Abstract
Pigment epithelium‐derived factor (PEDF), an angiogenesis inhibitor with multiple other functions, balances angiogenesis in the eye and blocks tumor progression. Retinoblastoma, an angiogenesis‐dependent tumor, is the most common ocular cancer in children without effective treatment. It has been reported that PEDF can induce neuronal differentiation of retinoblastoma cells; however, its anti‐angiogenic potential for inhibition of retinoblastoma growth in vivo has not been elucidated. The present study was designed to investigate the effect of PEDF on growth of retinoblastoma and the possible molecular mechanism. Soluble and non‐fusion recombinant PEDF were generated in E. coli. Recombinant PEDF dose‐dependently inhibited proliferation and induced apoptosis of endothelial cells. PEDF had no effects on the proliferation and apoptosis of retinoblastoma cell line SO‐Rb50. Intraperitoneal injection of PEDF resulted in growth inhibition of heterotopic retinoblastoma xenografts at 68.78%. MVD in tumor tissues treated with PEDF was significantly decreased. These results suggested that PEDF suppressed tumor growth by blocking angiogenesis instead of a direct cytotoxic effect on tumor cells. Vascular endothelial growth factor (VEGF), a major angiogenic stimulator, was down‐regulated by PEDF in both SO‐Rb50 cells and retinoblastoma xenografts. Hypoxia‐inducible factor (HIF)‐1α, a crucial transcriptional factor for VEGF expression, was also down‐regulated by PEDF both in vitro and in vivo. PEDF reduced HIF‐1α nuclear translocation, which may be responsible for the down‐regulation of VEGF. Down‐regulation of VEGF expression in tumor cells through inhibiting HIF‐1α, thus attenuating the paracrine effect of VEGF on endothelial cell proliferation and vascular permeability in tumor tissues, may represent a mechanism for the anti‐angiogenic activity of PEDF. (Cancer Sci 2009; 100: 2419–2425)
Retinoblastoma is the most common malignant intraocular tumor in children. In developed countries, the survival rate of children with retinoblastoma is over 95%,( 1 ) while in developing countries, due to delayed detection, only 50% of the children survive this tumor.( 2 , 3 ) Although several treatments are available for retinoblastoma, including chemotherapy, external beam radiotherapy (EBR), and plaque radiotherapy, each of them has major drawbacks in pediatric patients, such as bone marrow suppression, second cancer occurrence, cataracts, retinopathy, and recurrence of the primary tumor.( 1 , 4 ) Finding a safer and more efficient treatment remains a major challenge. In this study, we demonstrated that PEDF suppressed retinoblastoma growth by anti‐angiogenesis.
Pigment epithelium‐derived factor (PEDF), a member of the serpin super family, is known for its multiple biological activities including neuroprotective,( 5 ) neurotrophic,( 6 ) neuronal differentiation‐induction,( 6 ) neural stem cell self‐renewal,( 7 ) anti‐inflammatory,( 8 ) anti‐angiogenic,( 9 ) and antitumor activities.( 10 ) PEDF is a potent endogenous angiogenic inhibitor. It has been shown that PEDF can inhibit the proliferation and migration of, and induce the apoptosis of, endothelial cells. Moreover, the underlying mechanism of the effect PEDF on endothelial cells has been reported by several papers. It is known that the Fas/Fas‐L caspase‐8 apoptotic pathway is involved in the apoptosis of PEDF‐induced endothelial cells. Immature endothelial cells express a relative high level of the Fas receptor on the cell surface. PEDF induces an increase in surface expression of Fas‐L of immature endothelial cells, which can initiate a caspase‐dependent apoptotic cascade, and result in cell death.( 11 ) The MAPK pathway is also involved in the anti‐angiogenesis activity of PEDF. The MAPK pathway is an essential signal pathway in regulating cell proliferation, survival, and apoptosis, and plays an important role in vascular endothelial growth factor (VEGF) regulation, especially in endothelial cells. Our previous study showed that PEDF down‐regulated the expression of VEGF in endothelial cells and in the retina of rats with oxygen‐induced retinopathy (OIR), which partially contributed to the decrease of p42/p44 phosphorylation.( 12 ) Recently, Ho et al. showed that PEDF induced apoptosis of endothelial cells through the cytosolic calcium‐dependent phospholipase A2‐alpha (cPLA2‐α) pathway. PEDF induced the activation of cPLA2‐α by enhancing the phosphorylation level of cPLA2‐α and promoting the nuclear translocation of cPLA2‐α. The activated cPLA2‐α acted as a bridge to activate p38 MAPK, peroxisome proliferator‐activated receptor gamma (PPAR‐γ), p53, and caspase‐3.( 13 , 14 )
However, the potential of PEDF for the treatment of retinoblastoma has not been well explored. The present study was designed to investigate the effect of recombinant PEDF on the neovascularization and growth of retinoblastoma.
PEDF suppresses tumor growth by several mechanisms. It has been reported that PEDF can induce the neuronal differentiation of the retinoblastoma cell line Y79.( 15 ) Our previous study showed that PEDF inhibited retinal angiogenesis and decreased vascular permeability by reducing VEGF production in endothelial cells.( 12 ) Recent studies showed that PEDF suppressed tumor growth by inhibiting VEGF expression in melanoma and neuroblastoma tumor cells.( 16 , 17 ) However, the effect of PEDF on VEGF expression of retinoblastoma cells has not been investigated. Therefore, the possible molecular mechanism of PEDF for the anti‐angiogenesis effect in retinoblastoma was further explored in this study.
Material and Methods
Cell culture. The human retinoblastoma cell line (SO‐Rb50) was provided by the Department of Pathology of Zhongshan Ophthalmic Center, Guangzhou, and maintained in RPMI‐1640 medium (Gibco, Gaithersburg, MD, USA) with 10% (v/v) fetal bovine serum (FBS; Gibco), and incubated at 37°C in a humidified incubator at 5% CO2. Human umbilical vein endothelial cells (HUVEC) were freshly isolated from human umbilical cord obtained from the Department of Obstetrics and Gynecology (Second Affiliated Hospital, Sun Yat‐sen University, Guangzhou, China), as previously described,( 18 ) and cultured in M199 medium (Gibco) supplemented with 15% FBS and endothelial cell growth supplement (ECGS; BD Biosciences, Bedford, MA, USA), and also incubated at 37°C in a humidified incubator with 5%CO2. For HUVEC, all experiments were carried out between cell passages 2 and 6.
Recombination protein expression and purification. The human PEDF gene was acquired from the recombinant pET‐22b (+)/PEDF plasmid constructed previously( 19 ) using PCR reaction with Taq DNA polymerase (Takara Biotechnology, Dalian, China). The PCR product was cloned into the pET‐30a (+) vector (Novagen, Madison, WI, USA) at the EcoRI and HindIII sites in frame with the sequence encoding the 6 × His‐tag at its 3′ end. T4 DNA ligase, dNTP mixure, endonuclease EcoRI and HindIII, and DNA and protein markers were from the Takara Biotechnology. The pET30a (+)/PEDF construct was introduced into the E. coli strain BL‐21/DE3 (Novagen). The soluble recombinant PEDF was expressed in E. coli, and purified with the His. Bind affinity column (Novagen) according to the manufacturer’s recommendations. Recombinant protein was confirmed by Western blotting analysis using rabbit antihuman PEDF polyclonal antibody that we made previously.
Cell proliferation assay. Both HUVECs and SO‐Rb 50 cells were seeded in 24‐well plates with 2 × 104 cells per well and in triplicate. Cells were maintained in the growth medium until they reached 60–70% confluence. The culture medium was replaced with M199 medium with 2% FBS added for HUVECs and supplemented with various concentrations of PEDF (0, 10, 20, 40, 80, 160, and 320 nmol/L). For SO‐Rb 50cells, the growth medium was replaced with serum‐free medium supplemented with the same concentrations of PEDF as HUVECs. Both the cell cultures were incubated at 37°C in a humidified incubator with 5%CO2 for 72 h. Cell viability were measured by MTT (3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐dephenyl tetrazolium bromide) assay (Sigma, St. Louis, MO, USA), according to the manufacturer’s protocol. Data represents absorbance and is expressed as percentages of respective controls. The inhibitory effects of PEDF are expressed as IC50 values, which were determined from three independent tests.
AnnexinV‐FITC apoptosis assay HUVECs were seeded at 2.5 × 104, and SO‐Rb50 at 5 × 104 per well in six‐well plates. Twenty‐four hours later, both cell cultures were treated with PEDF at the concentration of 200 nmol/L for another 24 h. Cells were harvested for flow cytometry using the AnnexinV‐FITC Apoptosis Detection Kit (Bender Med Systems, Vienna, Austria) according to the manufacturer’s recommendation. Cells treated with 25 μmol/L colchicines were used as positive controls, and cells treated with PBS as negative controls.
Heterotopic tumor growth assay. SO‐Rb50 cells (2 × 107/0.2 mL) in R1640 medium were inoculated subcutaneously into the left inguinal region of 4‐ to 6‐week‐old male athymic mice (BALB/c‐nu) (Laboratory Animal Center of Guangdong Province, Guangzhou, China). When tumors reached 50mm3, the mice were randomized into two groups with five mice in each group: one group receiving peritoneal injection with PEDF, the other group receiving PBS. Mice received eight times injections every 4 days. The total dose of PEDF was 12 mg/kg. Tumor growth was monitored by external measurement in two‐dimensions every other day. Tumor volume was determined according to the equation: volume = (length × width2) × 0.5. Five weeks after the first injection of PEDF, the mice were executed and tumors were dissected, weighed, and stored at –80°C for Western blotting and immunohistochemistry analysis. The tumor inhibition ratio was calculated as follows: inhibition ratio (%) = [(C − T)/C] × 100%, where C is the average tumor weight of the control group and T is the average tumor weight of the PEDF treated group. All animal studies were performed under institutionally approved protocol at the State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat‐sen University, China, according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Microvessel density (MVD) assay. Frozen tissues were cut into 10‐μm sections, fixed in acetone at 4°C for 5 min, and blocked for endogenous peroxidase. Sections were treated with normal serum for 10 min. Tumor sections were incubated with the rat monoclonal antibody against mouse CD31 (BD Pharmingen, San Diego, CA, USA) at 1:100 dilutions at 4ºC. After rinsing with PBS, sections were incubated with biotinylated rabbit antirat immunoglobulins (Dako, Glostrup, Denmark) at 1:1000 dilutions for 30 min at room temperature followed by incubation with horseradish peroxidase–labeled streptavidin–biotin complex for 30 min. Peroxidase reaction was visualized by the use of diaminobenzidine. Tumor MVD was quantified as tumor vasculature by Weidner’s method.( 20 ) In negative‐control staining, the primary antibodies were omitted.
Western blot analysis. SO‐Rb50 cells, 1 × 107, were seeded in 100‐mm plates and incubated under normoxic (20%O2, v/v) or hypoxic (1%O2, v/v) conditions with or without PEDF at the concentration of 100 nmol/L at 37°C for 24 h. The cells were lysed and the total proteins were extracted. Tumor tissues were similarly homogenized with homogenizer, and total tissue proteins were extracted. Protein concentration was determined using the Bio‐Rad protein assay kit (Bio‐Rad, Hercules, CA, USA). VEGF and hypoxia‐inducible factor (HIF)‐1α expression were determined using an anti‐VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1:500 or an anti‐HIF‐1α antibody (Santa Cruz Biotechnology) at a dilution of 1:500, respectively. The same membrane was stripped and re‐blotted with an anti‐β‐actin antibody (1:10 000; Sigma) for normalization.
Immunocytochemistry assay. SO‐Rb50 cells were seeded on the cover‐slips coated with poly‐d‐lysine (Sigma) for 24 h. The cells were then incubated under normoxic (20%O2, v/v) or hypoxic (1%O2, v/v) conditions with or without 100 nmol/L PEDF at 37°C for 10 h. The changes of VEGF and HIF‐1α expression were detected by immunocytochemistry assay as described previously (anti‐VEGF 1:100, and anti‐HIF‐1α antibody 1:50, Santa Cruz Biotechnology).( 18 ) The result was analyzed by IPP (Image Plus Pro 6.0, Bethesda, MD, USA).
Statistical analysis. All data are expressed as mean ± SD. SPSS 13.0 software was used for the Student’s t‐test in all statistical analyses (SPSS, Chicago, IL, USA). A P‐value of <0.05 was considered statistically significant.
Results
Expression and purification of soluble recombinant human PEDF. PEDF was expressed in E. coli and purified to apparent homogeneity by affinity chromatography using the His binding affinity column. Based on Coomassie‐stain SDS–PAGE, the purified recombinant PEDF protein showed an apparent molecular weight of 50 kDa (Fig. 1a), matching the calculated molecular weight from the sequence. The identity of the protein was confirmed by Western blotting analysis using an anti‐PEDF polyclonal antibody (Fig. 1b). An average of 15 mg of purified PEDF in soluble form was obtained from 1‐L culture and over 90% purity was achieved for recombinant PEDF. To our knowledge, this is the first time the soluble form of PEDF has been obtained in an E. coli expression system outside of glutathione S‐transferase (GST)–PEDF fusion protein.
Figure 1.
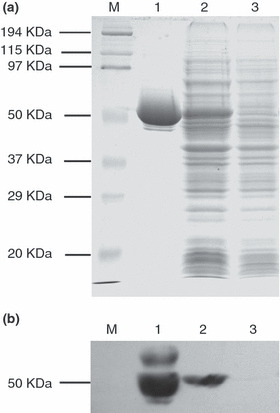
SDS–PAGE and Western blot analysis of purified recombinant pigment epithelium‐derived factor (PEDF). Recombinant PEDF protein was expressed in E. coli and purified by affinity chromatography using the His binding affinity column (a) SDS–PAGE with Coomassie blue staining; (b) Western blotting analysis with antibody specific to PEDF. Lane 1, purified PEDF recombinant protein; Lane 2, the cytoplasmic soluble part of the bacterial proteins of pET‐30a(+)/PEDF after IPTG induction; Lane 3, the cytoplasmic soluble part of the bacterial proteins of pET‐30a(+)/PEDF before IPTG induction; M, Protein marker. IPTG, Isopropyl‐β‐D‐thiogalactopyranoside.
Specific inhibition of endothelial cells by recombinant PEDF. PEDF treatment resulted in fewer viable HUVECs in a dose‐dependent manner. Data represent absorbance as percentages of respective controls (means ± SD, n = 3), and values statistically different from the control are indicated (P < 0.05, P < 0.01). The IC50 of PEDF on HUVECs proliferation was approximately 80 nmol/L. However, PEDF had no anti‐proliferation effect on SO‐Rb50 cells at the same concentrations (Fig. 2). Similarly, PEDF had a specific effect on the apoptosis of endothelial cells (P < 0.01). As shown in Figure 3, the percentages of apoptotic HUVEC cells in negative control, positive control, and PEDF (200 nmol/L) treatment were 6.47 ± 0.48%, 26.05 ± 2.32%, and 17.18 ± 2.74%, respectively. For SO‐Rb50 cells, the percentages of apoptotic cells of negative control, positive control, and PEDF (200 nmol/L) treatment were 2.5 ± 0.04%, 14.1 ± 0.4%, and 3.3 ± 0 .3%, respectively (Fig. 4). These results suggested that PEDF specifically inhibits proliferation and induces apoptosis of endothelial cells while it has no these effects on retinoblastoma cells.
Figure 2.
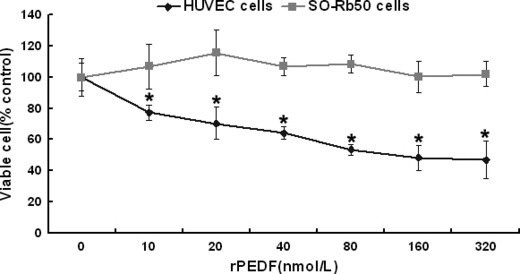
Effect of pigment epithelium‐derived factor (PEDF) on cell proliferation. Primary human umbilical vein endothelial cells (HUVECs) and SO‐Rb50 cells were treated with PEDF at concentrations indicated for 72 h. The viable cells were quantified by MTT. Data represents absorbance as percentages of respective controls (mean ± SD, n = 3), and values statistically different from the control are indicated (*P < 0.05). PEDF inhibited proliferation of HUVECs in a dose‐dependent manner, but had no effect on proliferation of SO‐Rb50 cells.
Figure 3.
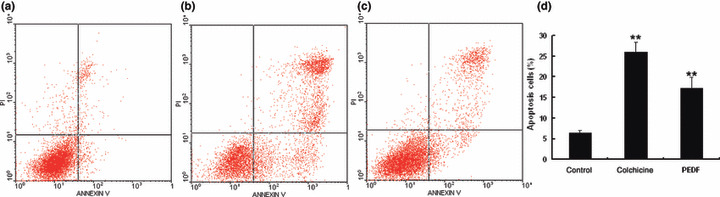
Quantitative analysis of endothelial cell apoptosis induced by pigment epithelium‐derived factor (PEDF). Human umbilical vein endothelial cells (HUVECs) were treated with PEDF for 24 h. Apoptotic cells were quantified by flow cytometry. (a) Negative control treated with PBS; (b) positive control treated with colchicine; (c) HUVECs treated with PEDF at 200 nmol/L; (d) statistical analysis of apoptosis results. Values significantly higher than controls (**P < 0.01) are indicated.
Figure 4.
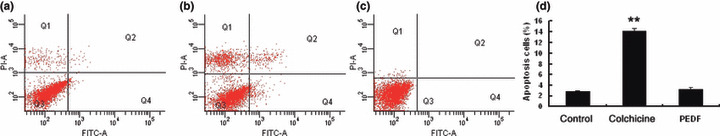
Quantitative analysis of retinoblastoma cell apoptosis induced by pigment epithelium‐derived factor (PEDF). SO‐Rb50 cells were treated by PEDF for 24 h. Apoptotic cells were quantified by flow cytometric analysis. (a) Negative control treated with PBS; (b) positive control treated with colchicine; (c) cells treated with PEDF at 200 nmol/L; (d) statistical analysis. Values significantly higher than controls (**P < 0.01) are indicated.
PEDF suppresses growth of human retinoblastoma. To evaluate the effect of PEDF on tumor growth, heterotopic tumor xenografts of retinoblastoma were established and treated with PEDF or PBS. At the 35th day after the first injection, there was no significant difference in body weight between PEDF‐treated mice and PBS‐treated mice. The average body weights of the PBS‐treated and PEDF‐treated groups were 25.1 ± 1.9 g and 25.8 ± 1.2 g, respectively. However, compared to the PBS‐treated group, the tumor weight in the PEDF‐treated group was significantly decreased, and an average of 68.78% suppression of primary tumor growth was observed in the PEDF‐treated group (P < 0.01, n = 5, Fig. 5a,c). After the 15th day from the first injection, the average tumor volume of the PEDF group was significantly lower than that of the PBS‐treated group (P < 0.05, Fig. 5b).
Figure 5.
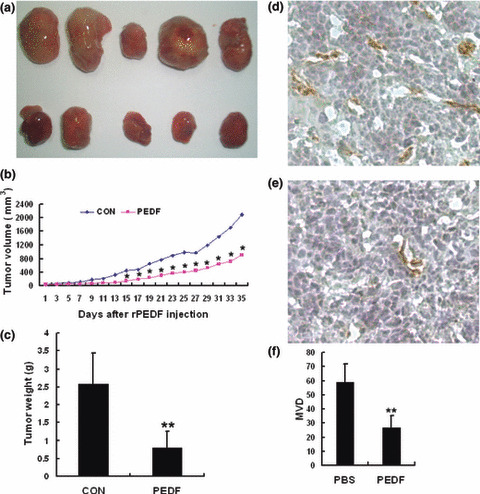
Pigment epithelium‐derived factor (PEDF) inhibits tumor growth and angiogenesis in retinoblastoma tissue. SO‐Rb 50 cell heterotopic transplanted tumors were developed as described in Materials and Methods. Mice were treated with PEDF (12 mg/kg) for 7–14 days after transplantation. Tumor growth was monitored, and tumor tissues were collected and weighted on the 35th day after the first injection of PEDF. (a) Tumor tissues treated with PBS (top) and PEDF (bottom) on 35th day after treatment. (b) Tumor growth curves: volumes of PBS‐ vs PEDF‐treated group on days indicated *P < 0.05. (c) An average of 68.78% suppression of primary tumor growth was observed. Data are presented as mean ± SD (n = 5). Microvessel density (MVD) in tumor tissues was determined by immunohistochemical staining by an endothelial‐specific antibody CD31. (d) PBS group (×200) and (e) PEDF group (×200). (f) Quantitative analysis of MVD. Data are presented as mean ± SD (*P < 0.05, **P < 0.01).
PEDF inhibits tumor angiogenesis. The effect of PEDF on tumor angiogenesis was evaluated by CD31 immunostaining for capillaries in tumor tissues. The amount of CD31‐stained tumor capillaries in the PEDF‐treated group was less than that in the PBS‐treated group (Fig. 5d,e). The MVD of the PEDF‐treated group was significantly reduced compared to the PBS‐treated group (P < 0.01, Fig. 5f). These data demonstrate that PEDF inhibits the neovascularization of retinoblastoma.
PEDF down‐regulates VEGF expression of retinoblastoma both in vitro and in vivo. Immunocytochemical analysis indicated that VEGF expression in SO‐Rb50 cells was up‐regulated under hypoxia compared to normoxia (Fig. 6a). PEDF markedly suppressed the expression of VEGF under hypoxia at the dose of 100 nmol/L (Fig. 6a). Western blotting analysis also showed that expression of VEGF both in SO‐Rb50 cells (Fig. 6b) and retinoblastoma tissues (Fig. 6c) were significantly down‐regulated by PEDF. PEDF injection reduced the level of VEGF protein to approximately 30.4% of the control in heterotopic transplanted tumor (Fig. 6b). These results suggested that the anti‐angiogenesis activities of PEDF could occur through the down‐regulation of VEGF expression.
Figure 6.
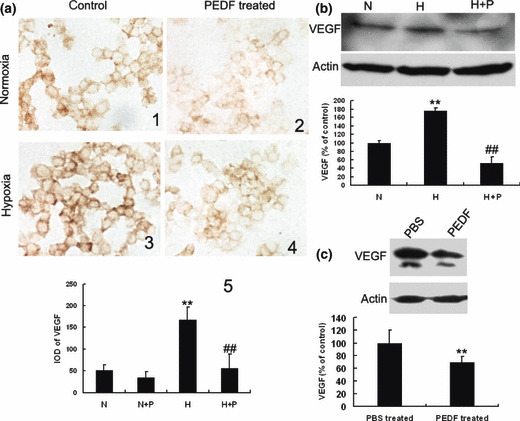
Pigment epithelium‐derived factor (PEDF) down‐regulates vascular endothelial growth factor (VEGF) of retinoblastoma both in vitro and in vivo. SO‐Rb50 cells were incubated with or without PEDF (100 nmol/L) for 10 h under normoxia and hypoxia. Immunocytochemistry and western blotting assays were explored to analyze the effect of PEDF on VEGF. (a) Immunocytochemistry analysis of VEGF expression in SO‐Rb50 cells (×640). (b,c) Western blotting analysis for VEGF in tumor cells and tumor tissues, respectively. H, hypoxia; H + P, hypoxia + PEDF (100 nmol/L); N, normoxia; N + P, normoxia + PEDF (100 nmol/L). Values significantly lower than control (**P < 0.01, ##P < 0.01, *vs normoxia control, # vs hypoxia control, *P < 0.05, #P < 0.05) are indicated.
PEDF inhibits the expression and nuclear translocation of HIF‐1α both in vitro and in vivo. To elucidate whether PEDF down‐regulated expression of VEGF through HIF‐1α, we investigated the amount and location of HIF‐1α in SO‐Rb50 cells by immunocytochemistry. As shown in Figure 7(a), HIF‐1α protein was mainly located in cytoplasm under normoxia, and hypoxia induced HIF‐1α expression in cytoplasm and promoted the nuclear translocation of HIF‐1α. PEDF reduced HIF‐1α protein translocation into the nucleus under hypoxia (Fig. 7a). Western blotting analysis also showed that the level of HIF‐1α expression was decreased by PEDF in xenografted tumor tissues (Fig. 7c). These data suggested that PEDF regulates VEGF through inhibiting the expression and nuclear translocation of HIF‐1α.
Figure 7.
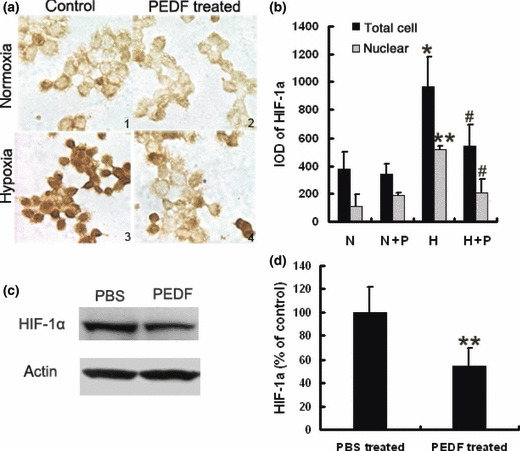
Pigment epithelium‐derived factor (PEDF) down‐regulates hypoxia‐induced factor (HIF)‐1α in retinoblastoma cells and xenografted tissues. Immunocytochemistry analysis of HIF‐1α expression in SO‐Rb 50 cells (a,b). SO‐Rb50 cells were incubated with or without PEDF (100 nmol/L) for 10 h under normoxia and hypoxia (×640). HIF‐1α protein level highlighted by immunocytochemistry. a1, a2, a3, a4 correspond to N, N + P, H, and H + P in graph B, respectively. Values significantly lower than controls (*P < 0.05, **P < 0.01, #P < 0.05, ##P < 0.01, *vs group N, # vs group H) are indicated. Western blotting analysis for HIF‐1α in xenografted tumor tissues (c,d). Values significantly lower than control (**P < 0.01) are indicated.
Discussion
Being an important multifunctional factor, recombinant PEDF was used widely in many investigations. Therefore, many different expression systems have been used to express recombinant PEDF, such as E. coli,( 19 , 21 ) HED‐293T,( 22 , 23 , 24 ) BHK cells,( 25 ) and yeast.( 26 ) In the eukaryotic expression system, the purification processes are complicated and time‐consuming with low yield for PEDF. Our previous study showed that in the prokaryotic system, recombinant human PEDF with a His‐tag was expressed mainly in the form of inclusion bodies by using a bacterial expression vector pET‐22b (+) in E. coli. The soluble recombinant protein was harvested by denaturation using 6 mol/L urea and renaturation by dialysis.( 19 ) Zhang et al. ( 21 ) obtained soluble GST fusion PEDF in the E. coli system. However, the GST–PEDF fusion protein, which is larger than native PEDF, had to be cleaved by thrombin and purified again by anion‐exchange chromatography. In this study, we developed a new simple method to obtain soluble PEDF in the pET‐30a (+) expression vector prokaryotic system. The recombinant protein was mainly in a soluble form according to SDS–PAGE and Western blot analysis (Fig. 1). An average of 15 mg of purified PEDF in soluble form was obtained from 1 L of culture. To our knowledge, it is the first time that a soluble form of PEDF in an E. coli expression system except GST–PEDF fusion protein has been obtained. The recombinant PEDF exhibited the same biological activities as native PEDF, including inhibition of proliferation and apoptosis induction of HUVEC in a dose‐ and time‐dependent manner (2, 3).
Angiogenesis, the growth of new blood vessels from preexisting capillaries, is necessary for solid tumor growth and metastasis.( 20 , 27 ) Anti‐angiogenesis therapy provides a novel approach for cancer management.( 20 ) Retinoblastoma, originating from retina, maintains itself from retinal vasculature initially, and as the tumor grows and outstrips the retina, neovascularization in retinoblastoma becomes the source of tumor survival and malignant progression. Studies have shown that inhibition of the angiogenesis of retinoblastoma could be a new strategy for retinoblastoma therapy.( 28 ) In the present study, we reported for the first time that intraperitoneal injection of PEDF inhibited retinoblastoma growth in xenografted mice. MVD in tumor tissues was been decreased by PEDF (Fig. 5). Moreover, we found that PEDF did not affect the proliferation and apoptosis of retinoblastoma cell line SO‐Rb50 at a concentration of 200 nmol/L which is a dose effective on HUVEC proliferation and apoptosis (2, 4). PEDF did not induce significant apoptosis of retinoblastoma cells even prolonging the incubation time to 72 h (data not shown). These results suggested that PEDF suppressed tumor growth by blocking angiogenesis instead of through a direct cytotoxic effect on tumor cells. Studies have shown that PEDF induced tumor apoptosis via the Fas–Fas‐L pathway in human melanoma cells and osteosarcoma cells at the concentration of 100 nmol/L.( 17 ) Y79, the commonly used human retinoblastoma cell line, has been identified as expressing correct Fas protein, but is resistant to the apoptosis induced by the Fas–Fas‐L pathway, due to caspase‐8 gene silencing via hypermethylation.( 29 ) SO‐Rb50 may have a similar mechanism to resist the effect of apoptosis induced by PEDF. These data demonstrated that PEDF suppressed retinoblastoma growth mainly by anti‐angiogenesis activity.
A common mechanism for neovascularization is the disturbed regulation of angiogenesis which is controlled by a delicate balance between angiogenic stimulators such as VEGF and angiogenic inhibitors such as PEDF.( 30 ) Stimulators are increased while angiogenic inhibitors are decreased under pathological conditions such as tumors. These changes break the balance in angiogenesis control and consequently, result in over proliferation of capillary endothelial cells and abnormal formation of new blood vessels.( 31 ) PEDF can restore the balance between angiogenesis stimulators and inhibitors in pathological neovascularization, by down‐regulating the expression of proangiogenic factors.( 9 )
VEGF is the most potent and specific known proangiogenic factor and is secreted by almost all solid tumor cells.( 32 ) Studies have shown that VEGF is highly expressed in retinoblastoma.( 33 ) Transfection of VEGF siRNA to SO‐Rb50 cells led to the inhibition of tumor growth via reduction in neovascularization.( 34 ) However, the effect of PEDF on the expression of VEGF in retinoblastoma has not been investigated. We demonstrated in this study that PEDF significantly down‐regulated VEGF expression both in SO‐Rb50 cells and in retinoblastoma tissues under hypoxia (Fig. 6).
HIF‐1α is a crucial transcriptional factor of VEGF expression. HIF‐1α is significantly expressed in retinoblastoma, but only weakly in the peritumoral areas of retinoblastoma, which has been believed to be closely related to tumor growth by pro‐angiogenesis.( 33 ) Previous studies have shown that inhibition of HIF1‐α attenuated VEGF expression and tumor growth.( 35 , 36 , 37 ) Our recently results also showed that Kallikrein‐binding protein (KBP), another angiogenic inhibitor, significantly reduced VEGF expression via inhibition of HIF‐1α in gastric and liver cancer.( 18 , 38 ) Therefore, the regulation of PEDF on HIF‐1α was examined in SO‐Rb50 cells and xenografted tissues in this study. We found that hypoxia induced significantly more HIF‐1α as compared to normoxia in SO‐Rb50 cells, and PEDF significantly reduced HIF‐1α expression and inhibited HIF‐1α nuclear translocation in retinoblastoma cells (Fig. 7a), and down‐regulated HIF‐1α expression in tumor tissues (Fig. 7c), consistent with the results for VEGF, These results suggested that PEDF down‐regulated VEGF through inhibition of HIF‐1α. The exact mechanism for the effect of PEDF on HIF‐1α is uncertain. PEDF might directly inhibit HIF‐1α expression or indirectly affect HIF‐1α degradation.
Taken together, we demonstrated for the first time that PEDF suppressed the growth of retinoblastoma by an anti‐angiogenesis mechanism. Down‐regulation of VEGF in tumor cells through inhibiting HIF‐1α, thus attenuating the paracrine effect of VEGF on endothelial cells in tumor tissues, may represent a novel mechanism for the anti‐retinoblastoma activity of PEDF.
Supporting information
Fig. S1. Effect of pigment epithelium‐derived factor (PEDF) on human umbilical vein endothelial cells (HUVECs) cell proliferation. Primary HUVECs were treated with PEDF at concentrations indicated for 24 h. The viable cells were quantified by MTT. Data represent absorbance as percentages of respective controls (mean ± SD, n = 3), and values statistically different from the control are indicated (*P < 0.05). PEDF inhibits proliferation of HUVECs in a dose‐dependent manner.
Fig. S2. Quantitative analysis of cell apoptosis induced by pigment epithelium‐derived factor (PEDF). Human umbilical vein endothelial cells (HUVECs) and SO‐Rb50 cells were treated with PEDF at 200 nnmol/L for 72 h, respectively. Apoptotic cells were dual‐stained with AnnexinV and PI, and quantified by flow cytometry. (a) Apoptosis analysis of HUVECs; (b) apoptosis analysis of SO‐Rb50 cells. Values significantly higher than control (**P < 0.01) are indicated.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Acknowledgments
This study was supported by the National Nature Science Foundation of China (grant numbers: 30370313, 30570372, 30600724, 30700120, 30872980); Program for Doctoral Station in University (grant numbers: 20070558209, 20070558215); Key Sci‐tech Research Project in University (grant number: 108104); Team Project of Nature Science Foundation of Guangdong Province, China (grant number: 06201946); Key Sci‐tech Research Project of Guangdong Province, China (grant number: 2008B080703027); Key Sci‐tech Research Project of Guangzhou Municipality, China (grant numbers: 2007Z3‐E5041, 2008Z1‐E231); and National Key Sci‐Tech Special Project of China (grant number: 2008ZX10002‐019).
References
- 1. Shields OL, Shields JA. Diagnosis and management of retinoblastoma. Cancer Control 2004; 11: 317–27. [DOI] [PubMed] [Google Scholar]
- 2. Schultz KR, Ranade S, Neglia JP et al. An increased relative frequency of retinoblastoma at a rural regional referral hospital in Miraj Maharashtra, India. Cancer 1993; 72: 282–6. [DOI] [PubMed] [Google Scholar]
- 3. Chantada G, Fandino A, Manzitti J et al. Late diagnosis of retinoblastoma in a developing country. Arch Dis Child 1999; 80: 171–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friedman DL, Himelstein B, Shields CL et al. Chemoreduction and local ophthalmic therapy for intraocular retinoblastoma. J Clin Oncol 2000; 18: 12–7. [DOI] [PubMed] [Google Scholar]
- 5. Cao W, Tombran‐Tink J, Elias R et al. In vivo protection of photoreceptors from light damage by pigmentepithelium‐derived factor. Invest Ophthalmol Vis Sci 2001; 42: 1646–52. [PubMed] [Google Scholar]
- 6. Tombran‐Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium‐derived factor with potent neuronal differentiative activity. Exp Eye Res 1991; 53: 411–4. [DOI] [PubMed] [Google Scholar]
- 7. Ramirez‐Castillejo C, Sanchez‐Sanchez F, Andreu‐Agullo C et al. Pigment epithelium‐derived factor is a niche signal for neural stem cell renewal. Nat Neurosci 2006; 9: 331–9. [DOI] [PubMed] [Google Scholar]
- 8. Zhang SX, Wang JJ, Gao G et al. Pigment epithelium‐derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 2006; 20: 323–5. [DOI] [PubMed] [Google Scholar]
- 9. Stellmach V, Crawford SE, Zhou W et al. Prevention of ischemia‐induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium‐derived factor. Proc Natl Acad Sci USA 2001; 98: 2593–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ek ET, Dass CR, Choong PF. PEDF: a potential molecular therapeutic target with multiple anti‐cancer activities. Trends Mol Med 2006; 12: 497–502. [DOI] [PubMed] [Google Scholar]
- 11. Volpert OV, Zaichuk T, Zhou W et al. Inducer‐stimulated Fas targets activated endothelium for destruction by anti‐angiogenic thrombospondin‐1 and pigment epithelium‐derived factor. Nat Med 2002; 8: 349–57. [DOI] [PubMed] [Google Scholar]
- 12. Zhang SX, Wang JJ, Gao G et al. Pigment epithelium‐derived factor downregulates vascular endothelial growth factor (VEGF) expression and inhibits VEGF‐VEGF receptor 2 binding in diabetic retinopathy. J Mol Endocrinol 2006; 37: 1–12. [DOI] [PubMed] [Google Scholar]
- 13. Ho TC, Chen SL, Yang YC et al. Cytosolic phospholipase A2‐α is an early apoptotic activator in PEDF‐induced endothelial cell apoptosis. Am J Physiol Cell Physiol 2009; 296: C273–84. [DOI] [PubMed] [Google Scholar]
- 14. Ho TC, Chen SL, Yang YC et al. PEDF induces p53‐mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovasc Res 2007; 76: 213–23. [DOI] [PubMed] [Google Scholar]
- 15. Steele FR, Chader GJ, Johnson LV et al. Pigment epithelium‐derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci USA 1993; 90: 1526–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia M, Fernandez‐Garcia NI, Rivas V et al. Inhibition of xenografted human growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium‐derived factor. Cancer Res 2004; 64: 5632–42. [DOI] [PubMed] [Google Scholar]
- 17. Streck CJ, Zhang Y, Zhou J et al. Adeno‐associated virus vector‐mediated delivery of pigment epithelium‐derived factor restricts neuroblastoma angiogenesis and growth. J Pediatr Surg 2005; 40: 236–43. [DOI] [PubMed] [Google Scholar]
- 18. Zhu B, Lu L, Cai W et al. Kallikrein‐binding protein inhibits growth of gastric carcinoma by reducing vascular endothelial growth factor production and angiogenesis. Mol Cancer Ther 2007; 6: 3297–306. [DOI] [PubMed] [Google Scholar]
- 19. Wang QS, Yang X, Yang ZH et al. Novel method for expression and purification of human pigment epithelium‐derived factor with biological activities in Escherichia coli. Prep Biochem Biotechnol 2006; 36: 127–38. [DOI] [PubMed] [Google Scholar]
- 20. Weidner N, Semple JP, Welch WR et al. Tumor angiogenesis and metastasis – correlation in invasive breast carcinoma. N Engl J Med 1991; 324: 1–8. [DOI] [PubMed] [Google Scholar]
- 21. Zhang T, Guang YL. Production of active pigment epithelium‐derived factor in E. coli . Biotechnol Lett 2005; 27: 403–7. [DOI] [PubMed] [Google Scholar]
- 22. Dawson DW, Volpert OV, Gillis P et al. Pigment epithelium‐derived factor: a potent inhibitor of angiogenesis. Science 1999; 285: 245–8. [DOI] [PubMed] [Google Scholar]
- 23. Duh EJ, Yang HS, Suzuma I et al. Pigment epithelium‐derived factor suppresses ischemia‐induced retinal neovascularization and VEGF‐induced migration and growth. Invest Ophthalmol Vis Sci 2002; 43: 821–9. [PubMed] [Google Scholar]
- 24. Wang L, Schmitz V, Perez‐Mediavilla A et al. Suppression of angiogenesis and tumor growth by adenoviral‐mediated gene transfer of pigment epithelium‐derived factor. Mol Ther 2003; 8: 72–9. [DOI] [PubMed] [Google Scholar]
- 25. Stratikos E, Alberdi E, Gettins PG et al. Recombinant human pigment epithelium‐derived factor (PEDF): characterization of PEDF overexpressed and secreted by eukaryotic cells. Protein Sci 1996; 5: 2575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sánchez‐Sánchez F, Aroca‐Aguilar JD, Segura I et al. Expression and purification of functional recombinant human pigment epithelium‐derived factor (PEDF) secreted by the yeast Pichia pastoris. J Biotechnol 2008; 134: 193–201. [DOI] [PubMed] [Google Scholar]
- 27. Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002; 29: 15–8. [DOI] [PubMed] [Google Scholar]
- 28. Apte RS, Harbour JW. Inhibiting angiogenesis in retinoblastoma. Ophthalmic Res 2007; 39: 188–90. [DOI] [PubMed] [Google Scholar]
- 29. Poulaki V, Mitsiades CS, McMullan C et al. Human retinoblastoma cells are resistant to apoptosis induced by death receptors: role of caspase‐8 gene silencing. Invest Ophthalmol Vis Sci 2005; 46: 358–66. [DOI] [PubMed] [Google Scholar]
- 30. Gao G, Li Y, Gee S et al. Down‐regulation of vascular endothelial growth factor and up‐regulation of pigment epithelium‐derived factor: a possible mechanism for the anti‐angiogenic activity of plasminogen kringle 5. J Biol Chem 2002; 277: 9492–7. [DOI] [PubMed] [Google Scholar]
- 31. Gao G, Li Y, Zhang D et al. Unbalanced expression of VEGF and PEDF in ischemia‐induced retinal neovascularization. FEBS Lett 2001; 489: 270–6. [DOI] [PubMed] [Google Scholar]
- 32. Leung DW, Cachianes G, Kuang WJ et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989; 246: 1306–9. [DOI] [PubMed] [Google Scholar]
- 33. Choi YK, Kim JH, Kim WJ et al. AKAP12 regulates human blood‐retinal barrier formation by downregulation of hypoxia‐inducible factor‐1alpha. J Neurosci 2007; 27: 4472–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jia RB, Zhang P, Zhou YX et al. VEGF‐targeted RNA interference suppresses angiogenesis and tumor growth of retinoblastoma. Ophthalmic Res 2007; 39: 108–15. [DOI] [PubMed] [Google Scholar]
- 35. Mizokami K, Kakeji Y, Oda S et al. Clinicopathologic significance of hypoxia‐inducible factor1 alpha overexpression in gastric carcinomas. J Surg Oncol 2006; 94: 149–54. [DOI] [PubMed] [Google Scholar]
- 36. Yeo EJ, Chun YS, Cho YS et al. YC‐1: a potential anticancer drug targeting hypoxia‐inducible factor 1. J Natl Cancer Inst 2003; 95: 516–25. [DOI] [PubMed] [Google Scholar]
- 37. Stoeltzing O, McCarty MF, Wey JS et al. Role of hypoxia‐inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst 2004; 96: 946–56. [DOI] [PubMed] [Google Scholar]
- 38. Lu L, Yang Z, Zhu B et al. Kallikrein‐binding protein suppresses growth of hepatocellular carcinoma by anti‐angiogenic activity. Cancer Lett 2007; 257: 97–106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of pigment epithelium‐derived factor (PEDF) on human umbilical vein endothelial cells (HUVECs) cell proliferation. Primary HUVECs were treated with PEDF at concentrations indicated for 24 h. The viable cells were quantified by MTT. Data represent absorbance as percentages of respective controls (mean ± SD, n = 3), and values statistically different from the control are indicated (*P < 0.05). PEDF inhibits proliferation of HUVECs in a dose‐dependent manner.
Fig. S2. Quantitative analysis of cell apoptosis induced by pigment epithelium‐derived factor (PEDF). Human umbilical vein endothelial cells (HUVECs) and SO‐Rb50 cells were treated with PEDF at 200 nnmol/L for 72 h, respectively. Apoptotic cells were dual‐stained with AnnexinV and PI, and quantified by flow cytometry. (a) Apoptosis analysis of HUVECs; (b) apoptosis analysis of SO‐Rb50 cells. Values significantly higher than control (**P < 0.01) are indicated.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item


