Abstract
Potent helper action is necessary for peptide‐based vaccines to efficiently induce antitumor immune responses against advanced cancer. A phase I trial for advanced esophageal squamous cell carcinoma was carried out for patients with HLA‐A*2402 using epitope peptides derived from novel cancer‐testis antigens, LY6K and TTK, in combination with CpG‐7909 (NCT00669292). This study investigated the feasibility and the toxicity as well as induction of tumor antigen‐specific immune responses. Nine patients were vaccinated on days 1, 8, 15, and 22 of each 28‐day treatment cycle with peptide LY6K‐177, peptide TTK‐567, and CpG‐7909 (level‐1; 0, level‐2; 0.02, level‐3; 0.1 mg/kg) and all were tolerated by this treatment. LY6K‐specific T cell responses in PBMCs were detected in two of the three patients in each level. In particular, two patients in level‐2/3 showed potent LY6K‐specific T cell responses. In contrast, only two patients in level‐2/3 showed TTK‐567‐specific T cell responses. The frequency of LY6K‐177 or TTK‐567‐specific CD8+ T cells increased in patients in level‐2/3 (with CpG). The vaccination with peptides and CpG‐7909 increased and activated both plasmacytoid dentritic cells and natural killer cells, and increased the serum level of α‐interferon. There were no complete response (CR) and partial response (PR), however, one of three patients in level‐1, and four of six patients in level‐2/3 showed stable disease (SD). In conclusion, vaccination with LY6K‐177 and TTK‐567 in combination with CpG‐7909 successfully elicited antigen‐specific CD8+ T cell responses and enhanced the innate immunity of patients with advanced esophageal squamous cell carcinoma. This vaccine protocol is therefore recommended to undergo further phase II trials. (Cancer Sci 2010; 101: 2510–2517)
Surgery is the mainstay of treatment for patients with esophageal carcinoma.( 1 , 2 ) Recently, definitive chemoradiotherapy has been reported to have greater effect than expected on resectable esophageal carcinoma.( 3 , 4 ) However, no promising strategy has been established for patients with unresectable or recurrent esophageal carcinoma, and their prognosis is still poor.( 2 ) Therefore, the development of a new approach to supplement surgery, chemotherapy, or chemoradiotherapy is necessary to improve the prognosis of patients with advanced esophageal carcinoma.
The discovery of the melanoma antigen‐associated gene family has led to the identification of a large number of tumor‐associated antigens in various carcinomas.( 5 , 6 , 7 , 8 , 9 ) In addition, MHC class I‐restricted epitope peptides have been identified from those tumor‐associated antigens, some of which have been used as cancer vaccines in clinical studies.( 10 , 11 , 12 , 13 ) Several reports have shown not only immunological responses but also clinical benefits in cancer vaccine studies.( 14 , 15 , 16 , 17 , 18 )
TTK protein kinase (TTK) and lymphocyte antigen 6 complex locus K (LY6K) are novel cancer‐testis antigens that were identified through cDNA microarray analysis.( 19 ) Both TTK and LY6K are highly expressed in esophageal squamous cell carcinoma (ESCC), but their expression was hardly detectable in normal organs except the testis. Therefore, they are considered to be suitable targets for specific immunotherapy; in fact, HLA‐A24‐restricted immunodominant peptides, TTK‐567 and LY6K‐177, have been identified from TTK and LY6K, respectively. These two peptides could stimulate CTLs that had a specific cytotoxic activity against ESCC cells in which these antigens were endogenously expressed.( 20 ) Moreover, T cells responding specifically to these epitope peptides were confirmed to exist in tumor infiltrating lymphocytes as well as lymphocytes in regional lymph node and peripheral blood that were obtained from ESCC patients with HLA‐A*2402.( 21 ) Therefore, these two peptides were considered to be clinically applicable as cancer vaccines.
Patients with advanced carcinoma are thought to have an impaired immune surveillance system, so potent helper action is required for induction of an antitumor immune response in such patients. This study used as an adjuvant CpG oligodeoxynucleotides (ODNs) containing unmethylated CG motifs that are similar to those observed in bacterial DNA. These CpG‐ODNs bind to Toll‐like receptor (TLR) 9 on plasmacytoid dentritic cells (pDCs) and B cells in humans, elicit T helper‐1 responses and pro‐inflammatory cytokine production, upregulate MHC and costimulatory molecules in antigen presenting cells, and induce the antigen‐specific T cell responses.( 22 )
CpG‐ODNs can be synthesized for therapeutic use and has been evaluated as vaccine adjuvant in several clinical studies. CpG‐7909, which belongs to the CpG‐B, acts as a very potent adjuvant in combination with Montanide (ISA51; Seppic, Paris, France), and was shown to promote strong antigen‐specific CD8+ T cell responses in melanoma patients.( 23 , 24 ) In addition, intradermal injections of CpG‐7909 around the excision site of melanoma activate the pDCs and myeloid DCs, and reduce the number of regulatory T cells in sentinel lymph nodes.( 25 ) Vaccination with NY‐ESO‐1 peptide in combination with CpG‐7909 was reported to successfully induce NY‐ESO‐1‐specific immune responses and reveal clinical benefit by extending survival in patients with NY‐ESO‐1‐positive cancers.( 26 )
The present study reports the results of a phase I clinical trial using tumor‐specific antigens, TTK‐567 and LY6K‐177, with CpG‐7909 in Montanide (ISA51) as a vaccine adjuvant for patients with advanced ESCC. We also describe the feasibility and the toxicity of this vaccine strategy, as well as the immune response and clinical response.
Materials and Methods
This phase I trial was approved by the Ethics Committee on Clinical Investigation of Wakayama Medical University Hospital (WMUH) (#409) (Wakayama, Japan). Patients with advanced or metastatic esophageal carcinoma and pathologically confirmed squamous cell carcinoma were enrolled in this study after appropriate informed consent was obtained. The eligibility criteria included: no expectation of the effect of other therapy such as chemotherapy or radiation therapy; performance status 0–1 (ECOG); age ≥20 and ≤80 years; measurable or evaluable disease; no prior therapy within 4 weeks; life expectancy ≥3 months; WBC ≥ 2000, ≤15 000, PLT ≥ 75 000; AST/ALT <3 times the institutional normal limits; Cr ≤ 2.0; HLA‐A*2402 positivity; and written informed consent. The exclusion criteria included: pregnancy (women of childbearing potential); serious infections requiring antibiotics; concomitant treatment with steroids or immunosuppressing agents; or determination of unsuitableness by a chief physician.
This study is registered with ClinicalTrials.gov, number NCT00669292.
Preparation of peptides and CpG‐B for vaccine. The peptides derived from LY6K‐177 (RYCNLEGPPI) and TTK‐567 (SYRNEIAYL), which have the ability to induce HLA‐A24‐restricted and tumor‐specific CTL activity, were synthesized as previously described.( 20 , 27 ) Quality assurance studies were carried out and determined to be within acceptable levels as Good Manufacturing Practice grade for the vaccines (NeoMPS, San Diego, CA, USA).( 27 ) Peptides were dissolved in DMSO at the concentration of 1 mg/10 μL. CpG‐7909 (TCGTCGTTTTGTCGTTTTGTC‐GTT; phosphorothioate backbone)( 28 ) was also synthesized by Hokkaido System Science (Sapporo, Japan) under the conditions of Good Manufacturing Practice, and quality control testing showed the conformation of identity, sterility, and high purity; HPLC purity testing 97.442%, endotoxin <0.01 EU/mg, bioburden <400 CFU/g, the heavy metal content less than reference value, and the residual solvent less than reference value.
Study protocol. The primary endpoint was to determine the safety and tolerability of escalating doses of CpG‐7909 (0, 0.02, and 0.1 mg/kg) with a fixed dose of peptides. The secondary endpoint was to determine the immune responses and the clinical responses. Patients received vaccinations weekly for four consecutive weeks of a 5‐week cycle. LY6K‐177 (1 mg) or TTK‐567 peptide (1 mg) was mixed with each dose of CpG‐7909 dissolved in lactated Ringer’s solution and emulsified in an incomplete Freund’s adjuvant (IFA) (Montanide*ISA51 VGF 1 mL), respectively, then injected s.c. in the axillary region separately in each vaccine. At least three patients were enrolled on three CpG‐7909 dose levels: namely, 0, 0.02, and 0.1 mg/kg. Additional patients were enrolled in the case of toxicity, which was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Dose‐limiting toxicity was defined as treatment‐related Grade 3 or 4 adverse events. The maximum tolerated dose was estimated as the highest dose at which fewer than two of six patients experienced dose‐limiting toxicity.
Toxicity was evaluated with symptoms, physical examinations, and laboratory examinations after a course of vaccination. Immunological responses were assessed before and after each course of vaccination. The clinical response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0.
Isolation of PBMC. Peripheral blood was taken from each patient before and after vaccination. The PBMCs were isolated using Ficoll‐Hypaque gradients (Amersham Biosciences, Piscataway, NJ, USA) and were frozen and stored until further processing.
Enzyme‐linked immunosorbent spot assay (ELISPOT). Tumor antigen‐specific T cell responses were assessed by γ‐interferon (IFN‐γ) ELISPOT assay. The PBMCs were treated with in vitro sensitization. A total of 5 × 104 PBMCs were cultured with 10 μg/mL LY6K‐177 or TTK‐567 peptide in medium containing 100 U/mL recombinant interleukin‐2 (rIL‐2; PeproTech, Rocky Hill, NJ, USA) at 37°C with 5% CO2 for 2 weeks. On day 7, the cells were restimulated with 10 μg/mL LY6K‐177 or TTK‐567 peptide in medium containing 100 U/mL rIL‐2. The harvested cells were used as responder cells and peptide‐pulsed TISI cells (IHWG Cell and Gene Bank, Seattle, WA, USA), which were HLA‐A*2402‐positive B‐lymphoblastoid cell lines, were used as stimulator cells. HLA‐A*2402‐restricted epitope peptide derived from HIV‐Env protein was used as the negative control peptide. The ELISPOT assays were carried out without in vitro stimulation in case 6 and case 8, because strong responses against LY6K were recognized with the in vitro stimulation with LY6K‐177‐pulsed TISI cells. Responder cells were mixed with stimulator cells (1 × 105 cells per well) at different ratios (responder/stimulator [R/S] ratio; 1.25, 2.5, 5, and 10), and incubated for 24 h in triplicate. An IFN‐γ ELISPOT kit and AEC substrate set (BD Biosciences PharMingen, San Diego, CA, USA) were used to measure the antigen‐specific T cell responses. The spots were captured and analyzed using an automated ELISPOT reader (ImmunoSPOT 4S; CTL, Cleveland, OH, USA). The number of specific spots was calculated as follows: Specific spots = Spots of positive target (LY6K‐177/TTK‐567) − Spots of negative control target (HIV peptide).
The assessment of the antigen‐specific T cell response was identified when the average spot‐forming cells per well in response to the LY6K‐177 or TTK‐567 peptide was ≥10 spot‐forming cells per well in response to the control peptide and, in addition, the specific spots were increased in an R/S ratio dependent manner.
Flow cytometry. A phenotypical analysis of PBMCs was carried out using a FACSCalibur (Becton Dickinson, Mountain View, CA, USA) using the CellQuest software package before and after vaccination. The PBMCs were incubated with specific antibodies in PBS for 30 min at 4°C, then analyzed. The following antibodies were used for flow cytometry: phycoerythrin (PE)‐conjugated anti‐human CD4 mAb; peridinin chlorophyll protein (PerCP)‐conjugated anti‐human CD8 mAb; FITC‐conjugated anti‐human CD11c mAb; PE‐conjugated anti‐human CD56 mAb; FITC‐conjugated anti‐human CD69 mAb; PE‐conjugated anti‐human CD80 mAb; PE‐conjugated anti‐human CD86 mAb (all from BD Biosciences PharMingen, Franklin Lakes, NJ, USA); FITC‐conjugated BDCA‐2 (Miltenyi Biotec, Bergisch Gladbach, Germany); and FITC‐conjugated anti‐human Foxp3 mAb (BioLegend, San Diego, CA, USA). All data were expressed as the percent change of the positive cell populations. When the percent change increased to 1.5 times, it was thus defined as an increase.
Serum cytokines and chemokines. Peripheral blood was from taken each patient before and after first vaccination, and serum levels of cytokines and chemokines were measured. Levels of IFN‐α, IFN‐γ, IL‐4, IL‐10, and IL‐12 were measured by ELISA (PBL Biomedical Laboratories, Piscataway, NJ, USA; BioSource Europe, Nivelles, Belgium; BD Biosciences PharMingen, San Diego; Endogen, Woburn, MA, USA). Interleukin‐6 levels were measured by a chemiluminescent enzyme immunoassay (CLEIA) with human IL‐6 CLEIA FUJIrebio (FUJIrebio, Tokyo, Japan). The IL‐10 levels were measured by an IL‐10 ELISA (Life Technologies Japan, Tokyo, Japan). The IL‐12 levels were measured by a Quantikine Human IL‐12 Immunoassay (R&D Systems, Minneapolis, MN, USA). MCP‐1 and IP‐10 were measured by human MCP‐1 and human CXCL10/IP10 Quantikine kits (R&D Systems). 2′–5′oligoadenylate synthetase (2–5AS) was measured by a 2–5A kit Eiken (ALOKA, Tokyo, Japan).
Pentamer staining. HLA‐A24/LY6K or TTK‐specific CD8+ T cell responses were assessed by a pentamer binding assay. The PE‐labeled HLA‐A*2402 presenting HIV/LY6K/TTK were obtained from ProImmune (Oxford, UK). The PBMCs (2 × 105) were incubated with 0.5 μg pentamer‐PE (HIV/LY6K/TTK) for 10 min at room temperature and rinsed twice. We then added and mixed 0.25 μg 7‐AAD (Sigma, St. Louis, MO, USA). Thereafter, the cells were incubated with Fluorotag (ProImmune) and FITC‐conjugated anti‐human CD8 mAb (BD Biosciences PharMingen) at 4°C for 20 min, and were analyzed using a FACSCalibur with the CellQuest software package (Becton Dickinson).
Results
Patients’ characteristics. The characteristics of the nine patients enrolled in this phase I trial are shown in Table 1. All patients had received at least one prior chemotherapy regimen for the treatment of advanced/metastatic disease.
Table 1.
Patient characteristics and adverse events
| Case | Age (years)/Gender | CpG (mg/kg) | Target organs | Courses | Systemic symptoms† | Injection site reactions† |
|---|---|---|---|---|---|---|
| 1 | 70/M | — | LN | 1 | — | — |
| 2 | 63/M | — | Primary tumor, LN | 3 | — | Redness (G1), swelling (G1) |
| 3 | 57/F | — | LN | 1 | — | — |
| 4 | 59/M | 0.02 | LN | 1 | Fever (G1) | — |
| 5 | 77/M | 0.02 | LN | 3 | — | Redness (G2), swelling (G2) |
| 6 | 57/F | 0.02 | LN | 3 | — | Redness (G2), swelling (G2) |
| 7 | 66/M | 0.10 | Primary tumor, LN | 1 | Fever (G1) | Redness (G2), swelling (G2) |
| 8 | 54/F | 0.10 | LN | 7 | Fever (G2), myalgia (G1), headache (G1) | Redness (G2), swelling (G2) |
| 9 | 66/M | 0.10 | LN, liver | 1 | Fever (G1) | Redness (G2), swelling (G2) |
†Adverse events graded according to the National Cancer Institute Common Terminology Criteria version 3.0. —, no symptoms; LN, lymph node.
Adverse events. A summary of the adverse events is shown in Table 1. One of three patients in level‐1 developed grade 1 skin reaction with redness at the injection sites. Five of six patients in level‐2/3 developed grade 1 or 2 skin reaction with redness and swelling. Only one patient (case 8) received the vaccines in the inguinal region after five cycles, because of the swelling of the injection sites in the axillary region. Flu‐like symptoms were observed in four patients who received CpG‐7909 injections; one patient in level‐2 developed grade 1 fever, all of the three patients in level‐3 developed grade 1 or 2 fever including one with grade 1 headache and myalgia. Most systemic symptoms were pronounced within several hours after the CpG was given, and disappeared within 2–3 days without any treatment. Vaccine treatment with or without CpG was well tolerated in all patients, and there were no dose‐limiting toxicities. Hence, the maximum tolerated dose was not reached in the dose range tested in this trial.
CD8+ T cell reactivity. The CD8+ T cell response induced by the vaccination was evaluated by monitoring LY6K‐177 or TTK‐567‐specific T cells in the PBMCs of the patients by IFN‐γ ELISPOT assays. Two of three patients in each level had an increased number of IFN‐γ‐producing LY6K‐specific T cells in their PBMCs. Importantly, two patients in level‐2/3 revealed a remarkable increase of IFN‐γ‐producing LY6K‐specific T cells (Fig. 1A). The number of IFN‐γ‐producing LY6K‐specific T cells was detected to be approximately four times higher than the controls after the third vaccinations by ELISPOT assays without any in vitro stimulation in these two cases. None of the patients in level‐1 showed TTK‐specific T cell responses in their PBMCs. However, one each of the three patients in level‐2 and level‐3 showed an increased number of IFN‐γ‐producing TTK‐567‐specific T cells (Fig. 1B).
Figure 1.
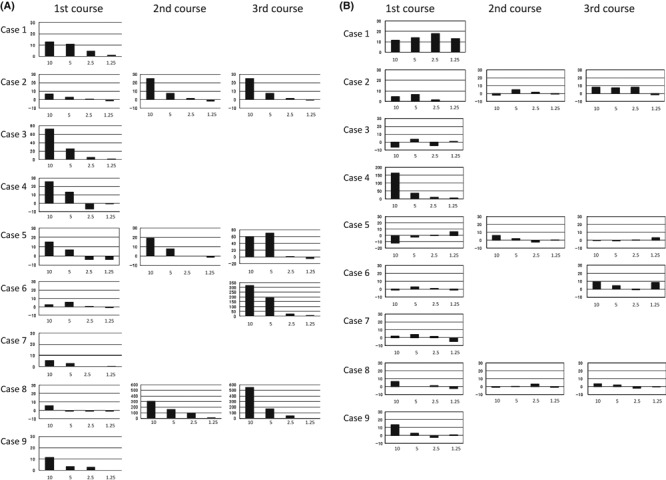
(A) Vaccine‐generated LY6K‐177‐specific T cell response. LY6K‐specific T cell responses were assessed by enzyme‐linked immunosorbent spot assay. The PBMCs were cultured with LY6K‐177 peptide for 2 weeks. On day 7, cells were restimulated with LY6K‐177 peptide. The harvested cells were used as responder cells and LY6K‐177‐pulsed TISI cells were used as stimulator cells. HLA‐A*2402‐restricted epitope peptide derived from HIV‐Env protein was used as the negative control peptide. Spots were captured and analyzed using ImmunoSPOT 4S. Responder cells were mixed with stimulator cells at different ratio, and incubated for 24 h. The X‐axis of the figure indicates the responder to stimulator (R/S) ratio. The Y‐axis indicates the number of specific spots. (B) Vaccine‐generated TTK‐567‐specific T cell response. TTK‐specific T cell responses were assessed by enzyme‐linked immunosorbent spot assay. The PBMCs were cultured with TTK‐567 peptide for 2 weeks. The cells were restimulated with TTK‐567 peptide on day 7. The harvested cells were used as responder cells and TTK‐567‐pulsed TISI cells were used as stimulator cells. HLA‐A*2402‐restricted epitope peptide derived from HIV‐Env protein was used as the negative control peptide. Responder cells were mixed with stimulator cells at different ratios, and incubated for 24 h. Spots were captured and analyzed using ImmunoSPOT 4S. The X‐axis of the figure indicates the R/S ratio. The Y‐axis indicates the number of specific spots.
HLA‐A*2402/LY6K‐177 or TTK‐567 pentamer staining was carried out for the enrolled patients, except case 1 (level‐1) and case 9 (level‐3), because we were unable to obtain the sufficient volume of blood samples from these two cases. One of the two patients in level‐1 revealed an increase in the frequencies of LY6K‐177 pentamer‐positive CD8+ T cells. Interestingly, four of the five patients in levels‐2 and ‐3 with the addition of CpG showed an increased number of LY6K pentamer‐positive CD8+ T cells in the PBMCs (Fig. 2A). Two patients, cases 6 and 8, who developed a significantly high number of IFN‐γ‐producing LY6K‐specific T cells by the ELISPOT assay, also had a high number of LY6K‐specific T cells in the PBMCs (Fig. 2B). In contrast, TTK‐567 induced lower levels of circulating antigen‐specific T cells even with adjuvant treatment of CpG (Fig. 2C). These data were consistent with the data obtained by IFN‐γ ELISPOT assays.
Figure 2.
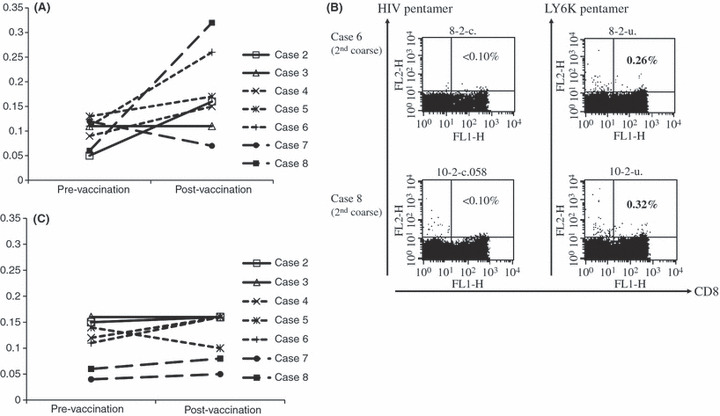
Frequencies of LY6K/TTK‐specific CD8+ T cells in patients with advanced esophageal squamous cell carcinoma, evaluated by HLA‐A*2402/LY6K‐177 (A) or TTK‐567 (C) pentamer staining. The PBMCs were incubated with phycoerythrin‐labeled HLA‐A*2402 presenting LY6K/TTK for 10 min. We added 7‐AAD, and cells were incubated with Fluorotag and FITC‐conjugated anti‐human CD8 mAb, then analyzed using a FACSCalibur. (B) The representative dot plot data of pentamer staining derived from two positive cases, case 6 and 8.
Effect of a vaccination on the population of PBMCs. We also examined the effect of vaccination on the changes of the subpopulaton on PBMCs of the nine patients. We counted the proportions of cells with CD8, Foxp3/CD4, BDCA2, CD80/BDCA2, CD86/BDCA2, CD56, and CD69/CD56. We observed no significant change in the phenotypes of PBMCs in the three patients in level‐1. In contrast, we detected some changes in the proportion of specific subtypes in PBMCs of the patients in levels‐2 and ‐3 (Fig. 3). We observed the increase of BDCA2+ pDCs in three of the six patients, that of CD80+/BDCA2+ cells in two patients, and that of CD86+/BDCA2+ cells in three patients. The increase of CD56+ natural killer (NK) cells and CD69+/CD56+ cells was observed in all patients in levels‐2 and ‐3. However, we observed no changes in the proportion of CD80+/CD11c+ cells and CD86+/CD11c+ cells in any patients. These results suggested that the peptide vaccination alone did not affect the population of PBMCs, but the vaccination in combination with CpG‐7909 might activate both pDCs and NK cells.
Figure 3.
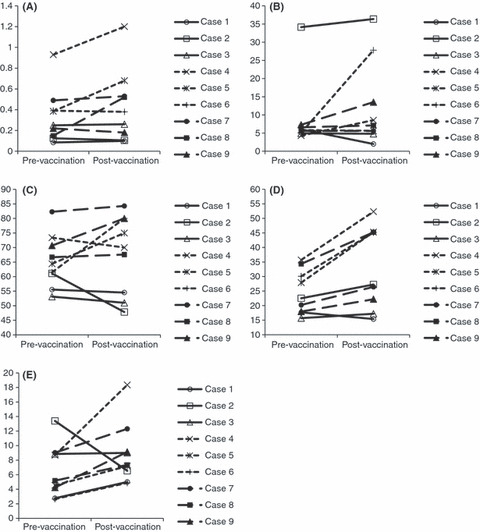
Effect of vaccination on the population of PBMCs in patients with advanced esophageal squamous cell carcinoma. The phenotypical analysis of PBMCs was carried out using a FACSCalibur with CellQuest software before and after vaccination. Data are expressed as the percent change of the positive cell populations. A percent change of 1.5 times was defined as increase. (A) BDCA2+. (B) CD80+/BDCA2+. (C) CD86+/BDCA2+. (D) CD56+. (E) CD69+/CD56+.
Serum levels of cytokines and chemokines. The serum levels of IFN‐α, IFN‐γ, IL‐4, IL‐6, IL‐10, IL‐12, and 2–5AS were measured to assess the effect of the vaccination on the serum cytokines that are related to Th1 and Th2 responses. The serum level of IFN‐α was under the measurable limit, 12.5 pg/mL, before and after the vaccination in all patients in level‐1/2, however, it was increased to approximately 20 pg/mL in two of the three patients in level‐3. The serum 2–5AS level was increased in all patients in levels‐2 and ‐3, although the increased levels were different (Table 2). Levels of IFN‐γ, IL‐4, IL‐6, IL‐10, and IL‐12 were under the detectable limits before and after the vaccination in all patients (data not shown).
Table 2.
Change in serum levels of cytokines and chemokines in patients with metastatic esophageal squamous cell carcinoma who were vaccinated with LY6K‐177 and TTK‐567 in combination with CpG‐7909
| Case | IFN‐α | 2–5AS | MCP‐1 | IP‐10 | ||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | ND | ND | 73 | 80 | 381 | 360 | 176 | 324 |
| 2 | ND | ND | 43 | 37 | 268 | 262 | 328 | 209 |
| 3 | ND | ND | 80 | 91 | 199 | 220 | 378 | 341 |
| 4 | ND | ND | 124 | 608 | 394 | 454 | 253 | 270 |
| 5 | ND | ND | 48 | 141 | 332 | 567 | 121 | 326 |
| 6 | ND | ND | 86 | 177 | 140 | 270 | 42 | 144 |
| 7 | ND | 23.9 | 56 | 440 | 218 | 928 | 136 | 628 |
| 8 | ND | 22.2 | 122 | 145 | 172 | 857 | 118 | 628 |
| 9 | ND | ND | 58 | 246 | 334 | 1040 | 148 | 623 |
Peripheral blood was taken from each patient before and after the first vaccination, and the serum levels of cytokines and chemokines were measured. ND, under detectable level (12.5 pg/mL).
The serum levels of MCP‐1 and IP‐10, which are inducible by IFN‐α, were also measured. The serum levels of both MCP‐1 and IP‐10 slightly increased in all patients in level‐2 and significantly increased in all patients in level‐3 (Table 2).
Clinical outcomes. The clinical response of the vaccination was evaluated according to RECIST version 1.0. No complete response (CR) or partial response (PR) cases were observed. However, one of the three patients in level‐1, and two each of the three patients in levels‐2 and ‐3 showed stable disease (SD). The median survival time was 3.7 months. One patient (case 8) showed a long survival period (630 days alive).
Discussion
This study represents the first trial of vaccination with the epitope peptides derived from novel cancer‐testis antigens in combination with CpG‐ODN for patients with advanced esophageal cancer. The study used HLA‐A24‐restricted immunodominant peptides, TTK‐567 and LY6K‐177, which were identified from TTK and LY6K, respectively. Recently, it has been reported that vaccination with multiple peptides including TTK‐567 and LY6K‐177 combined with IFA induced antigen‐specific T cell responses in most patients;( 27 ) no antigen‐specific T cell responses were recognized in one patient, whose tumor lost MHC class I expression, and in another patient, the expression of MHC class I was significantly downregulated in the hepatic metastatic tumor after relapse from the complete response (CR) condition after the vaccination. They noted the importance of the preservation of MHC class I expression in cancer cells to achieve the success of therapeutic cancer vaccine.( 27 )
CpG ODNs can stimulate both innate immunity and adoptive immune responses through endosomal TLR9, which is expressed in pDCs and B cells in humans. Plasmacytoid dendritic cells produce high levels of type I interferons, as well as a variety of other cytokines and chemokines to promote Th1‐like immune responses involving other cell types, including additional dendritic cell subsets, monocytes, NK cells, and neutrophils.( 29 , 30 , 31 ) Therefore, CpG‐ODN is considered to play important roles as an adjuvant for cancer vaccines using epitope peptides. CpG‐7909, which belongs to the CpG‐B family, showed a detectable adjuvant effect in the first human trial of CpG‐ODN combined with the Melan‐A analogue peptide, and elicited rapid Melan‐A‐specific CD8+ T cell responses.( 23 ) Another vaccine trial, in which vaccination with the NY‐ESO‐1 analogue peptide combined with CpG7909 was applied, revealed induction of NY‐ESO‐1‐specific CD8+ T cell responses, although the vaccination of NY‐ESO‐1 analogue peptide alone or CpG7909 alone could not induce. Hence, the vaccine strategy using the epitope peptide with CpG‐B seemed to induce higher levels of NY‐ESO‐1‐specific CD8+ T cells than the majority of published cancer vaccine studies in which cancer antigen‐derived epitopes and IFA were used.( 32 ) The present study found that the LY6K‐specific T cell responses were induced in most of the enrolled patients. In particular, two patients (case 6 in level‐2 and case 8 in level‐3) who received vaccines with LY6K‐peptide and CpG‐B revealed very strong LY6K‐specific T cell responses; IFN‐γ‐producing LY6K‐specific T cells were detected even without in vitro sensitization in these two patients. However, the immune response to TTK‐567 was not so strong as that to LY6K‐177. But the present study was still a phase I trial, and we will consecutively observe whether the immune response to TTK‐567 can be induced by this vaccine protocol in the next phase II study.
Synthetic peptides with adjuvants such as IFA were reported to induce circulating antigen‐specific T cells at a very low level. A small number of studies described ex vivo detectable T cell responses, but the T cell frequency was 10–100 times lower than that observed in the trial of vaccination using Melan‐A analogue peptide combined with CpG‐7909 in melanoma patients.( 23 ) The frequencies of antigen‐specific T cells were increased in four of the six patients in levels‐2 and ‐3 who received vaccination with CpG in the present study, although we also observed the increase of the frequencies of antigen‐specific pentamer‐positive CD8+ T cells in one of the three patients in level‐1 without CpG. However, the very significant induction of LY6K‐specific T cells observed in cases 6 and 8 of level‐2/3 were not detected in any patients in level‐1. Taken together, CpG‐7909 would be a potent adjuvant for peptide‐based vaccine therapy.
Previous trials of vaccines using tumor antigen‐derived epitope peptides combined with CpG‐ODN did not evaluate the innate immunity.( 23 , 26 , 32 ) However, one of the most important effects of CpG on the human immune system is known to be the activation of the innate immunity. Systemic TH1‐like innate immunity is induced by giving CpG‐7909 s.c. in normal healthy volunteers.( 33 ) Several doses of CpG‐7909 were tested in this phase I clinical trial for healthy volunteers, and the results indicated that serum levels of the IFN‐inducible chemokines, IP‐10 and MCP‐1, were slightly increased at the dose of 0.02 mg/kg, which corresponded to level‐2 in the current protocol. A significant increase was observed at the dose of 0.08 mg/kg, which was similar to level‐3 in the current protocol, although the effect to increase serum IFN‐α at the dose of 0.08 mg/kg was very modest. The results of the present study were consistent with this previous report, although the current study was applied to patients with advanced esophageal cancer. The serum levels of both MCP‐1 and IP‐10 revealed a slight increase in patients in level‐2, and were significantly increased in patients in level‐3. The serum level of IFN‐α was under the detectable level before and after the vaccination in patients in level‐2, but it rose to the detectable level in two of the three patients in level‐3. The observation that the serum 2–5AS level, a surrogate marker of IFN‐α, was increased in all patients in levels‐2 and ‐3 suggested that pDCs activated by CpG‐7909 produced some levels of IFN‐α. However, 0.02 mg/kg CpG‐7909 (level‐2) seemed to be insufficient and 0.1 mg/kg CpG‐7909 (level‐3) would be an adequate dose for effective activation of innate immunity. In addition, IFN‐α was reported to upregulate the MHC class I molecules on various neoplastic cells, and the treatment of the cells with IFN‐α was shown to restore MHC class I expression and T cell recognition.( 34 , 35 ) It is expected that a sufficient amount of IFN‐α might be needed to restore the downregulation or loss of the cell surface expression of MHC class I antigens in tumor cells in patients with cancer. Therefore, 0.1 mg/kg CpG‐7909 would be an adequate dose for effective activation of acquired immunity.
It was indicated that CpG‐ODNs stimulated pDCs through interaction with endosomal TLR9. Therefore, pDCs were activated and matured to be more effective antigen presenting cells (APCs). In addition, pDCs secrete type I IFN, which activates NK cells.( 36 ) However, there have been few clinical trials of cancer vaccines using low‐dose CpG‐ODNs, such as the current protocol, to investigate the effects on populations of PBMCs. Vaccination with epitope peptides alone (level‐1) did not cause a change in the population of PBMCs in the present study. However, the vaccination with peptides in combination with CpG‐7909 (level‐2/3) increased and activated pDC populations and NK cell populations. These results closely corresponded with the profiles of the cytokines and chemokines in the serum.
The current treatment was well tolerated in all patients, and the maximum tolerated dose was not reached in the maximum level. In a phase II trial of CpG‐B monotherapy for melanoma, 6 mg/body PF‐3512676 (CpG‐7909) was given weekly by s.c. injection, and no serious adverse effect related to PF‐3512676 was observed. Grade 3/4 adverse effects of laboratory study occurred in 25% of the enrolled patients, which were all normalized without further intervention.( 37 ) Accordingly, a higher dose of CpG‐7909 than 0.1 mg/kg (level‐3 in the present study) may not have any advantage as a vaccine adjuvant; in fact, it may cause some problems in terms of toxicity. The present serum cytokines and chemokines profiles indicate that level‐3 is recommended for the further phase II study.
Recent vaccine trials using multiple peptides, including TTK‐567 and LY6K‐177, showed favorable objective responses in patients with advanced esophageal cancer.( 27 ) Therefore, the comprehensive clinical results need to be evaluated in further phase II studies.
In conclusion, the current series showed that immunization with two kinds of squamous cell carcinoma‐specific peptides, LY6K‐177 and TTK‐567, in combination with CpG‐7909 successfully elicited antigen‐specific CD8+ T cell responses in patients with advanced ESCC. In addition, IFN‐α and its related chemokines were upregulated and, correspondingly, NK cells were activated. These results suggest that not only the tumor‐specific acquired immunity but also innate immunity was enhanced by this vaccination. This vaccine protocol of LY6K and TTK peptides with CpG‐7909 is safe and feasible for patients with advanced ESCC.
Acknowledgments
The authors sincerely thank Professor Yusuke Nakamura and Dr. Takuya Tsunoda (University of Tokyo, Tokyo, Japan) for their valuable advice.
References
- 1. Akiyama H, Tsurumaru M, Udagawa H, Kajiyama Y. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994; 220: 364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ando N, Iizuka T, Ide H et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study – JCOG9204. J Clin Oncol 2003; 21: 4592–6. [DOI] [PubMed] [Google Scholar]
- 3. Hironaka S, Ohtsu A, Boku N et al. Nonrandomized comparison between definitive chemoradiotherapy and radical surgery in patients with T2‐3Nany M0 squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2003; 57: 425–33. [DOI] [PubMed] [Google Scholar]
- 4. Bedenne L, Michel P, Bouché O et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007; 25: 1160–8. [DOI] [PubMed] [Google Scholar]
- 5. Boon T. Tumor antigens recognized by cytolytic T lymphocytes: present perspectives for specific immunotherapy. Int J Cancer 1993; 54: 177–80. [DOI] [PubMed] [Google Scholar]
- 6. Boon T, Van Der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med 1996; 183: 725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen YT, Scanlan MJ, Sahin U et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A 1997; 94: 1914–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kawakami Y, Eliyahu S, Delgado CH et al. Identification of a human melanoma antigen recognized by tumor‐infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A 1994; 91: 6458–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Der Bruggen P, Traversari C, Chomez P et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991; 254: 1643–7. [DOI] [PubMed] [Google Scholar]
- 10. Jager E, Gnjatic S, Nagata Y et al. Induction of primary NY‐ESO‐1 immunity: CD8+ T lymphocyte and antibody responses in peptide‐vaccinated patients with NY‐ESO‐1+ cancers. Proc Natl Acad Sci U S A 2000; 97: 12198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawakami Y, Eliyahu S, Sakaguchi K et al. Identification of the immunodominant peptides of the MART‐1 human melanoma antigen recognized by the majority of HLA‐A2‐restricted tumor infiltrating lymphocytes. J Exp Med 1994; 180: 347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kikuchi M, Nakao M, Inoue Y et al. Identification of a SART‐1‐derived peptide capable of inducing HLA‐A24‐restricted and tumor‐specific cytotoxic T lymphocytes. Int J Cancer 1999; 81: 459–66. [DOI] [PubMed] [Google Scholar]
- 13. Shichijo S, Nakao M, Imai Y et al. A gene encoding antigenic peptides of human squamous cell carcinoma recognized by cytotoxic T lymphocytes. J Exp Med 1998; 187: 277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barve M, Bender J, Senzer N et al. Induction of immune responses and clinical efficacy in a phase II trial of IDM‐2101, a 10‐epitope cytotoxic T‐lymphocyte vaccine, in metastatic non‐small‐cell lung cancer. J Clin Oncol 2008; 26: 4418–25. [DOI] [PubMed] [Google Scholar]
- 15. Bernhardt SL, Gjertsen MK, Trachsel S et al. Telomerase peptide vaccination of patients with non‐resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer 2006; 95: 1474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bocchia M, Gentili S, Abruzzese E et al. Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial. Lancet 2005; 365: 657–62. [DOI] [PubMed] [Google Scholar]
- 17. Uemura H, Fujimoto K, Tanaka M et al. A phase I trial of vaccination of CA9‐derived peptides for HLA‐A24‐positive patients with cytokine‐refractory metastatic renal cell carcinoma. Clin Cancer Res 2006; 12: 1768–75. [DOI] [PubMed] [Google Scholar]
- 18. Yajima N, Yamanaka R, Mine T et al. Immunologic evaluation of personalized peptide vaccination for patients with advanced malignant glioma. Clin Cancer Res 2005; 11: 5900–11. [DOI] [PubMed] [Google Scholar]
- 19. Kikuchi T, Daigo Y, Katagiri T et al. Expression profiles of non‐small cell lung cancers on cDNA microarrays: identification of genes for prediction of lymph‐node metastasis and sensitivity to anti‐cancer drugs. Oncogene 2003; 22: 2192–205. [DOI] [PubMed] [Google Scholar]
- 20. Suda T, Tsunoda T, Uchida N et al. Identification of secernin 1 as a novel immunotherapy target for gastric cancer using the expression profiles of cDNA microarray. Cancer Sci 2006; 97: 411–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mizukami Y, Kono K, Daigo Y et al. Detection of novel cancer‐testis antigen‐specific T‐cell responses in TIL, regional lymph nodes, and PBL in patients with esophageal squamous cell carcinoma. Cancer Sci 2008; 99: 1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol 2004; 4: 249–58. [DOI] [PubMed] [Google Scholar]
- 23. Speiser DE, Lienard D, Rufer N et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 2005; 115: 739–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Valmori D, Souleimanian NE, Tosello V et al. Vaccination with NY‐ESO‐1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross‐priming. Proc Natl Acad Sci U S A 2007; 104: 8947–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molenkamp BG, Sluijter BJ, Van Leeuwen PA et al. Local administration of PF‐3512676 CpG‐B instigates tumor‐specific CD8+ T‐cell reactivity in melanoma patients. Clin Cancer Res 2008; 14: 4532–42. [DOI] [PubMed] [Google Scholar]
- 26. Karbach J, Gnjatic S, Bender A et al. Tumor‐reactive CD8+ T‐cell responses after vaccination with NY‐ESO‐1 peptide, CpG 7909 and Montanide ISA‐51: association with survival. Int J Cancer 2010; 126: 909–18. [DOI] [PubMed] [Google Scholar]
- 27. Kono K, Mizukami Y, Daigo Y et al. Vaccination with multiple peptides derived from novel cancer‐testis antigens can induce specific T‐cell responses and clinical responses in advanced esophageal cancer. Cancer Sci 2009; 100: 1502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paul S. Technology evaluation: CpG‐7909, Coley. Curr Opin Mol Ther 2003; 5: 553–9. [PubMed] [Google Scholar]
- 29. Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 2002; 20: 709–60. [DOI] [PubMed] [Google Scholar]
- 30. Ballas ZK, Krieg AM, Warren T et al. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J Immunol 2001; 167: 4878–86. [DOI] [PubMed] [Google Scholar]
- 31. Weber JS, Zarour H, Redman B et al. Randomized phase 2/3 trial of CpG oligodeoxynucleotide PF‐3512676 alone or with dacarbazine for patients with unresectable stage III and IV melanoma. Cancer 2009; 115: 3944–54. [DOI] [PubMed] [Google Scholar]
- 32. Fourcade J, Kudela P, Andrade Filho PA et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen‐specific CD8+ T cells in melanoma patients. J Immunother 2008; 31: 781–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Krieg AM, Efler SM, Wittpoth M, Al Adhami MJ, Davis HL. Induction of systemic TH1‐like innate immunity in normal volunteers following sub‐cutaneous but not intravenous administration of CPG 7909, a synthetic B‐class CpG oligodeoxynucleotide TLR9 agonist. J Immunother 2004; 27: 460–71. [DOI] [PubMed] [Google Scholar]
- 34. Brouwer RE, Van Der Heiden P, Schreuder GM et al. Loss or downregulation of HLA class I expression at the allelic level in acute leukemia is infrequent but functionally relevant, and can be restored by interferon. Hum Immunol 2002; 63: 200–10. [DOI] [PubMed] [Google Scholar]
- 35. Yang I, Kremen TJ, Giovannone AJ et al. Modulation of major histo‐compatibility complex Class I molecules and major histocompatibility complex‐bound immunogenic peptides induced by interferon‐alpha and interferon‐gamma treatment of human glioblastoma multiforme. J Neurosurg 2004; 100: 310–9. [DOI] [PubMed] [Google Scholar]
- 36. Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest 2007; 117: 1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pashenkov M, Goess G, Wagner C et al. Phase II trial of a toll‐like receptor 9‐activating oligonucleotide in patients with metastatic melanoma. J Clin Oncol 2006; 24: 5716–24. [DOI] [PubMed] [Google Scholar]


