Abstract
Metastasis in sentinel lymph nodes indicates the initial spread of tumors from a primary site. The recent discovery of tumor‐associated growth of lymphatic vessels clarified that tumor lymphangiogenesis actively promotes enhanced draining/sentinel lymph node metastasis. Studies of experimental carcinogenesis have further established that tumors continue to induce lymphangiogenesis in metastatic foci such as draining lymph nodes. Lymphangiogenesis within draining lymph nodes probably contributes to enhanced distant lymph node and distant organ metastases. Lymph node lymphangiogenesis has recently been identified in several human malignancies, such as cutaneous malignant melanoma. Tumor‐associated lymphangiogenesis thus has potential significance not only at the primary site, but also in lymph nodes. Primary tumors induce new lymphatic vessel growth in draining lymph nodes before metastasis. The remarkable enlargement of sinusoidal lymphatic endothelium might facilitate tumor cell transport to the lymph nodes, and potentially contribute to the migration, residence, and/or survival of metastatic tumor cancer stem cells by inducing a specific tumor microenvironment. Therefore, the novel concept of ‘lymphvascular niche’ is proposed herein to explain lymphatic network expansion. This concept might help to improve understanding of the molecular mechanism of lymph node metastasis, and change therapeutic approaches to treating cancer metastasis. (Cancer Sci 2009; 100: 983–989)
Lymphatic vessels comprise an invisible but essential component of the vascular system
The vascular system comprises two types of vascular structures, namely blood and lymphatic vessels. Blood vessels form a closed circulation so that blood can travel from the heart to peripheral organs such as the skin.( 1 ) However, blood must also provide oxygen and nutrients to cells within tissues, including the epidermis, which consist of layers of keratinocytes. Therefore, blood capillaries extravasate plasma so that oxygen and nutrients can be effectively transported to targeted cells, and homeostasis is maintained by draining interstitial tissue fluid from the peripheral tissues. Hence, the lymphatic vessels play essential roles in absorbing interstitial tissue fluid and macromolecules, as well as in transporting ‘lymph’ to the bloodstream by way of the subclavian vein.
Tumors induce new blood vessel formation from pre‐existing vessels in a process called tumor angiogenesis( 2 ) to obtain sufficient oxygen and nutrients for growth and survival within primary sites. However, newly‐formed blood vessels play a pivotal role in promoting enhanced tumor metastasis to distant organs, the primary cause of poor patient outcomes.( 3 ) The mechanism of tumor angiogenesis has been extensively characterized based on such clinical and biological significance, however, it is unknown whether lymphatic vessels remain quiescent in tumors, or actively promote their lymphatic metastasis.
Tumor lymphangiogenesis: A potent inducer of tumor metastasis to sentinel lymph nodes and a novel risk factor for patient survival
Tumors induce the growth of new lymphatic vessels in experimental mouse models and in human cancers. Increasing evidence, particularly regarding lymphatic vessel‐specific markers and lymphangiogenic growth factors, led to the identification of tumor‐associated lymphangiogenesis in 2001( 4 , 5 , 6 , 7 ). Pioneering molecular cloning technologies developed during the 1990s have identified several genes, such as Prox1,( 8 ) T1α/podoplanin,( 9 ) the lymphatic vessel endothelial hyaluronan receptor (LYVE1),( 10 ) and Fms‐related tyrosine kinase 4 (FLT4)/vascular endothelial growth factor receptor‐3 (VEGFR3),( 11 ) that are specifically expressed in lymphatic endothelial cells, and/or play crucial roles in the development of lymphatic vessels. Prox1 is a homeobox transcription factor that specifies lymphatic vessels from blood vessels during mouse embryogenesis and postnatal growth.( 12 , 13 ) The origin of the mammalian lymphatic endothelium is venous during development.( 14 ) The inactivation of Prox1 in mice showed that Prox1 null‐lymphatic progenitor cells do not bud off the cardinal vein, leading to a loss of the lymphatic vascular system, resulting in lymphedema, a distinct phenotype of defective lymphatic vessels.( 12 ) Therefore, Prox1 is a key transcription factor in lymphatic vessel development, and its expression confirms lymphatic identity.
Sox18, a developmental transcription factor in the SRY‐related HMG domain family, has recently been identified as a novel protein that trans‐activates Prox1 expression exclusively in the lymphatic endothelial cells of mice.( 15 )
T1α/podoplanin is a mutin‐type glycoprotein that is abundantly expressed on lymphatic endothelial cells, and that potently distinguishes lymphatic vessels from blood vessels in the normal skin.( 16 ) Although expressed in various cell types under both physiological and pathological conditions, T1α/podoplanin is widely accepted as a marker of tumor‐associated lymphatic vessels, particularly in human cancers. The monoclonal anti‐T1α/podoplanin antibodies D2‐40( 16 ) or NZ‐1( 17 ) are currently used to label lymphatic endothelium in paraffin‐embedded tumor specimens. Furthermore, LYVE‐1 has been identified as a glycoprotein that is specifically expressed on lymphatic endothelial cells.( 10 ) This glycoprotein is generally accepted as a marker of lymphatic capillaries( 18 ) and of tumor‐associated lymphatic vessels in mice,( 19 ) although its precise function remains unclear. Moreover, FLT4/VEGFR‐3, a receptor tyrosine kinase among three VEGF receptors, was originally identified as a homolog of FLT1,( 20 ) the receptor tyrosine kinase for angiogenesis factors VEGF‐A, a placenta growth factor, and VEGF‐B (Fig. 1). VEGF‐C( 21 ) and VEGF‐D( 22 ) were identified as specific ligands for VEGFR‐3 that is expressed in the normal lymphatic vessels. Targeted VEGF‐C or VEGF‐D overexpression in the skin induces the remarkable growth of new lymphatic vessels,( 23 , 24 ) leading to the notion that the VEGF‐C,‐D‐VEGFR‐3 pathway plays a key role in promoting lymphangiogenesis. Experimental tumor models that overexpress VEGF‐C or VEGF‐D obviously induce tumor‐associated lymphatic vessel growth, confirming their roles as tumor‐associated lymphangiogenesis factors. Furthermore, VEGF‐C or VEGF‐D overexpression in these experimental tumor studies increased the frequency of draining lymph node metastasis,( 5 , 6 , 7 ) suggesting that tumor lymphangiogenesis promotes enhanced sentinel or regional lymph node metastasis. Importantly, recent experimental studies have identified that functional blockade of the VEGF‐C,‐D‐VEGFR‐3 pathway in mouse tumor models results in significant suppression of tumor lymphangiogensis and of regional lymph node metastasis using soluble VEGFR‐3 – immunoglobulin chimeric proteins/VEGF‐C,‐D trap,( 1 , 25 ) multikinase inhibitors targeting VEGFR‐3,( 26 ) neutralizing antibodies against VEGFR‐3( 27 , 28 ) or VEGF‐D,( 7 ) or etodolac, a cyclooxygenase‐2 inhibitor that decreases VEGF‐C levels by tumor‐associated macrophages.( 29 ) Moreover, the VEGF‐C coreceptor neuropilin‐2 is induced in tumor‐associated lymphatic vessels in mice( 30 ) (Fig. 1), and suppression of the VEGF‐C,‐D‐VEGFR‐3 or neuropilin‐2 pathways reduces the formation of lymph node metastasis and/or distant organ metastasis.( 1 , 7 , 30 ) Taken together, increasing evidence reveals that VEG‐C,‐D‐VEGFR‐3 signals could be a therapeutic target for the prevention of lymph node metastasis, and for monitoring cancer metastasis through the newly formed tumor‐associated lymphatic vessels.
Figure 1.
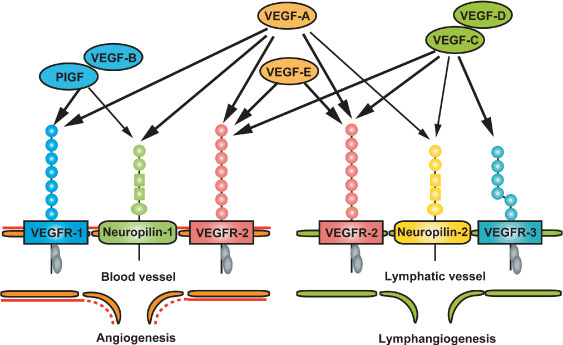
Schematic vascular endothelial growth factor (VEGF) ligand and receptor family members in lymphangiogenesis and angiogenesis. Both VEGF‐C and VEGF‐D, specific ligands for VEGF receptor 3 (VEGFR‐3), potently stimulate tumor lymphangiogenesis. Neuropilin‐2, the VEGF‐C coreceptor, is induced in tumor‐associated lymphatic endothelial cells, leading to enhanced tumor lymphangiogenesis. Major ligand VEGF‐A for receptor tyrosine kinases VEGFR‐1 and VEGFR‐2, positively mediates angiogenesis, as well as lymphangiogenesis in experimental tumor models. PIGF, placenta growth factor.
Tumor lymphangiogenesis and its potential role in promoting lymph node metastasis in human malignancy were originally identified in cutaneous malignant melanomas.( 31 ) In 37 patients with melanoma, the formation of tumor‐associated lymphatic vessels increased within the primary sites of those with regional lymph node metastasis. Importantly, increased tumor lymphangiogenesis is significantly associated with reduced patient survival. Thereafter, increasing evidence generated from studies of human cancers has confirmed that tumor lymphangiogenesis is a novel risk factor for patient survival and a feasible target for the prevention of lymph node metastasis.
Identification of VEGF‐A as a potent tumor lymphangiogenesis factor
Initial experimental studies in vivo emphasized that the VEGF‐C,‐D‐VEGFR‐3 pathway plays a crucial role in physiological and tumor‐associated lymphangiogenesis.( 4 , 5 , 6 , 7 , 24 , 32 ) However, the absence of cultured lymphatic endothelial cells has prevented elucidation of the molecular mechanisms that regulate multiple signal transduction pathways and biological functions in the lymphatic endothelium. Therefore, our research group, along with others, have developed in vitro technology to isolate cultured lymphatic endothelial cells from human skin.( 33 , 34 , 35 ) Fresh lymphatic endothelial cells maintained a lineage‐specific gene expression profile compared with blood vascular endothelial cells. This finding indicate that cultured lymphatic endothelial cells could contribute to elucidating the underlying mechanisms that regulate lymphatic vessel development, and the biological functions that play crucial roles in maintaining the fundamental function of the vasculature under physiological and/or pathological conditions.
Cultured lymphatic endothelial cells rapidly proliferate in the presence of VEGF‐A under physiological conditions.( 35 ) Lymphatic endothelial cells express considerable amounts of VEGFR‐2, a functional receptor for VEGF‐A, under adenoviral transduction with VEGF‐A in mice( 36 ) (Fig. 1). Therefore, the question arose as to whether VEGF‐A promotes tumor lymphangiogenesis as well as angiogenesis, and whether it enhances draining lymph node metastases.( 37 )
Chemically induced multistep skin carcinogenesis is an expedient model that has been applied to investigating consecutive tumor progression from initial development to distant advanced metastasis. Squamous cell carcinomas (SCCs) induced by cancer initiation and promotion potently induce tumor angiogenesis and lymphangiogenesis,( 19 ) leading to subsequent metastases to lymph nodes and distant organs in immunocompetent mice (Fig. 2). We then elucidated the biological effect of VEGF‐A on tumor‐associated lymphatic vessel growth and lymphatic metastasis using transgenic mice under a carcinogenesis regimen.
Figure 2.
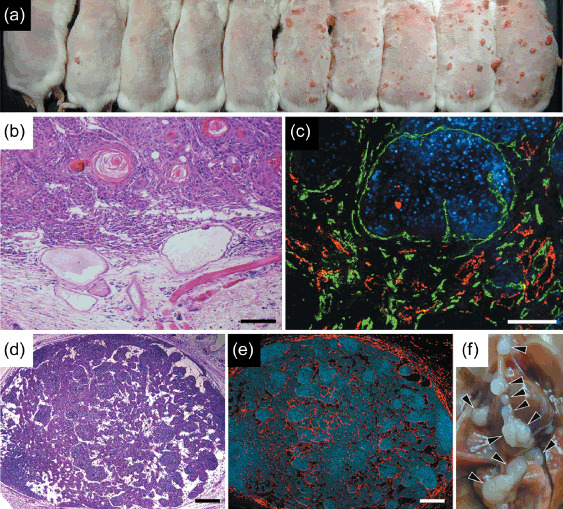
Targeted overexpression of vascular endothelial growth factor A (VEGF‐A) promotes tumor and lymph node lymphangiogenesis in chemically‐induced skin carcinogenesis. (a) The photograph shows wild‐type mice on the left (n = 5) and K14‐VEGF‐A mice on the right (n = 5) at 12 weeks during cancer promotion. Targeted overexpression of VEGF‐A induced increased number of red cutaneous tumors compared to wild‐type mice, indicating that strong induction of tumor angiogenesis by VEGF‐A leads to an accelerated formation of highly vascularized tumors in the skin. (b) Hematoxylin–eosin stains showed a representative formation of cutaneous squamous cell caricinomas (SCCs) in VEGF‐A transgenic mice during carcinogenesis study. Note that VEGF‐A overexpressing SCCs induced marked tissue edema in tumor‐associated stroma and remarkable vascular enlargement. (c) Immunofluorescence stains with antibodies against CD31 (green) and lymphatic vessel endothelial hyaluronan receptor (LYVE‐1; red) respectively indicate prominent tumor angiogenesis and lymphangiogenesis in SCCs of VEGF‐A transgenic mice. (d) Routine hematoxylin–eosin stains show remarkable enlargement of vasculature within draining lymph nodes in VEGF‐A transgenic mice during skin carcinogenesis regimen. (e) Immunofluorescence analysis with antibody against LYVE‐1 (red) by serial sections confirmed a striking formation of new lymphatic vessels within the lymph nodes even before SCCs metastasized. Scale bars = 200 µm (b–e). Nuclei are labeled blue (diethylenetriaminepentaacetic acid stain). (f) Distant lymph node metastases (arrowheads) were markedly increased in SCC‐bearing VEGF‐A transgenic mice.
Targeted VEGF‐A overexpression in the skin promotes prominent tumor angiogenesis and lymphangiogenesis.( 38 ) The formation of highly vascularized tumors is accelerated in VEGF‐A transgenic, compared with wild‐type mice (Fig. 2a). Such enhanced tumor angiogenesis appears to promote tumor formation, leading to increased SSC formation in chemically induced skin carcinogenesis. The active proliferation of tumor‐associated lymphatic endothelial cells induced by VEGF‐A, along with the marked enlargement of lymphatic vessels in SCCs, indicate that VEGF‐A could be a tumor lymphangiogenesis factor (Fig. 2b,c).
An initial observation in experimental tumor models showed that tumor‐associated lymphangiogenesis is potently induced by VEGF‐D, and not by VEGF‐A, in immunodeficient mice( 7 ) and that VEGF‐A actively promotes tumor lymphangiogenesis in immunocompetent or syngeneic mice.( 38 , 39 ) Therefore, a sufficient immune response probably promotes VEGF‐A‐induced tumor lymphangiogenesis in mice. Furthermore, targeted VEGF‐A overexpression in the skin of mice promotes enhanced tissue inflammation in the delayed‐type hypersensitivity reaction or after ultraviolet‐B irradiation, leading to the remarkable induction of lymphangiogenesis.( 40 , 41 ) In accordance with this notion, VEGF‐A silencing combined with VEGF‐C inactivation by short interfering RNA caused a significant reduction of tumor lymphangiogenesis and subsequent lymph node metastasis in a syngeneic tumor model.( 42 ) In fact, levels of VEGF‐C mRNA and polypeptides are increased in SCCs overexpressing VEGF‐A in mice with chemically induced skin carcinogenesis compared with SCCs in wild‐type mice.( 38 ) Therefore, direct and indirect effects of VEGF‐A in tumor lymphangiogenesis might result in the prominent enlargement of tumor‐associated lymphatic vessels in VEGF‐A transgenic mice. Several experimental studies of the potential effects of VEGF‐A upon lymphangiogenesis found increased VEGF‐C levels, revealing that VEGF‐A can spontaneously attract macrophages as a major source of VEGF‐C and VEGF‐D towards inflamed tissue,( 29 , 43 ) or that tumor‐associated macrophages produce abundant VEGF‐A, VEGF‐C, and VEGF‐D.( 44 )
Tumor‐associated lymphangiogenesis in lymph nodes: A potential mediator of tumor metastasis to distant sites
Tumor lymphangiogenesis induced by VEGF‐A enhances draining lymph node metastasis in the carcinogenesis model.( 38 ) Importantly however, the frequency of distant lymph node and distant organ metastases was significantly higher in VEGF‐A transgenic mice than in wild‐type mice (Fig. 2f). Both tumor‐associated lymphangiogenesis and angiogenesis within draining lymph nodes are prominently enhanced in VEGF‐A transgenic mice bearing SCC.( 38 ) Furthermore, targeted VEGF‐C overexpression in the skin promotes enhanced intranodal lymphangiogenesis in the presence of metastatic SCCs in draining lymph nodes, resulting in a significantly increased frequency of distant lymph node and distant organ metastases compared with wild‐type mice.( 45 ) Therefore, enhanced lymphangiogenesis within draining lymph nodes likely promotes tumor metastasis to distant sites.
The new concept of lymph node lymphangiogenesis described herein might alter clinical management and the understanding of therapeutic targets in efforts to prevent cancer metastasis. Many of the investigations that have been directed towards tumor progression and metastasis have focused on the mechanisms of solid tumor metastasis from primary sites to draining/sentinel lymph nodes. However, tumors can be removed from primary lesions and regional lymph nodes by surgical resection and/or by radiation therapy, and patients can be cured. In contrast, the presence of distant lymph nodes and/or distant organ metastases offers the choice of several chemotherapeutic strategies that aim to prolong the lives of cancer patients rather than to achieve complete remission. Therefore, the mechanism that promotes distant metastasis needs to be identified (Fig. 3). Thus, lymphangiogenesis within regional lymph nodes should receive more focus in the context of tumor metastasis, as it could be a novel therapeutic target of strategies aimed at preventing the spread of cancer to distant sites.
Figure 3.
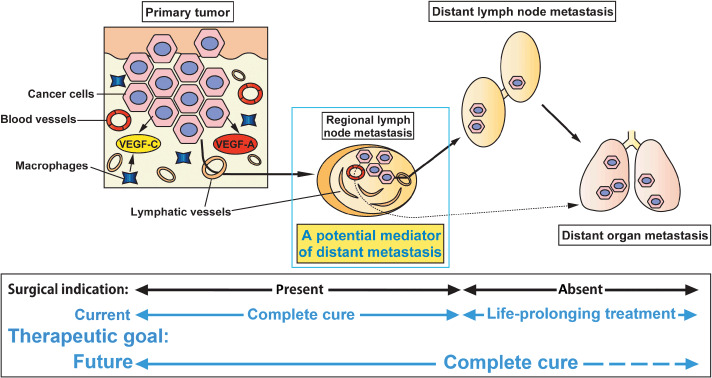
Lymph node lymphangiogenesis potently mediates tumor metastasis to distant sites. New lymphatic vessel growth is enhanced after tumor cell metastasis to regional lymph nodes. Tumor‐associated lymphangiogenesis within lymph nodes can positively mediate distant lymph nodes and/or distant organ metastasis.
The initial observation of intranodal lymphangiogenesis in experimental tumor models has led to the finding that cutaneous malignant melanomas in human patients induce tumor‐associated lymphangiogenesis within sentinel lymph nodes.( 46 ) Routine hematoxylin–eosin staining showed tumor metastases within sentinel lymph nodes (Fig. 4e), and double immunofluorescence staining using the monoclonal antibodies HMB‐45 for melanoma cells (Fig. 4f, red) and NZ‐1 for podoplanin (Fig. 4f, green) showed that such metastatic foci are closely associated with lymphatic vessels that surround tumors, and not adjacent to blood vessels (Fig. 4g, red), indicating a distinct role of tumor‐associated lymphatic vessels that actively grow (Fig. 4h) in lymph nodes.
Figure 4.
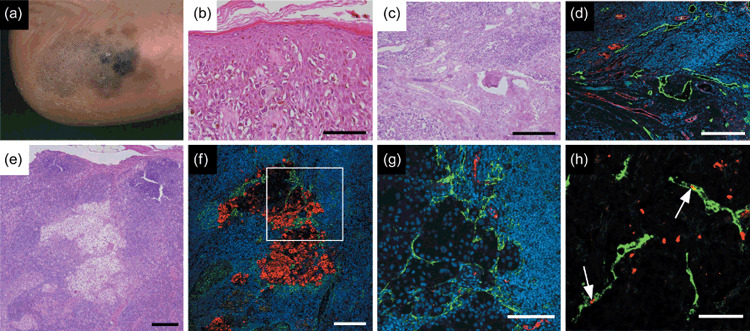
Tumor and nodal lymphangiogenesis in cutaneous malignant melanoma. (a) Macroscopic appearance of early malignant melanoma in heel. Pigmented macules with irregular outline and remarkable variation in color illustrate superficial melanocyte spread during tumor progression. (b) Rounded melanocytes with atypical, hyperchromatic nuclei and abundant cytoplasm are scattered in a pagetoid pattern throughout epidermis. (c) Dermal invasion of melanoma cells develops tumor nests surrounded by tumor‐associated stroma during vertical growth in malignant progression. (d) Double immunofluorescence staining with anti‐von Willebrand factor antibody (red) shows enlarged blood vasculature. Podoplanin staining (green) shows prominent lymphatic vessel growth in tumor‐associated tissue. Tumor lymphangiogenesis can actively promote sentinel lymph node metastasis by tumorigenic melanoma cells. (e) Regional lymph nodes with metastatic cutaneous malignant melanoma (hematoxylin–eosin staining). (f) Double immunofluorescence staining using HMB‐45, a melanoma‐specific marker (red) in the corresponding area indicates metastatic melanoma cells that are closely associated with tumor‐associated podoplanin‐positive lymphatic vessels (green). (g) Vasculature in the corresponding field of Fig. 4f visualized using anti‐von Willebrand factor antibody for blood vessels (red), and D2‐40 for lymphatic vessels (green). Striking enlargement of tumor‐associated lymphatic vasculature might be associated with further distant spread of tumors. (h) Podoplanin‐positive lymphatic vessels (green) within metastatic lymph nodes stained with Ki‐67 (red) indicate active lymphatic proliferation (arrows). Scale bars, 100 µm (b,g); 200 µm (c–f); 50 µm (h). Nuclei are stained blue (diethylenetriaminepentaacetic acid).
Lymphangiogenesis within sentinel lymph nodes induces enhanced metastasis within non‐sentinel/regional/axillary lymph nodes in patients with inflammatory breast cancer.( 47 ) However, the significance of tumor‐associated lymphangiogenesis within lymph nodes in distant metastases remains unclear, particularly in patients with cancer. Therefore, further investigation should address the fundamental question of whether sentinel and/or regional lymph nodes positively mediate distant lymph node and/or distant organ metastasis by elucidating a subset of human malignancies.
Intranodal lymphangiogenesis occurs prior to tumor metastasis
Experimental carcinogenesis studies have indicated that primary tumors induce new lymphatic vessel growth within draining lymph nodes before they metastasize.( 38 , 45 ) This finding is of particular importance because it means that primary tumors can actively modify future metastatic sites for preferential relocation. The ‘seed and soil’ hypothesis proposed by the English surgeon Stephan Paget in 1889 suggested that specific tumors (seeds) have preferential sites for metastasis (soil).( 48 ) This concept has become widely accepted, in particular, with respect to understanding specific microenvironments for cancer metastasis. However, primary tumors seem to spontaneously alter draining lymph nodes by inducing lymphangiogenesis. This enlargement of the lymphatic endothelium likely promotes efficient transport and migration of metastatic tumor cells within lymph nodes. Therefore, lymph nodes in terms of clinical relevance could serve not only as an initial indicator of tumor metastasis, but also as mediators of lymphatic metastasis.
Lymphvascular niche: A potential microenvironment for lymph node metastasis
Lymphatic vessels can grow within lymph nodes, and newly‐formed lymphatic endothelium probably promotes the lymphatic spread of tumors. However, it is still unclear whether nodal lymphangiogenesis alters the site‐specific microenvironment in lymph nodes from that of an intrinsic immune system against tumor metastasis to a preferential site for the expansion of metastasis. Recent studies on cancer biology directed much attention to cancer stem cells that represent tumorigenic and stem‐cell like properties in primary and metastatic tumors.( 49 , 50 , 51 , 52 ) A cancer stem cell that possesses the capacity to self‐renew within a tumor is capable of causing the heterogeneous lineages of cancer cells that comprise the tumor.( 52 ) Importantly, cancer stem cells have been identified in leukemia and several types of human solid tumors including malignant melanoma.( 53 ) In contrast however, it remains unclear whether the tumor‐associated microenvironment might serve a niche that allows growth and survival of cancer stem cells. Therefore, to better understand the specific microenvironment, particularly within lymph nodes with regard to tumor metastasis, the novel concept of a ‘lymphvascular niche’ that potentially promotes the retention and growth of cancer stem cells with metastatic potential is required, and presented herein (Fig. 5).
Figure 5.
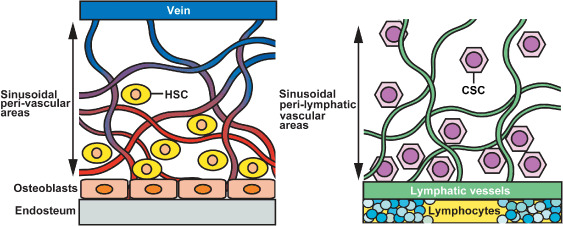
Specific microenvironment lymphyascular niche in lymph nodes during tumor metastasis. Bone marrow vascular niche (left), comprising specialized sinusoidal endothelium of venous origin (purple), forms a microenvironment for colonization with and maintenance of hematopoietic stem cells (HSCs). In contrast, tumor‐associated lymphangiogenesis within lymph nodes has induced remarkable enlargement of lymphatic network (right, green) composed of sinusoidal lymphatic endothelium. This specific microenvironment ‘lymphvascular niche’ might contribute to serve as a sanctuary for residence and survival of metastatic cancer stem cells in lymph nodes. CSC, cancer stem cell.
Lymphatic vessels in the skin originate as blunt‐ended thin‐walled capillaries without continuous vascular basement membranes. This anatomical feature enables lymphatic capillaries to absorb interstitial tissue fluid, macromolecules, and immune cells under physiological conditions, but also allows cancer cells to invade lymphatic vessels associated with tumors. The lymphatic endothelium of lymph nodes is composed of ‘sinusoids’ that do not have abundant extracellular matrix components, resulting in close contact with parenchymal cells. Increasing evidence, particularly from stem cell biology, indicates that sinusoidal endothelial cells in the bone marrow form a specialized microenvironment for the maintenance of hematopoietic stem cells (HSCs), called the vascular niche.( 54 , 55 , 56 ) HSCs are located in sinusoidal perivascular areas, or in the trabecular endosteum of adult bone marrow (Fig. 5). The structure of the vascular niche consists of a network of thin‐walled and fenestrated sinusoidal vessels that allow cells in the venous circulation to extravasate into hematopoietic tissues. The bone marrow vascular niche is thought to support HSC development,( 57 ) proliferation, and mobilization to the peripheral blood circulation,( 58 ) whereas the endosteal zone (osteoblastic niche) maintains HSC quiescence.( 58 ) The structural similarity between the vascular niche in the bone marrow and the lymphvascular niche in the lymph nodes suggests that the lymphvascular niche serves as a location where metastatic cancer stem cells can be retained and/or survive. Furthermore, recent studies have shown that the bone marrow vascular niche secretes high levels of CXC chemokine ligand (CXCL) 12,( 59 ) a ligand for the chemokine receptor CXCR4 that is expressed by HSCs and subsequently required for colonization in bone marrow.( 60 ) Importantly, lymph nodes produce abundant CXCL12, whereas metastatic breast cancer cells express CXCR4,( 61 ) indicating that a CXCL12‐rich micoenvironment in lymph nodes might contribute to the formation of a lymphvascular niche that attracts and maintains a subset of cancer stem cells that potently initiates a new tumor in distant sites. Moreover, perivascular sites during tumor progression recruit myelomonocytic VEGFR‐1+ cells derived from bone marrow.( 62 ) This non‐neoplastic cell population forms premetastatic niches in distant organs, thus promoting the establishment of tumor metastasis in mice and in patients with cancer.( 63 ) Brain tumor stem cells that are rare fractions of tumor‐initiating nestin+CD133+ cells reside adjacent to blood capillaries composing a perivascular niche, leading to accelerated tumor formation.( 64 ) Thus, increasing evidence about the vascular niche and tumor metastasis has facilitated further investigations of the precise contribution of the lymphvascular niche in the formation of lymph node metastasis.
Conclusions
The identification of tumor‐associated lymphangiogenesis within lymph nodes will lead to elucidation of its potential role in human cancers, understanding of the molecular mechanisms of the lymphvascular niche in promoting lymph node metastasis, and the development of therapeutic approaches to prevent lymph node metastasis. Understanding of the lymphatic vessels and their potential role in tumor metastasis has historically been hampered by technological difficulties. However, the recent identification of lymphatic vessel‐specific markers and growth factors has widened the scope of research and illuminated not only the development and physiological functions of the lymphatic vessels, but also the molecular mechanisms of tumor‐associated lymphatic vessel growth. Furthermore, several studies revealed that previously known angiogenesis factors such as platelet‐derived growth factor‐BB,( 65 ) hepatocyte growth factor,( 66 ) angiopoietins,( 67 , 68 ) and insulin‐like growth factors 1 and 2( 69 ) are able to induce lymphangiogenesis in experimental murine models. Moreover, transforming growth factor‐β has been shown as a potent inhibitor of lymphangiogenesis notably in experimental cancer models,( 70 ) indicating that multiple growth factor–receptor pathways are extensively involved in physiological and pathological lymphatic vessel growth. Importantly, tumor‐associated lymphangiogenesis actively promotes sentinel lymph node metastasis in several human cancers. Lymphangiogenesis in lymph nodes might positively mediate distant lymph node metastasis. Therefore, studies particularly focusing on genes expressed in sinusoidal lymphatic endothelial cells might further reveal the functional roles of the lymphatic endothelium in tumor metastasis.
Pathological lymphatic vessel growth, including tumor lymphangiogenesis, is closely associated with tissue inflammation. Macrophages potently induce lymphangiogenesis by secreting VEGF‐C. However, numerous types of immune cells such as lymphocytes are deployed in inflammatory tissue responses. Thus, other cell types or factors might induce lymphangiogenesis not only in inflamed tissue, but also in tumor microenvironments. Cell–cell interaction is notably active in tumor invasion and/or metastasis. Based on the finding that a sinusoidal lymphatic network exists in lymph nodes during the metastatic process, it is proposed that the lymphvascular niche, described herein, is a specific location that potentially maintains tumor cell residence and expansion, resulting in their systemic spread.
Presence of distant lymph node and/or distant organ metastases presents a therapeutic limitation. Thus, lymph node lymphangiogenesis could be a crucial mechanism that specifies two clinical categories of cancer metastasis: one at an early stage in which surgical resection of regional lymph nodes and primary site can aim to cure with complete remission; and another at an advanced stage in which treatment can simply be aimed at prolonging life. Therefore, this new concept could have considerable impact on understating the molecular mechanism of lymph node metastasis, and might change therapeutic approaches to cancer metastasis.
Acknowledgments
The author thanks Dr Michael Detmar for his critical reading and comments of the manuscript, and for providing materials and photographs on mouse skin carcinogenesis studies. The author is grateful to Dr Koji Hashimoto for his comprehensive organization of the department and continuous support of our research projects, to Dr Shigeki Higashiyama for his expert advice regarding the manuscript, and to Dr Hisayo Fukuda and Dr Hidenori Okazaki for their helpful discussions and technical assistance. A rat monoclonal antibody NZ‐1 was kindly provided by Dr Yukinari Kato. This study was supported by a Grant‐in‐Aid for Scientific Research on Priority Areas (MEXT20013032) and a Grant‐in‐Aid for Scientific Research (C20591348), Ministry of Education, Culture, Sports, Science and Technology.
References
- 1. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature 2005; 438: 946–53. [DOI] [PubMed] [Google Scholar]
- 2. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med 1971; 285: 1182–6. [DOI] [PubMed] [Google Scholar]
- 3. Folkman J. Clinical applications of research on angiogenesis. N Engl J Med 1995; 333: 1757–62. [DOI] [PubMed] [Google Scholar]
- 4. Karpanen T, Egeblad M, Karkkainen MJ et al . Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res 2001; 61: 1786–90. [PubMed] [Google Scholar]
- 5. Mandriota SJ, Jussila L, Jeltsch M et al . Vascular endothelial growth factor‐C‐mediated lymphangiogenesis promotes tumour metastasis. EMBO J 2001; 20: 672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skobe M, Hawighorst T, Jackson DG et al . Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 7. Stacker SA, Caesar C, Baldwin ME et al . VEGF‐D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001; 7: 186–91. [DOI] [PubMed] [Google Scholar]
- 8. Oliver G, Sosa‐Pineda B, Geisendorf S, Spana EP, Doe CQ, Gruss P. Prox 1, a prospero‐related homeobox gene expressed during mouse development. Mech Dev 1993; 44: 3–16. [DOI] [PubMed] [Google Scholar]
- 9. Rishi AK, Joyce‐Brady M, Fisher J et al . Cloning, characterization, and developmental expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev Biol 1995; 167: 294–306. [DOI] [PubMed] [Google Scholar]
- 10. Banerji S, Ni J, Wang SX et al . LYVE‐1, a new homologue of the CD44 glycoprotein, is a lymph‐specific receptor for hyaluronan. J Cell Biol 1999; 144: 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pajusola K, Aprelikova O, Korhonen J et al . FLT4 receptor tyrosine kinase contains seven immunoglobulin‐like loops and is expressed in multiple human tissues and cell lines. Cancer Res 1992; 52: 5738–43. [PubMed] [Google Scholar]
- 12. Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 1999; 98: 769–78. [DOI] [PubMed] [Google Scholar]
- 13. Johnson NC, Dillard ME, Baluk P et al . Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 2008; 22: 3282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srinivasan RS, Dillard ME, Lagutin OV et al . Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 2007; 21: 2422–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Francois M, Caprini A, Hosking B et al . Sox18 induces development of the lymphatic vasculature in mice. Nature 2008; 456: 643–7. [DOI] [PubMed] [Google Scholar]
- 16. Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M. Up‐regulation of the lymphatic marker podoplanin, a mucin‐type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol 2005; 166: 913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kato Y, Kaneko MK, Kuno A et al . Inhibition of tumor cell‐induced platelet aggregation using a novel anti‐podoplanin antibody reacting with its platelet‐aggregation‐stimulating domain. Biochem Biophys Res Commun 2006; 349: 1301–7. [DOI] [PubMed] [Google Scholar]
- 18. Tammela T, Saaristo A, Holopainen T et al . Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nat Med 2007; 13: 1458–66. [DOI] [PubMed] [Google Scholar]
- 19. Oliver G, Detmar M. The rediscovery of the lymphatic system. Old and new insights into the development and biological function of the lymphatic vascular system. Genes Dev 2002; 16: 773–83. [DOI] [PubMed] [Google Scholar]
- 20. Shibuya M, Yamaguchi S, Yamane A et al . Nucleotide sequence and expression of a novel human receptor‐type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 1990; 5: 519–24. [PubMed] [Google Scholar]
- 21. Joukov V, Pajusola K, Kaipainen A et al . A novel vascular endothelial growth factor, VEGF‐C, is a ligand for the Flt4 (VEGFR‐3) and KDR (VEGFR‐2) receptor tyrosine kinases. EMBO J 1996; 15: 1751. [PMC free article] [PubMed] [Google Scholar]
- 22. Achen MG, Jeltsch M, Kukk E et al . Vascular endothelial growth factor D (VEGF‐D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci USA 1998; 95: 548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jeltsch M, Kaipainen A, Joukov V et al . Hyperplasia of lymphatic vessels in VEGF‐C transgenic mice. Science 1997; 276: 1423–5. [DOI] [PubMed] [Google Scholar]
- 24. Veikkola T, Jussila L, Makinen T et al . Signalling via vascular endothelial growth factor receptor‐3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J 2001; 20: 1223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He Y, Kozaki K, Karpanen T et al . Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst 2002; 94: 819–25. [DOI] [PubMed] [Google Scholar]
- 26. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi‐kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA‐MB‐231 via inhibition of vascular endothelial growth factor‐receptor (VEGF‐R) 2 and VEGF‐R3 kinase. Clin Cancer Res 2008; 14: 5459–65. [DOI] [PubMed] [Google Scholar]
- 27. Shimizu K, Kubo H, Yamaguchi K et al . Suppression of VEGFR‐3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci 2004; 95: 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burton JB, Priceman SJ, Sung JL et al . Suppression of prostate cancer nodal and systemic metastasis by blockade of the lymphangiogenic axis. Cancer Res 2008; 68: 7828–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iwata C, Kano MR, Komuro A et al . Inhibition of cyclooxygenase‐2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res 2007; 67: 10181–9. [DOI] [PubMed] [Google Scholar]
- 30. Caunt M, Mak J, Liang WC et al . Blocking neuropilin‐2 function inhibits tumor cell metastasis. Cancer Cell 2008; 13: 331–42. [DOI] [PubMed] [Google Scholar]
- 31. Dadras SS, Paul T, Bertoncini J et al . Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol 2003; 162: 1951–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Makinen T, Jussila L, Veikkola T et al . Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor‐3. Nat Med 2001; 7: 199–205. [DOI] [PubMed] [Google Scholar]
- 33. Kriehuber E, Breiteneder GS, Groeger M et al . Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J Exp Med 2001; 194: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Petrova TV, Makinen T, Makela TP et al . Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox‐1 homeobox transcription factor. EMBO J 2002; 21: 4593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirakawa S, Hong YK, Harvey N et al . Identification of vascular lineage‐specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol 2003; 162: 575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nagy JA, Vasile E, Feng D et al . Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med 2002; 196: 1497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Detmar M, Hirakawa S. The formation of lymphatic vessels and its importance in the setting of malignancy. J Exp Med 2002; 6: 713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF‐A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med 2005; 201: 1089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bjorndahl MA, Cao R, Burton JB et al . Vascular endothelial growth factor‐a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res 2005; 65: 9261–8. [DOI] [PubMed] [Google Scholar]
- 40. Kunstfeld R, Hirakawa S, Hong YK et al . VEGF‐A plays a key role in the induction of chronic inflammation and the associated lymphangiogenic response. Blood 2004; 104: 1048–57. [DOI] [PubMed] [Google Scholar]
- 41. Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor‐A mediates ultraviolet B‐induced impairment of lymphatic vessel function. Am J Pathol 2006; 169: 1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shibata MA, Morimoto J, Shibata E, Otsuki Y. Combination therapy with short interfering RNA vectors against VEGF‐C and VEGF‐A suppresses lymph node and lung metastasis in a mouse immunocompetent mammary cancer model. Cancer Gene Ther 2008; 15: 776–86. [DOI] [PubMed] [Google Scholar]
- 43. Cursiefen C, Chen L, Borges LP et al . VEGF‐A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest 2004; 113: 1040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jeon BH, Jang C, Han J et al . Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res 2008; 68: 1100–9. [DOI] [PubMed] [Google Scholar]
- 45. Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF‐C‐induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007; 109: 1010–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dadras SS, Lange‐Asschenfeldt B, Velasco P et al . Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol 2005; 18: 1232–42. [DOI] [PubMed] [Google Scholar]
- 47. Van den Eynden GG, Vandenberghe MK, Van Dam PJ et al . Increased sentinel lymph node lymphangiogenesis is associated with nonsentinel axillary lymph node involvement in breast cancer patients with a positive sentinel node. Clin Cancer Res 2007; 13: 5391–7. [DOI] [PubMed] [Google Scholar]
- 48. Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889; 133: 571–3. [PubMed] [Google Scholar]
- 49. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med 2006; 355: 1253–61. [DOI] [PubMed] [Google Scholar]
- 50. Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med 2008; 359: 2814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease. J Cell Mol Med 2008; 12: 374–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Clarke MF, Dick JE, Dirks PB et al . Cancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006; 66: 9339–44. [DOI] [PubMed] [Google Scholar]
- 53. Schatton T, Murphy GF, Frank NY et al . Identification of cells initiating human melanomas. Nature 2008; 451: 345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arai F, Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann NY Acad Sci 2007; 1106: 41–53. [DOI] [PubMed] [Google Scholar]
- 55. Kopp HG, Avecilla ST, Hooper AT, Rafii S. The bone marrow vascular niche: home of HSC differentiation and mobilization. Physiology (Bethesda) 2005; 20: 349–56. [DOI] [PubMed] [Google Scholar]
- 56. Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature 2005; 438: 937–45. [DOI] [PubMed] [Google Scholar]
- 57. Takakura N, Huang XL, Naruse T et al . Critical role of the TIE2 endothelial cell receptor in the development of definitive hematopoiesis. Immunity 1998; 9: 677–86. [DOI] [PubMed] [Google Scholar]
- 58. Arai F, Hirao A, Ohmura M et al . Tie2/angiopoietin‐1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 2004; 118: 149–61. [DOI] [PubMed] [Google Scholar]
- 59. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12–CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006; 25: 977–88. [DOI] [PubMed] [Google Scholar]
- 60. Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. Long‐term hematopoietic stem cells require stromal cell‐derived factor‐1 for colonizing bone marrow during ontogeny. Immunity 2003; 19: 257–67. [DOI] [PubMed] [Google Scholar]
- 61. Muller A, Homey B, Soto H et al . Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410: 50–6. [DOI] [PubMed] [Google Scholar]
- 62. Lyden D, Hattori K, Dias S et al . Impaired recruitment bone‐marrow‐derived endothelial hematopoietic precursor cells blocks tumor angiogenesis growth. Nat Med 2001; 7: 1194–201. [DOI] [PubMed] [Google Scholar]
- 63. Kaplan RN, Riba RD, Zacharoulis S et al . VEGFR1‐positive haematopoietic bone marrow progenitors initiate the pre‐metastatic niche. Nature 2005; 438: 820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Calabrese C, Poppleton H, Kocak M et al . A perivascular niche for brain tumor stem cells. Cancer Cell 2007; 11: 69–82. [DOI] [PubMed] [Google Scholar]
- 65. Cao R, Bjorndahl MA, Religa P et al . PDGF‐BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 2004; 6: 333–45. [DOI] [PubMed] [Google Scholar]
- 66. Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J 2005; 24: 2885–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gale NW, Thurston G, Hackett SF et al . Angiopoietin‐2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by angiopoietin‐1. Dev Cell 2002; 3: 411–23. [DOI] [PubMed] [Google Scholar]
- 68. Morisada T, Oike Y, Yamada Y et al . Angiopoietin‐1 promotes LYVE‐1‐positive lymphatic vessel formation. Blood 2005; 105: 4649–56. [DOI] [PubMed] [Google Scholar]
- 69. Bjorndahl M, Cao R, Nissen LJ et al . Insulin‐like growth factors 1 and 2 induce lymphangiogenesis in vivo . Proc Natl Acad Sci USA 2005; 102: 15593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Oka M, Iwata C, Suzuki HI et al . Inhibition of endogenous TGF‐β signaling enhances lymphangiogenesis. Blood 2008; 111: 4571–9. [DOI] [PubMed] [Google Scholar]


