Abstract
The tetraspanin protein superfamily member KAI1 suppresses tumor growth and metastasis in animal models and is downregulated in various human malignancies. In breast cancer, KAI1 is preferentially lost in estrogen receptor (ER)‐positive tumors. Interestingly, most ER‐negative primary breast cancers retain KAI1 expression. This study aimed to evaluate whether or not KAI1 is downregulated during progression to metastasis of these carcinomas. Expression of KAI1, ER, progesterone receptor, c‐ErbB2, and Ki67 was analyzed in tissue microarrays comprising a large collection of distant organ metastases from human breast cancers (n = 92) by immunohistochemistry. Results were compared with a previously characterized set of primary breast tumors (n = 209). Immunoreactivity for KAI1 was observed in one‐third of the metastases and was associated with lack of ER expression (P = 0.005). The high frequency of KAI1‐positive cases in ER‐negative primary tumors was maintained in ER‐negative metastases. Expression of KAI1 was also observed in MDA‐MB‐468 and SK‐BR‐3, two ER‐negative breast cancer cell lines of metastatic origin. Moreover, a reanalysis of independent microarray gene expression data indicated maintenance of KAI1 mRNA expression in metastases from ER‐negative breast cancers. Furthermore, in a series of matched pairs of mammary carcinomas and metachronous distant metastases, all metastases from KAI1‐positive/ER‐negative primary tumors were KAI1‐positive as well. Collectively, these findings demonstrate that the expression of KAI1 is maintained during progression to metastasis in a large proportion of ER‐negative mammary carcinomas. This has significant implications for the use of KAI1 as a clinical marker and the understanding of the metastatic process in human breast cancer. (Cancer Sci 2009; 100: 1767–1771)
Few genes have so far been characterized as metastasis suppressor genes, one of which is KAI1/CD82. ( 1 , 2 ) KAI1 encodes a cell‐surface protein of the tetraspanin superfamily and was first identified as a suppressor of the metastatic phenotype of AT6.1 prostate cancer cells.( 3 ) Ectopic expression of KAI1 also inhibits growth and metastasis of LNCaP, B16BL6, and MDA‐MB‐435/M14 cancer cells.( 4 , 5 , 6 ) Upon stimulation with appropriate ligands, KAI1 mediates growth‐suppressive signals which lead to induction of p21 and inhibition of cell proliferation.( 5 ) In addition, KAI1 interferes with the activation of the tyrosine kinase c‐Met.( 7 ) Analyses of clinical tumor specimens revealed that KAI1 is gradually downregulated during the progression of prostate cancer.( 8 , 9 , 10 ) Ueda and colleagues reported an inverse correlation of KAI1 expression and histological grade in prostate cancer.( 8 ) Bouras et al. likewise observed reduced expression of KAI1 in poorly differentiated prostate cancer.( 9 ) Dong and colleagues reported that the expression of KAI1 was uniformly lost in prostate cancer metastases.( 10 ) In line with these findings, White et al. demonstrated that KAI1 was downregulated in 16 of 18 human cancer cell lines of metastatic origin derived from lung, ovarian, endometrial, bladder, and prostate cancer.( 11 ) Subsequently, KAI1 has been proposed to represent a promising biomarker for the metastatic potential of various human malignancies, including breast cancer.( 1 , 2 , 12 ) However, in comparison to prostate cancer, the role of KAI1 in breast cancer is more complex. KAI1 is expressed throughout the normal mammary epithelium but is downregulated in the majority of human primary breast tumors.( 13 , 14 ) Downregulation of KAI1 preferentially occurs in estrogen receptor (ER)‐positive cancers and involves ER‐mediated gene repression.( 13 , 14 ) Interestingly, ER‐negative mammary carcinomas, which represent a biologically and clinically distinct entity, mostly retain expression of KAI1.( 13 , 14 ) Given the metastasis‐suppressive function of ectopically expressed KAI1 in experimental animal models, it seems reasonable to assume that ER‐negative breast tumors would rarely metastasize. Yet, in the clinics, ER‐negative breast cancers are clearly associated with metastasis and an adverse outcome.( 15 , 16 , 17 , 18 , 19 ) Paradoxically, ER‐negative mammary carcinomas frequently express the metastasis suppressor KAI1.( 13 , 14 ) This raises the question whether KAI1 is downregulated in distant metastases from ER‐negative breast cancers. As only few cancer patients have distant metastases surgically removed, little is known about the expression of KAI1 in metastatic breast cancer tissue.( 20 ) These considerations prompted us to study the expression of KAI1 in clinical specimens of distant metastases from human breast cancers.
Materials and Methods
Tissue microarrays and cell lines. With approval of the local ethics committee, tissue microarrays containing 1.4 mm (diameter) core biopsies from representative tumor areas were constructed as described previously.( 14 , 19 ) Microarrays comprised 92 metastases resected from the liver or central nervous system of female patients diagnosed with invasive breast cancer at the Hannover Medical School (Suppl. Table 1). Additional microarrays comprised a cohort of 209 unselected M0/Mx primary breast tumors, which were characterized earlier.( 14 ) A third set of tumor specimens included 25 metachronously metastasized primary breast tumors (Suppl. Table 2), of which a total of 14 cases were matched primary tumors corresponding to a particular metachronous distant metastasis included in the collection of 92 metastases (Suppl. Table 3). The breast cancer cell lines MDA‐MB‐468 and MDA‐MB‐453 were obtained from ATCC (Manassas, VA, USA). The breast cancer cell lines SK‐BR‐3, MDA‐MB‐231, CAL‐51, MDA‐MB‐157 and MCF‐7, and their culture conditions have been described earlier.( 21 )
Immunological reagents and immunohistochemistry. Detection of KAI1 was performed with the monoclonal anti‐KAI1 antibody G2 (Santa Cruz, Santa Cruz, CA, USA) using paraffin‐embedded UACC‐893 and MCF‐7 cells as internal controls as described previously.( 14 ) Detection of ER, progesterone receptor (PR), c‐ErbB2, and Ki67 was performed with the SP1 (Lab Vision, Fremont, CA, USA), PgR‐636 (DakoCytomation, Hamburg, Germany), NCL‐c‐erbB‐2p (Novocastra, Newcastle, UK), and MIB‐1 (DakoCytomation) antibodies. Four‐micrometer sections of tissue microarrays were mounted on poly‐L‐lysine‐coated slides and were incubated with the primary antibodies outlined above. Immunoreactions were detected with the ZytoChem‐Plus HRP Kit (Zytomed, Berlin, Germany). Membranous KAI1 immunoreactivity was evaluated using an immunoreactivity score (IRS) as described by Remmele and Stegener.( 14 ) Tumor samples with an IRS ≤2 were considered as KAI1‐negative, whereas those with an IRS ≥3 were considered as KAI1‐positive. Overexpression of c‐ErbB2 and high/low Ki67 labeling index (LI) were defined as described earlier.( 14 ) Statistical analyses were carried out using GraphPad Prism software and the χ2‐test.
Microarray gene expression data. cDNA microarray gene expression data covering more than 18 000 human transcripts in a previously described set of matched primary breast cancers (n = 8) and corresponding metastases (n = 8) were obtained from the Netherlands Cancer Institute's webpage (http://www.nki.nl/nkidep/pa/microarray).( 22 ) Normalized expression ratios from all samples and cDNA clones were uploaded into BRB‐ArrayTools version 3.5.0‐Patch_2 for retrieval of KAI1 gene expression ratios and further analyses with GraphPad Prism software.
Quantitative reverse transcription PCR and western blot. The cDNA synthesis was performed using Superscript II reverse transcriptase (Invitrogen, Karlsruhe, Germany) and 1 µg DNAse I‐treated total RNA as template in a 20‐µL reaction volume. Standard endpoint reverse transcription PCR was performed with 1 µL cDNA as template in a 25‐µL reaction volume using Platinum Taq DNA polymerase (Invitrogen). Quantitative assessment of KAI1 gene expression normalized to the housekeeping gene β‐GUS was performed with Sybr Green I (Invitrogen) as described previously.( 14 ) Primers for the amplification of full‐length KAI1 have been described elsewhere.( 23 ) For western blotting, cells were lysed in radioimmunoprecipitation assay (RIPA) buffer and 40 µg total cellular protein was separated by 12% SDS‐PAGE and transferred to nitrocellulose membranes. KAI1 and β‐actin protein were detected with the G2 and AC‐15 (Acris, Hiddenhausen, Germany) antibody, respectively.
Results
KAI1 expression in distant metastases. Expression of KAI1 was evaluated by immunohistochemistry in a large collection of 92 distant metastases from human breast cancers. Figure 1(a) shows representative examples of KAI1 immunohistochemical stainings in a KAI1‐negative and a KAI1‐positive liver metastasis ( Fig. 1a ). An overview of the immunoreactivity profile of the whole metastasis set is shown in Figure 1(b). Overall, KAI1 immunoreactivity was observed in 33% of the metastases, while 67% were considered as KAI1‐negative. KAI1‐positive metastases were clearly associated with an ER‐negative phenotype (P = 0.005) (Table 1). Expression of KAI1 mRNA and protein was also observed in MDA‐MB‐468 and SK‐BR‐3, two ER‐negative breast cancer cell lines of metastatic origin ( Fig. 1c,d ). In MDA‐MB‐468, wild‐type KAI1 mRNA was confirmed by sequencing (not shown).
Figure 1.
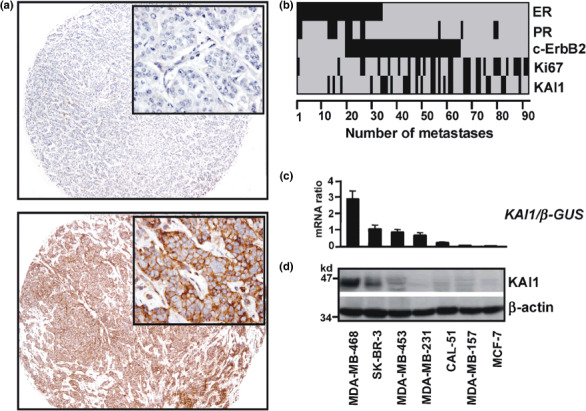
Expression of KAI1 in clinical specimens of distant metastases from breast and in breast cancer cell lines of metastatic origin. (a) Representative examples of KAI1 immunohistochemical stainings in formalin‐fixed paraffin‐embedded breast cancer metastases resected from the liver. The top panel shows a KAI1‐negative metastasis and the bottom panel a KAI1‐positive metastasis (magnification, ×50; insets: magnification, ×400). (b) Overview of the immunoreactivity profiles of 92 distant metastases on tissue microarrays for estrogen receptor (ER), progesterone receptor (PR), c‐ErbB2, Ki67, and KAI1. Black: ER, positive; PR, positive; c‐ErbB2, 2+/3+; Ki67, labeling index (LI) ≥35. Grey: ER, negative; PR, negative; c‐ErbB2, 0/1+; Ki67, LI <35. (c) Total RNA from breast cancer cell lines of metastatic origin was subjected to quantitative reverse transcription PCR. Normalization was performed using the housekeeping gene β‐GUS as reference. Bars represent mean expression of KAI1. Error bars represent the SEM. (d) Western blot. Whole cell lysates were separated by 12% SDS‐PAGE and probed with the monoclonal anti‐KAI1 antibody G2. Detection of β‐actin verified equal loading.
Table 1.
Relationship between KAI1 expression and clinicopathological factors in breast cancer metastases
| KAI1‐negative | KAI1‐positive | P‐values | |
|---|---|---|---|
| Cases | 62 (67%) | 30 (33%) | |
| Age | P = 0.070 | ||
| <60 years | 31 (60%) | 21 (40%) | |
| ≥60 years | 31 (77%) | 9 (23%) | |
| Metastasis localization | |||
| Liver | 29 (81%) | 7 (19%) | |
| Cerebrum | 19 (54%) | 16 (46%) | |
| Cerebellum | 10 (63%) | 6 (37%) | |
| Meninges | 4 (80%) | 1 (20%) | |
| Estrogen receptor | P = 0.005 | ||
| Negative | 33 (57%) | 25 (43%) | |
| Positive | 29 (85%) | 5 (15%) | |
| Progesterone receptor | P = 0.255 | ||
| Negative | 50 (65%) | 27 (35%) | |
| Positive | 12 (80%) | 3 (20%) | |
| c‐ErbB2 expression | P = 0.657 | ||
| 0, 1+ | 32 (70%) | 14 (30%) | |
| 2+, 3+ | 30 (65%) | 16 (35%) | |
| Ki67 labeling index (LI) | P = 0.067 | ||
| <35 | 45 (74%) | 16 (26%) | |
| ≥35 | 17 (55%) | 14 (45%) | |
χ2‐test.
KAI1 expression in distant metastases versus primary tumors. To gain insight into the role of KAI1 in breast cancer progression, the immunoreactivity profile of the metastasis set was compared with the immunoreactivity profile of a previously described set of 209 unselected M0/Mx primary breast tumors analyzed in the same institution.( 14 ) Interestingly, this comparison revealed an approximately 1.4‐fold excess of KAI1‐positive cases in the metastasis set ( Fig. 2a ). A similar excess of KAI1‐positive cases was also observed in metachronously metastasized primary breast tumors relative to unselected M0/Mx primary breast tumors (Suppl. Table 4). However, these comparisons were biased by the well‐known overrepresentation of ER‐negative cases among breast cancer patients with metastatic disease.( 17 , 18 , 19 ) Hence, this analysis was further refined by subdividing the metastasis set as well as the primary tumor set in an ER‐positive and an ER‐negative subgroup (Fig. 2b,c). In the ER‐positive subgroup, immunoreactivity for KAI1 was observed in 8% of the primary tumors and in 15% of the metastases (Fig. 2b). This was not a statistically significant difference (P = 0.195). In the ER‐negative subgroup, immunoreactivity for KAI1 was observed in 57% of the primary tumors and in 43% of the metastases (Fig. 2c). This was also not a statistically significant difference (P = 0.126), suggesting that the expression of KAI1 is maintained during progression to metastasis in a large proportion of ER‐negative mammary carcinomas.
Figure 2.
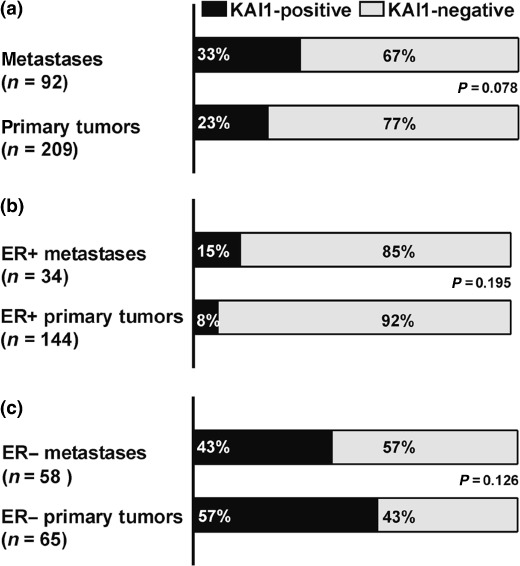
Comparison of KAI1 immunoreactivity in distant metastases from breast cancer and in a cohort of unselected M0/Mx primary mammary carcinomas. The set of primary tumors has been described previously.( 14 ) Statistical analysis was carried out using the actual numbers of observed cases and the χ2‐test. (a) Frequency of KAI1‐positive cases in the whole metastasis set versus frequency of KAI1‐positive cases in the whole set of primary mammary carcinomas. (b) Frequency of KAI1‐positive cases in the ER‐positive subgroup of the metastasis set versus frequency of KAI1‐positive cases in the ER‐positive subgroup of the set of primary mammary carcinomas. (c) Frequency of KAI1‐positive cases in the ER‐negative subgroup of the metastasis set versus frequency of KAI1‐positive cases in the ER‐negative subgroup of the set of primary mammary carcinomas.
KAI1 expression in matched pairs of primary tumors and distant metastases. To further substantiate this finding, matched pairs of primary tumors and corresponding distant metastases were examined. First, the cDNA microarray gene expression data provided by Weigelt et al. comprising four ER‐negative and four ER‐positive primary breast cancers and corresponding metastases, were re‐evaluated.( 22 ) In line with our immunohistochemical finding, KAI1 expression ratios were considerably higher in ER‐negative metastases compared to ER‐positive metastases (P = 0.047), but not in ER‐negative primary tumors compared to ER‐negative metastases (P = 0.835) (Fig. 3a). However, the limited number of available human metastases samples restricted the informative value of this independent dataset.( 22 ) Hence, we examined KAI1 expression in additional matched pairs of primary tumors and metchronous distant metastases by immunohistochemistry. From the set of 92 metastases described above, 14 metastases were analyzed side‐by‐side with the corresponding primary or locally recurrent breast tumor of the respective patient (Fig. 3b,c). Notably, all metastases from KAI1‐positive/ER‐negative primary tumors were KAI1‐positive as well (Fig. 3b,c).
Figure 3.
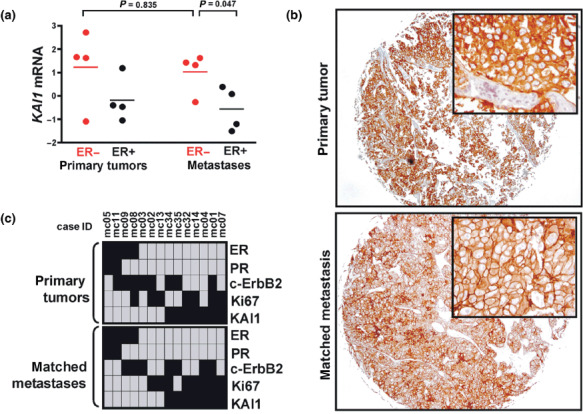
Expression of KAI1 in matched pairs of primary mammary carcinomas and corresponding distant metastases. (a) Reanalysis of the cDNA microarray expression data of Weigelt et al.( 22 ) Red, ER‐negative; black, ER‐positive. Shown is the KAI1 gene expression (clone ID: 20990) of a particular sample relative to a reference pool of 60 unrelated breast cancers.( 22 ) Statistical significance was determined using the unpaired t‐test. (b) Representative example of a KAI1 immunohistochemical staining in an ER‐negative mammary carcinoma (matched case ID: #mc32) and its corresponding metachronous cerebral metastasis (magnification, ×50; insets: magnification, ×400). (c) Overview of the immunoreactivity profiles of the series of 14 matched pairs of primary tumors (upper panel) and corresponding metachronous distant metastases (lower panel). Black: ER, positive; PR, positive; c‐ErbB2, 2 + /3 + ; Ki67, labeling index (LI) ≥35. Grey: ER, negative; PR, negative; c‐ErbB2, 0/1+; Ki67, LI <35.
Discussion
KAI1 suppresses tumor growth and metastasis in experimental animal models and has been proposed as a clinical marker of low metastatic potential in various human malignancies, including breast cancer.( 1 , 2 , 12 ) However, we and others have recently shown that the more aggressive ER‐negative mammary carcinomas commonly express KAI1.( 13 , 14 ) The present study sought to clarify whether or not KAI1 is downregulated during the progression to metastasis of these carcinomas. Therefore, KAI1 expression was analyzed in tissue microarrays comprising a large collection of clinical specimens of distant organ metastases from human breast cancers. KAI1 immunoreactivity was observed in one‐third of the metastases. Like in primary breast tumors, expression of KAI1 was clearly associated with an ER‐negative phenotype in breast cancer metastases (P = 0.005). The high frequency of KAI1‐positive cases in ER‐negative primary tumors was maintained in ER‐negative metastases, arguing against a downregulation of KAI1 during progression to metastasis of ER‐negative mammary carcinomas. In line with a previous report, expression of full‐length KAI1 mRNA and protein was also observed in ER‐negative breast cancer cell lines of metastatic origin.( 13 ) Moreover, a reanalysis of independent, publicly available microarray gene expression data also indicated that KAI1 mRNA expression levels are maintained in distant metastases from ER‐negative breast cancers.( 22 ) Furthermore, in a series of matched pairs of primary tumors and corresponding metachronous distant metastases, all metastases from KAI1‐positive/ER‐negative primary tumors were KAI1‐positive as well. Collectively, these findings demonstrate that the expression of KAI1 is maintained during progression to metastasis in a large proportion of ER‐negative mammary carcinomas. We conclude that the downregulation of KAI1 is dispensable for ER‐negative mammary carcinomas to progress and to metastasize in breast cancer patients. Possibly, KAI1 is functionally inactivated in ER‐negative mammary carcinomas by antagonistic and metastasis‐enhancing tetraspanins like KITENIN or CD151.( 24 , 25 ) Contrary to the notion gained by the clear metastasis‐suppressive function of ectopically expressed KAI1 in experimental animal models, the results reported herein show that KAI1 is not useful as a marker of low metastatic potential in breast cancer. Moreover, this strongly underscores the importance of analyzing clinical metastasis specimens for the evaluation of factors that regulate tumor progression and metastasis in experimental models.
Supporting information
Table S1. Clinicopathological parameters of the breast cancer metastasis set.
Table S2. Clinicopathological parameters of the set of metachronously metastasized primary breast cancers.
Table S3. Characteristics of the set of metachronously metastasized primary breast cancers and their matched distant metastases.
Table S4. Immunoreactivity profiles of distant organ metastases from breast cancers, unselected primary breast cancers (M0/MX), and metachronously metastasized primary breast cancers.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
References
- 1. Steeg PS, Ouatas T, Halverson D, Palmieri D, Salerno M. Metastasis suppressor genes: basic biology and potential clinical use. Clin Breast Cancer 2003; 4: 51–62. [DOI] [PubMed] [Google Scholar]
- 2. Debies MT, Welch DR. Genetic basis of human breast cancer metastasis. J Mammary Gland Biol Neoplasia 2001; 6: 441–51. [DOI] [PubMed] [Google Scholar]
- 3. Dong JT, Lamb PW, Rinker‐Schaeffer CW et al . KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science 1995; 268: 884–6. [DOI] [PubMed] [Google Scholar]
- 4. Kim JH, Kim B, Cai L et al . Transcriptional regulation of a metastasis suppressor gene by Tip60 and beta‐catenin complexes. Nature 2005; 434: 921–6. [DOI] [PubMed] [Google Scholar]
- 5. Bandyopadhyay S, Zhan R, Chaudhuri A et al . Interaction of KAI1 on tumor cells with DARC on vascular endothelium leads to metastasis suppression. Nat Med 2006; 12: 933–8. [DOI] [PubMed] [Google Scholar]
- 6. Yang X, Wei LL, Tang C, Slack R, Mueller S, Lippman ME. Overexpression of KAI1 suppresses in vitro invasiveness and in vivo metastasis in breast cancer cells. Cancer Res 2001; 61: 5284–8. [PubMed] [Google Scholar]
- 7. Sridhar SC, Miranti CK. Tetraspanin KAI1/CD82 suppresses invasion by inhibiting integrin‐dependent crosstalk with c‐Met receptor and Src kinases. Oncogene 2006; 25: 2367–78. [DOI] [PubMed] [Google Scholar]
- 8. Ueda T, Ichikawa T, Tamaru J et al . Expression of the KAI1 protein in benign prostatic hyperplasia and prostate cancer. Am J Pathol 1996; 149: 1435–40. [PMC free article] [PubMed] [Google Scholar]
- 9. Bouras T, Frauman AG. Expression of the prostate cancer metastasis suppressor gene KAI1 in primary prostate cancers: a biphasic relationship with tumour grade. J Pathol 1999; 188: 382–8. [DOI] [PubMed] [Google Scholar]
- 10. Dong JT, Suzuki H, Pin SS et al . Down‐regulation of the KAI1 metastasis suppressor gene during the progression of human prostatic cancer infrequently involves gene mutation or allelic loss. Cancer Res 1996; 56: 4387–90. [PubMed] [Google Scholar]
- 11. White A, Lamb PW, Barrett JC. Frequent downregulation of the KAI1 (CD82) metastasis suppressor protein in human cancer cell lines. Oncogene 1998; 16: 3143–9. [DOI] [PubMed] [Google Scholar]
- 12. Yang X, Welch DR, Phillips KK, Weissman BE, Wei LL. KAI1, a putative marker for metastatic potential in human breast cancer. Cancer Lett 1997; 119: 149–55. [DOI] [PubMed] [Google Scholar]
- 13. Huang H, Groth J, Sossey‐Alaoui K, Hawthorn L, Beall S, Geradts J. Aberrant expression of novel and previously described cell membrane markers in human breast cancer cell lines and tumors. Clin Cancer Res 2005; 11: 4357–64. [DOI] [PubMed] [Google Scholar]
- 14. Christgen M, Bruchhardt H, Ballmaier M et al . KAI1/CD82 is a novel target of estrogen receptor‐mediated gene repression and downregulated in primary human breast cancer. Int J Cancer 2008; 123: 2239–46. [DOI] [PubMed] [Google Scholar]
- 15. Callagy GM, Pharoah PD, Pinder SE et al . Bcl‐2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res 2006; 12: 2468–75. [DOI] [PubMed] [Google Scholar]
- 16. Weigelt B, Peterse JL, Van't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer 2005; 5: 591–602. [DOI] [PubMed] [Google Scholar]
- 17. Koenders PG, Beex LV, Langens R, Kloppenborg PW, Smals AG, Benraad TJ. Steroid hormone receptor activity of primary human breast cancer and pattern of first metastasis. The Breast Cancer Study Group. Breast Cancer Res Treat 1991; 18: 27–32. [DOI] [PubMed] [Google Scholar]
- 18. James JJ, Evans AJ, Pinder SE et al . Bone metastases from breast carcinoma: histopathological – radiological correlations and prognostic features. Br J Cancer 2003; 89: 660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gaedcke J, Traub F, Milde S et al . Predominance of the basal type and HER‐2/neu type in brain metastasis from breast cancer. Mod Pathol 2007; 20: 864–70. [DOI] [PubMed] [Google Scholar]
- 20. Stark AM, Tongers K, Maass N, Mehdorn HM, Held‐Feindt J. Reduced metastasis‐suppressor gene mRNA‐expression in breast cancer brain metastases. J Cancer Res Clin Oncol 2005; 131: 191–8. [DOI] [PubMed] [Google Scholar]
- 21. Christgen M, Ballmaier M, Bruchhardt H, Von Wasielewski R, Kreipe H, Lehmann U. Identification of a distinct side population of cancer cells in the Cal‐51 human breast carcinoma cell line. Mol Cell Biochem 2007; 306: 201–12. [DOI] [PubMed] [Google Scholar]
- 22. Weigelt B, Glas AM, Wessels LF, Witteveen AT, Peterse JL, Van't Veer LJ. Gene expression profiles of primary breast tumors maintained in distant metastases. Proc Natl Acad Sci USA 2003; 100: 15901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takaoka A, Hinoda Y, Sato S et al . Reduced invasive and metastatic potentials of KAI1‐transfected melanoma cells. Jpn J Cancer Res 1998; 89: 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee JH, Park SR, Chay KO et al . KAI1 COOH‐terminal interacting tetraspanin (KITENIN), a member of the tetraspanin family, interacts with KAI1, a tumor metastasis suppressor, and enhances metastasis of cancer. Cancer Res 2004; 64: 4235–43. [DOI] [PubMed] [Google Scholar]
- 25. Yang XH, Richardson AL, Torres‐Arzayus MI et al . CD151 accelerates breast cancer by regulating alpha 6 integrin function, signaling, and molecular organization. Cancer Res 2008; 68: 3204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinicopathological parameters of the breast cancer metastasis set.
Table S2. Clinicopathological parameters of the set of metachronously metastasized primary breast cancers.
Table S3. Characteristics of the set of metachronously metastasized primary breast cancers and their matched distant metastases.
Table S4. Immunoreactivity profiles of distant organ metastases from breast cancers, unselected primary breast cancers (M0/MX), and metachronously metastasized primary breast cancers.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item


