed] [Google Scholar]
- 4. Peacock CD, Watkins DN. Cancer stem cells and the ontogeny of lung cancer. J Clin Oncol 2008; 26: 2883–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weissman IL, Anderson DJ, Gage F. Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Ann Rev Cell Dev Biol 2001; 17: 387–403. [DOI] [PubMed] [Google Scholar]
- 6. Atsumi N, Ishii G, Kojima M, Sanada M, Fujii S, Ochiai A. Podoplanin, a novel marker of tumor‐initiating cells in human squamous cell carcinoma A431. Biochem Biophys Res Commun 2008; 373: 36–41. [DOI] [PubMed] [Google Scholar]
- 7. Pellegrini G, Dellambra E, Golisano O et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA 2001; 98: 3156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prince ME, Sivanandan R, Kaczorowski A et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 2007; 104: 973–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kahn HJ, Marks A. A new monoclonal antibody, D2‐40, for detection of lymphatic invasion in primary tumors. Lab Invest 2002; 82: 1255–7. [DOI] [PubMed] [Google Scholar]
- 10. Kaneko MK, Kato Y, Kitano T, Osawa M. Conservation of a platelet activating domain of Aggrus/podoplanin as a platelet aggregation‐inducing factor. Gene 2006; 378: 52–7. [DOI] [PubMed] [Google Scholar]
- 11. Martin‐Villar E, Scholl FG, Gamallo C et al. Characterization of human PA2.26 antigen (T1alpha‐2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer 2005; 113: 899–910. [DOI] [PubMed] [Google Scholar]
- 12. Weber GF, Bronson RT, Ilagan J, Cantor H, Schmits R, Mak TW. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res 2002; 62: 2281–6. [PubMed] [Google Scholar]
- 13. Fasano M, Sabatini MT, Wieczorek R, Sidhu G, Goswami S, Jagirdar J. CD44 and its v6 spliced variant in lung tumors: a role in histogenesis? Cancer 1997; 80: 34–41. [PubMed] [Google Scholar]
- 14. Mylona E, Giannopoulou I, Fasomytakis E et al. The clinicopathologic and prognostic significance of CD44+/CD24(‐/low) and CD44‐/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol 2008; 39: 1096–102. [DOI] [PubMed] [Google Scholar]
- 15. Boldrup L, Coates PJ, Gu X, Nylander K. DeltaNp63 isoforms regulate CD44 and keratins 4, 6, 14 and 19 in squamous cell carcinoma of head and neck. J Pathol 2007; 213: 384–91. [DOI] [PubMed] [Google Scholar]
- 16. Irwin MS, Kaelin WG. p53 family update: p73 and p63 develop their own identities. Cell Growth Differ 2001; 12: 337–49. [PubMed] [Google Scholar]
- 17. Wang BY, Gil J, Kaufman D, Gan L, Kohtz DS, Burstein DE. P63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol 2002; 33: 921–6. [DOI] [PubMed] [Google Scholar]
- 18. Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev 2000; 1: 199–207. [DOI] [PubMed] [Google Scholar]
- 19. Hibi K, Trink B, Patturajan M et al. AIS is an oncogene amplified in squamous cell carcinoma. Proc Natl Acad Sci USA 2000; 97: 5462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ito T, Ishii G, Nagai K et al. Low podoplanin expression of tumor cells predicts poor prognosis in pathological stage IB squamous cell carcinoma of the lung, tissue microarray analysis of 136 patients using 24 antibodies. Lung Cancer. 2008; 3: 418–24. [Google Scholar]
- 21. Yuan P, Temam S, El‐Naggar A et al. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer 2006; 107: 563–9. [DOI] [PubMed] [Google Scholar]
- 22. Durchdewald M, Guinea‐Viniegra J, Haag D et al. Podoplanin is a novel fos target gene in skin carcinogenesis. Cancer Res 2008; 68: 6877–83. [DOI] [PubMed] [Google Scholar]
- 23. Dumoff KL, Chu CS, Harris EE et al. Low podoplanin expression in pretreatment biopsy material predicts poor prognosis in advanced‐stage squamous cell carcinoma of the uterine cervix treated by primary radiation. Mod Pathol 2006; 19: 708–16. [DOI] [PubMed] [Google Scholar]
- 24. Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature 2008; 456: 593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martin‐Villar E, Megias D, Castel S, Yurrita MM, Vilaro S, Quintanilla M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial‐mesenchymal transition. J Cell Sci 2006; 119: 4541–53. [DOI] [PubMed] [Google Scholar]


