Abstract
Because stroma in tumor tissues can promote prostate cancer development, modulation of tumor–stromal cell interactions may represent an attractive new strategy for cancer treatment. Here, we report that phthoxazolin A and its analog inthomycin B inhibit the growth of human prostate cancer DU‐145 cells by modulating tumor–stromal cell interactions. Using an in vitro coculture system, in which prostate cancer cell growth is upregulated by prostate stromal cells (PrSC), we found that phthoxazolin A and inthomycin B strongly inhibited the growth of DU‐145 cells when in coculture with PrSC compared to DU‐145 cells cultured alone. Although PrSC consist of both fibroblasts and myofibroblasts, phthoxazolin A and inthomycin B inhibited the expression of smooth muscle α‐actin, a myofibroblast marker, without affecting vimentin and β‐actin expression. Because myofibroblasts secrete various factors that can promote tumor cell growth, we examined whether the inhibitory compounds affected the secretion of such factors from PrSC. Proteomic analysis and reverse transcription–polymerase chain reaction revealed that phthoxazolin A and inthomycin B inhibited the expression of several insulin‐like growth factor binding proteins and insulin‐like growth factor (IGF)‐I by PrSC. Transforming growth factor‐β1 increased myofibroblast numbers and IGF‐I levels in PrSC. Phthoxazolin A inhibited transforming growth factor‐β1 activity without altering phosphorylation of the downstream molecule smad2. Furthermore, conditioned medium from phthoxazolin A‐treated PrSC failed to increase the phosphorylation of IGF‐IR and Akt in DU‐145 cells. Taken together, our results suggested that phthoxazolin A acts as a small‐molecule modulator of tumor–stromal cell interactions that can indirectly suppress prostate cancer cell growth through inhibition of IGF‐I production by PrSC. (Cancer Sci 2009; 100: 150–157)
There is increasing evidence that the stroma is involved in the growth and metastasis of several cancers, including colorectal,( 1 ) breast,( 2 , 3 ) pancreatic,( 4 ) and prostate cancer.( 5 ) The stroma consists of a variety of components, including fibroblasts, macrophages, endothelial cells, and extracellular matrix.( 6 , 7 ) Of these, the fibroblasts play a critical role in the regulation of tumor growth. Although certain types of fibroblasts appear to enhance tumor growth, others suppress it.( 2 , 8 , 9 , 10 ) Fibroblasts that positively modulate tumor growth are called cancer‐associated fibroblasts or reactive stroma,( 11 , 12 ) and are distinct from normal fibroblasts as they express both vimentin and smooth muscle (SM) α‐actin, indicating a myofibroblast phenotype.( 7 ) Such cells secrete various factors favorable for tumor cell growth, such as growth factors, cytokines, and adhesion molecules.( 7 , 12 ) Thus, tumor–stromal cell interactions can promote tumor growth through secreted factors and cell–cell adhesion.( 9 , 10 , 12 , 13 )
Various lines of evidence have suggested the involvement of the insulin‐like growth factor (IGF) axis in prostate cancer development.( 14 ) We previously reported that prostate stromal cells (PrSC) secrete IGF‐I that promotes the growth of prostate cancer cells.( 15 ) Transforming growth factor (TGF)‐β is a well‐known inducer of myofibroblasts that stimulates fibroblasts to differentiate into myofibroblasts.( 16 , 17 ) TGF‐β has multiple functions and can either enhance or suppress tumor growth.( 18 , 19 , 20 , 21 ) We recently found that TGF‐β1 increased the expression of IGF‐I and IGF binding protein (IGFBP)‐3, a negative regulator of IGF‐I, such that the balance between the levels of these proteins is critical to the regulation of prostate cancer growth.( 22 )
Because stromal cells can regulate tumor development positively or negatively, the modulation of tumor–stromal cell interactions could be an attractive new strategy for the treatment of cancer. For instance, reports show that hepatocyte growth factor (HGF) acts as a mediator in tumor–stromal cell interactions and that an HGF antagonist, NK4, suppresses some cancers.( 23 , 24 ) We previously found that interleukin (IL)‐1β significantly inhibited the growth of human prostate cancer LNCaP cells during coculture with stromal cells.( 25 ) Mechanistic experiments revealed that IL‐1β‐stimulated stromal cells secreted IL‐6, and that the synergistic action of IL‐1β and IL‐6 induced cancer cell apoptosis.( 25 , 26 ) Thus, our findings and studies by others have led to the hypothesis that small molecules that modulate tumor–stromal cell interactions could act as novel anticancer drugs. To explore this hypothesis, we used an in vitro coculture system of prostate cancer cells and PrSC, designed to mimic the characteristics of tumors in vivo.( 15 , 27 ) Using this system, we searched for small molecules that suppressed prostate cancer growth via the modulation of tumor–stromal cell interactions. We have previously reported that phthoxazolin A, a small natural compound, suppressed prostate cancer cell growth more strongly when cancer cells were cocultured with PrSC than when cultured alone.( 27 ) However, the precise mechanisms underlying this finding remained unclear. Here, we report that phthoxazolin A suppressed the growth of prostate cancer cells in coculture with PrSC indirectly by inhibiting IGF‐I production by PrSC. Thus, our results suggest that phthoxazolin A acts as a small‐molecule modulator of tumor–stromal cell interactions in prostate cancer.
Materials and Methods
Reagents. Rhodanile blue was purchased from Aldrich (Milwaukee, WI, USA). Insulin, hydrocortisone, and SB431542 were obtained from Sigma (St Louis, MO, USA). Transferrin was obtained from Wako Pure Chemical Industries (Tokyo, Japan). Recombinant human basic fibroblast growth factor (bFGF), recombinant human IGF‐I, and recombinant human TGF‐β1 were purchased from Pepro Tech (London, UK). The antibodies used were: anti‐vimentin, anti‐SM α‐actin, anti‐β‐actin, and anti‐vinculin (Sigma); anti‐smad2, anti‐phospho‐smad2, anti‐smad4, anti‐Akt, and anti‐phospho‐Akt (Thr308) (Cell Signaling Technology, Danvers, MA, USA); anti‐IGF‐IRβ (sc‐713) (Santa Cruz Biotechnology, Santa Cruz, CA, USA); and anti‐IGFBP‐5 (06‐110) and anti‐phosphotyrosine (05‐321) (Upstate Biotechnology, Lake Placid, NY, USA). Phthoxazolin A and inthomycin B were purified from microbial cultured broth in our laboratory.
Cells. The human prostate cancer cell line DU‐145 was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; ICN Biomedicals, Aurora, OH, USA), 100 U/mL penicillin G, and 100 µg/mL streptomycin at 37°C with 5% CO2. Human normal PrSC were obtained from Bio Whittaker (Walkersville, MD, USA) and maintained in DMEM supplemented with 10% FBS, 100 U/mL penicillin G, 100 µg/mL streptomycin, ITH (5 µg/mL insulin, 5 µg/mL transferrin, and 1.4 µmol/L hydrocortisone), and 5 ng/mL bFGF at 37°C with 5% CO2.
Coculture experiments. A microplate assay method for the selective measurement of epithelial tumor cells in coculture with stromal cells using rhodanile blue dye was carried out as described previously.( 27 ) PrSC were first inoculated into 96‐well plates at 5000 cells per well in 100 µL DMEM supplemented with ITH and 0.1% FBS in the presence of various concentrations of test compounds. After 2 days, 10 µL aliquots of DU‐145 cell suspension (5000 cells) in serum‐free DMEM were inoculated onto monolayers of PrSC, and cells cultured for a further 3 days. For monoculture of DU‐145 cells, assay medium alone was first incubated in the presence of test compounds for 2 days at 37°C, DU‐145 cells were inoculated as described above, and then cultured for a further 3 days.
For the conditioned medium (CM) assay, PrSC were cultured with ITH and 0.1% FBS in the presence of various concentrations of test compounds for the indicated times as described above. CM was then transferred into new 96‐well plates, and 10‐µL aliquots of DU‐145 cell suspension (5000 cells) in serum‐free DMEM were inoculated and cultured for 3 days. Growth of DU‐145 cells was determined using 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium (MTT; Sigma) as described previously.( 28 )
Immunofluorescence. PrSC (1 × 105) were cultured on glass coverslips in DMEM supplemented with ITH and 0.1% FBS in the presence of various concentrations of test compounds for 3 days. The cells were then fixed with cold acetone and incubated with anti‐vimentin (IgM), followed by anti‐IgM Alexa Flour 488 (Molecular Probes, Eugene, OR, USA). Fixed cells were further incubated with anti‐SM α‐actin (IgG2a), followed by anti‐IgG2a Alexa Flour 546 (Molecular Probes) as described previously.( 22 )
Flow cytometry. PrSC (6 × 105) were cultured in DMEM supplemented with ITH and 0.1% FBS in the presence of various concentrations of test compounds for 3 days. Cells were then detached from the plastic culture plates using 0.2% ethylenediaminetetraacetic acid (EDTA)–phosphate‐buffered saline (PBS) and fixed with 1% formaldehyde–PBS for 15 min at 4°C. Cells were washed with 0.1% NaN3–1% FBS–PBS and incubated with anti‐vimentin or control IgM for 1 h at 4°C. Cells were washed and further incubated with anti‐IgM Alexa Flour 488 for 45 min at 4°C. After washing, the cells were analyzed using a flow cytometer (FACSCalibur; BD, Franklin Lakes, NJ, USA).
Preparation of cell lysates and western blotting. PrSC (3 × 105 cells) were cultured in DMEM supplemented with ITH and 0.1% FBS in the presence of various concentrations of test compounds for the indicated times. For preparation of PrSC‐CM, PrSC (3 × 105) were cultured in DMEM supplemented with 0.1% dialyzed FBS in the presence of various concentrations of test compounds for 2 days. DU‐145 cells (5 × 105) were cultured in serum‐free DMEM overnight and treated with PrSC‐CM for 30 min at 37°C. Cell lysates were prepared and used for western blotting with antibodies as described previously.( 29 )
Reverse transcription–polymerase chain reaction analysis. PrSC (2.5 × 105) or DU‐145 cells (2.5 × 105) were cultured in DMEM supplemented with ITH and 0.1% FBS in the presence of various concentrations of test compounds for the indicated times. Total RNA was isolated using the RNeasy Minikit (Qiagen, Hilden, Germany). cDNA was synthesized using avian myeloblastosis virus reverse transcriptase (Promega, Madison, WI, USA) from 1 µg aliquots of RNA and amplified using Taq DNA polymerase (Promega). The specific primers used were: SM α‐actin (590 bp), 5′‐GCTCACGGAGGCACCCCTGA‐3′ (sense) and 5′‐CTGATAGGACATTGTTAGCAT‐3′ (antisense); TGF‐β1 (352 bp), 5′‐TGGAAACCCACAACGAAATC‐3′ (sense) and 5′‐TTATCCCTGCTGTCACAGGAG‐3′ (antisense); TGF‐β2 (686 bp), 5′‐GCAGAACCCAAAAGCCAGAG‐3′ (sense) and 5′‐GGACACGCAGCAAGGAGAAG‐3′ (antisense); and other primers as reported elsewhere.( 15 ) Polymerase chain reactions were optimized for each set of primers using different numbers of cycles to ensure that amplification occurred in a linear range. After amplification, products were electrophoresed in 2% agarose gels, stained with SYBR Green I (Cambrex Bio Science, Rockland, ME, USA), and analyzed with a FLA‐5000 image analyzer (Fujifilm, Tokyo, Japan).
Dye exclusion assay. To enrich the myofibroblast population in PrSC, PrSC (5 × 104) were precultured in DMEM supplemented with ITH and 0.1% FBS for 3 days as described previously.( 22 ) Cells were cultured for a further 3 days in the presence of various concentrations of test compounds, suspended using tyrpsin, and then incubated with an equal volume of 0.4% Trypan blue‐PBS. Cell viability (%) was calculated by dividing the number of dead cells (stained) by the total number of cells.
Two‐dimensional gel electrophoresis. PrSC (107) were cultured in 100 mL DMEM supplemented with ITH and 0.1% FBS in the presence or absence of phthoxazolin A for 3 days. Resultant CM were treated with 80% ammonium sulfate and the precipitates analyzed using a Multiphor II electrophoresis unit (GE Healthcare, Buckinghamshire, UK) according to the manufacturer's instructions. Gels were stained with Coomassie blue and altered protein spots were excised and digested with trypsin. The digested peptides were then applied to MALDI‐TOF MS analysis (APRO Life Science Institute, Tokushima, Japan).
Measurement of IGF‐I. PrSC (105) were cultured in 1 mL DMEM supplemented with ITH and 0.1% dialyzed FBS in the presence or absence of phthoxazolin A for 2 days. The cell culture supernatants were withdrawn, and the amounts of IGF‐I in the supernatants were determined using a human IGF‐I Qunatikine kit (R. & D. Systems, Minneapolis, MN, USA).
Statistical analysis. All data are representative of two or three independent experiments with similar results. Statistical analysis was carried out using Student's t‐test.
Results
Effect of phthoxazolin A on DU‐145 cell and PrSC cocultures. During screening for modulators of tumor–stromal cell interactions associated with prostate cancer, we found one microbial cultured broth that inhibited the growth of DU‐145 cells more strongly when grown in coculture with PrSC than when grown alone (i.e. as a monoculture). We purified the active components, which were identified as phthoxazolin A (MW 290)( 27 ) and inthomycin B (MW 290) (Fig. 1a). Phthoxazolin A was originally discovered as an inhibitor of cellulose biosynthesis.( 30 , 31 , 32 ) As shown in Figure 1a, phthoxazolin A showed greater growth inhibition of DU‐145 cells when the DU‐145 cell line was grown as a coculture with PrSC than as a monoculture, without any effect on PrSC growth. DU‐145 cells stained with rhodanile blue in coculture were significantly decreased by phthoxazolin A, but were not affected in monocultures (Fig. 1b). Inthomycin B, an analog of phthoxazolin A, showed similar effects as phthoxazolin A, but at slightly higher effective doses (Fig. 1a).
Figure 1.
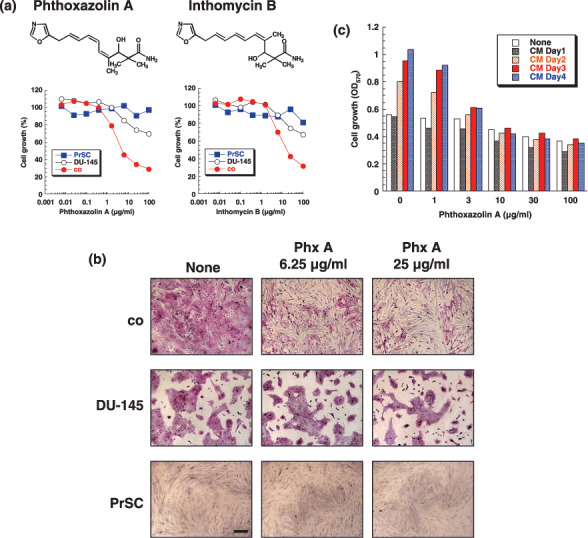
Effect of phthoxazolin A (Phx A) on cocultures of DU‐145 cells and prostate stromal cells (PrSC). (a) Effects of Phx A and inthomycin B on the growth of DU‐145 cells alone (open circles), DU‐145 cells co‐cultured with PrSC (closed red circles), or PrSC alone (closed blue squares) were determined using rhodanile blue staining. Values represent means of duplicate determinations (SE < 10%). (b) Representative photos of DU‐145 cells cocultured with PrSC (co), DU‐145 cells cultured alone (DU‐145), and PrSC cultured alone (PrSC) after staining with rhodanile blue. Cells were treated with the indicated concentrations of Phx A. Scale bar = 200 µm. (c) PrSC were precultured with Phx A for the indicated times. Cultured conditioned medium (CM) was collected, and DU‐145 cells were cultured in the CM for 3 days. Values represent means of duplicate determinations (SE < 10%). All data are representative of three independent experiments with similar results.
Conditioned medium prepared from PrSC increased the growth of DU‐145 cells, with the growth‐stimulatory effect positively correlated with the number of days the PrSC were cultured to prepare the CM (Fig. 1c). This growth‐stimulatory effect of the PrSC‐CM was diminished when the CM was passed through a membrane filter with a molecular cut‐off (MW 10 000) (data not shown). In contrast, CM prepared from phthoxazolin A‐treated PrSC lost the growth‐stimulatory effect (Fig. 1c). The effective concentrations of phthoxazolin A in Figure 1c correlated well with those in Figure 1a. Furthermore, addition of phthoxazolin A only into DU‐145 cell monocultures did not affect cell growth (Fig. 1c). These results suggested that phthoxazolin A inhibited the secretion of growth‐stimulatory factors from PrSC rather than having a direct effect on DU‐145 cells.
Phthoxazolin A inhibits myofibroblast differentiation of PrSC. Myofibroblasts have been reported to be associated with tumor progression through the secretion of various factors that favor tumor cell growth.( 7 , 12 ) Therefore, we examined the effect of phthoxazolin A on PrSC myofibroblast differentiation. As we reported recently,( 22 ) PrSC are a mixture of myofibroblasts expressing both vimentin and SM α‐actin, and fibroblasts expressing vimentin but not SM α‐actin (Fig. 2a). When PrSC were treated with phthoxazolin A for 3 days, the proportion of cells expressing both vimentin and SM α‐actin was found to be significantly decreased (Fig. 2a). Phthoxazolin A inhibited the expression of SM α‐actin without affecting β‐actin and vimentin expression (Fig. 2b,c). These results indicated that phthoxazolin A decreased the proportion of myofibroblasts within PrSC. Inthomycin B also inhibited the expression of SM α‐actin, but at higher doses than required for phthoxazolin A (Fig. 2).
Figure 2.
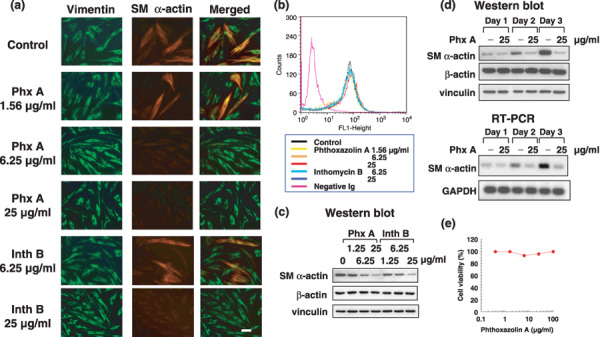
Effects of phthoxazolin A (Phx A) and inthomycin B (Inth B) on myofibroblast differentiation. (a) Prostate stromal cells (PrSC) were cultured with the indicated concentrations of test compounds for 3 days. The presence of myofibroblasts was evaluated by immunofluorescence using anti‐vimentin (green) and anti‐smooth muscle (SM) α‐actin (red) antibodies. Photos are representative immunofluorescence results of three independent experiments with similar results. Scale bar = 50 µm. (b) PrSC were cultured with test compounds for 3 days and the expression of vimentin was analyzed by flow cytometry. (c) PrSC were cultured with Phx A and Inth B for 3 days. Cell lysates were prepared and used for western blotting. (d) PrSC were cultured with 25 µg/mL Phx A for the indicated times. Cell lysates and total RNA were prepared and used for western blotting and reverse transcription–polymerase chain reaction (RT‐PCR), respectively. (e) PrSC were precultured for 3 days and then further cultured with the Phx A for 3 days. Cell viability was assessed using trypan blue. Values represent means of duplicate determinations (SE < 10%). All data are representative of three independent experiments with similar results. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
Under our experimental conditions, PrSC time‐dependently increased the expression of SM α‐actin, which demonstrated that PrSC fibroblasts gradually differentiated into myofibroblasts (Fig. 2d). Phthoxazolin A inhibited this increased SM α‐actin expression at the mRNA level (Fig. 2d). Pthoxazolin A also decreased the expression of SM α‐actin that was already elevated in myofibroblast‐enriched PrSC (Supporting Fig. S1). Furthermore, phthoxazolin A did not affect the viability of myofibroblast‐enriched PrSC (Fig. 2e), which indicated that phthoxazolin A did not show specific cytotoxicity against myofibroblasts; rather, phthoxazolin A suppressed the myofibroblast differentiation of PrSC.
Phthoxazolin A affects the expression of IGF axis molecules. We next examined the effect of phthoxazolin A on secreted proteins from PrSC. PrSC were treated with or without phthoxazolin A for 3 days. The CM was then screened for proteins that showed altered expression in response to phthoxazolin A treatment. Mass spectrum analysis results indicated that several proteins were affected by the phthoxazolin A treatment (Fig. 3a), and subsequent western blotting of PrSC‐CM confirmed that phthoxazolin A suppressed the secretion of IGFBP‐5 by PrSC (Fig. 3b).
Figure 3.
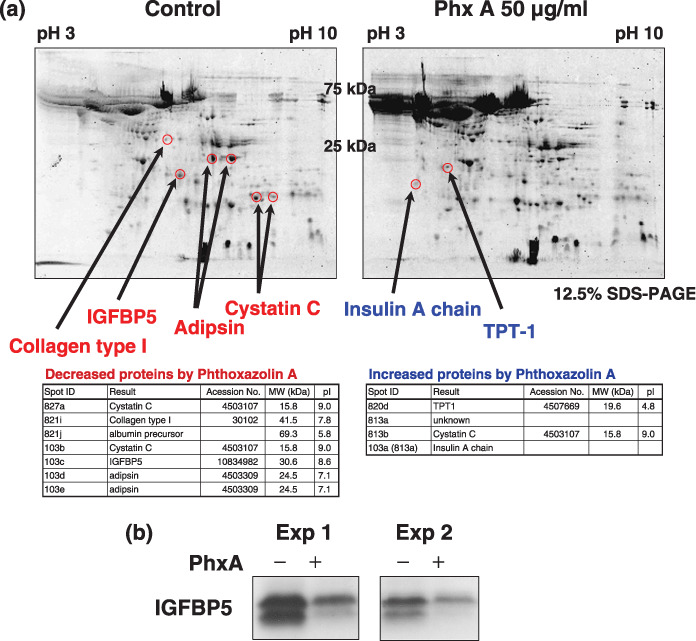
Two‐dimensional gel analysis of prostate stromal cell (PrSC)‐conditioned medium (CM). PrSC were cultured with 50 µg/mL phthoxazolin A (Phx A) for 3 days. Ammonium sulfate precipitates of the CM were analyzed by two‐dimensional gel analysis. (a) The representative photo of the gels and protein spots decreased by Phx A (red) and increased by Phx A (blue). (b) Ammonium sulfate precipitates of the CM were separated by sodium dodecylsulfate–polyacrylamide gel electrophoresis and immunoblotted with anti‐insulin‐like growth factor binding protein (IGFBP)‐5 antibodies. IGFBP‐5 protein was detected as a doublet of 33–35 kDa.( 38 ) TPT, tumor protein, translationally‐controlled.
As we have previously reported that the IGF axis plays a significant role in prostate cancer development through tumor–stromal cell interactions,( 15 ) we then examined the effect of phthoxazolin A on the expression of IGF axis molecules in PrSC. As shown in Figure 4a, in addition to IGFBP‐5, phthoxazolin A was found to decrease the expression of IGF‐1 and other IGFBP such as IGFBP‐2, ‐3, and ‐6. Of these, inhibition of IGF‐I expression by phthoxazolin A was the strongest, even after only 1 day of treatment (Fig. 4a,b). Inthomycin B also decreased the expression of IGF axis molecules, but at higher concentrations than required for phthoxazolin A (Fig. 4b). Phthoxazolin A did not affect the expression of IGF axis molecules by DU‐145 cells even after 2 days of treatment (Fig. 4c). Thus, our results suggested that phthoxazolin A modulated the expression of IGF axis molecules in PrSC but not in prostate cancer cells.
Figure 4.
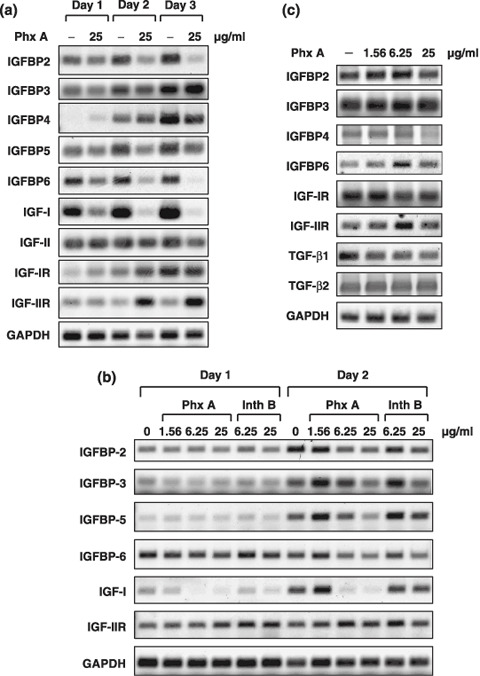
Effect of phthoxazolin A (Phx A) on the expression of insulin‐like growth factor (IGF) axis proteins in prostate stromal cells (PrSC) and DU‐145 cells. (a,b) PrSC were cultured with Phx A or inthomycin B (Inth B) for the indicated times. Total RNA was collected and reverse transcription–polymerase chain reaction (RT‐PCR) for the indicated molecules was carried out using specific primers. (c) DU‐145 cells were cultured with Phx A for 2 days and RT‐PCR analysis was carried out. All data are representative of two or three independent experiments with similar results. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; IGFBP, IGF binding protein; TGF, transforming growth factor.
Effect of phthoxazolin A on TGF‐β signaling. TGF‐β is a well‐known inducer of myofibroblasts( 16 , 17 ) and we recently reported that TGF‐β1 increased the expression of IGF‐I and IGFBP‐3.( 22 ) Therefore, we examined whether phthoxazolin A affected TGF‐β signaling in PrSC. As shown in Figure 5a, although TGF‐β1 increased the expression of SM α‐actin, IGF‐I, and IGFBP‐3, phthoxazolin A weakly suppressed the expression of these molecules compared with the strong inhibitory effect of SB431542, an inhibitor of TGF‐β receptor kinase.( 33 , 34 ) Western blotting revealed that TGF‐β1 increased the phosphorylation of smad2 in PrSC, whereas SB431542 completely inhibited this signal (Fig. 5b). In contrast, phthoxazolin A treatment had no effect on the phosphorylation of smad2 or expression of smad4, which indicated that phthoxazolin A did not directly inhibit TGF‐β receptor signaling (Fig. 5b). As DU‐145 cells express both TGF‐β1 and TGF‐β2,( 35 ) we examined the effect of phthoxazolin A on the expression of these molecules by DU‐145 cells. However, phthoxazolin A treatment failed to decrease TGF‐β1 and TGF‐β2 expression (Fig. 4c).
Figure 5.
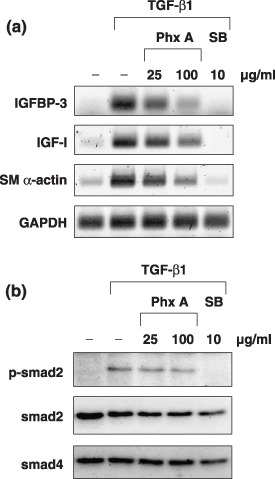
Effect of phthoxazolin A (Phx A) on transforming growth factor (TGF)‐β signaling. (a) Prostate stromal cells (PrSC) were cultured with Phx A or SB431542 (SB) in the presence of 10 ng/mL TGF‐β1 for 1 day. Total RNA was collected and reverse transcription–polymerase chain reaction for the indicated molecules was carried out using specific primers. (b) PrSC were pretreated with Phx A or SB for 30 min, and then treated with 10 ng/mL TGF‐β1 for 30 min. Cell lysates were prepared and used for western blotting. All data are representatives of three independent experiments with similar results. GAPDH, glyceraldehyde 3‐phosphate dehydrogenase; IGF, insulin‐like growth factor; IGFBP, IGF binding protein.
Inhibition of IGF‐1 action in PrSC‐CM by phthoxazolin A. Finally, we examined whether phthoxazolin A reduced IGF‐I activity in PrSC‐CM, as suggested by the downregulation of IGF axis molecules in Figure 4. When we measured the amounts of IGF‐I in PrSC‐CM, phthoxazolin A was found to decrease the secretion of IGF‐I from PrSC (Fig. 6a). We also assessed IGF‐I activity indirectly using a bioassay based on the activation of IGF‐IR in DU‐145 cells. Our results indicated that although PrSC‐CM increased the phosphorylation of IGF‐IR and Akt in DU‐145 cells, phthoxazolin A inhibited this increase (Fig. 6b). Furthermore, phthoxazolin A‐induced inhibition of DU‐145 cell growth in coculture with PrSC was recovered by the external addition of IGF‐I (Fig. 6c). Thus, it appeared that phthoxazolin A indirectly suppressed the growth of DU‐145 cells through the downregulation of IGF‐I activity.
Figure 6.
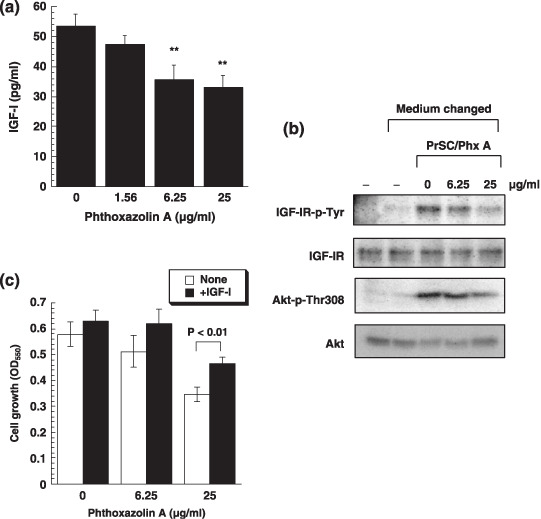
Inhibition of insulin‐like growth factor (IGF)‐I activity in prostate stromal cell (PrSC)‐conditioned medium (CM) by phthoxazolin A (Phx A). (a) PrSC were cultured with Phx A for 2 days and the amounts of IGF‐I in PrSC‐CM were measured. Values are means ± SD for triplicate determinations. **p<0.02 versus control. (b) CM was prepared from PrSC cultured with the indicated concentrations of Phx A for 2 days (PrSC/Phx A). DU‐145 cells were treated with CM or normal medium for 30 min (medium changed). Cell lysates were prepared and used for immunoprecipitation of IGF‐IR for the detection of tyrosine‐phosphorylated IGF‐IR and total IGF‐IR, or directly for the detection of phosphorylated Akt and total Akt. (c) PrSC were precultured with Phx A for 2 days, and then DU‐145 cells were cocultured with the PrSC by the external addition of IGF‐I at 500 ng/mL. Values are means ± SD for triplicate determinations. All data are representative of three independent experiments with similar results.
Discussion
Phthoxazolin A was originally discovered as an inhibitor of cellulose biosynthesis.( 30 , 31 , 32 ) Using an in vitro coculture system of prostate cancer cells and PrSC, in which the growth of prostate cancer cells is increased by the presence of PrSC,( 15 , 27 ) we identified phthoxazolin A and its analog inthomycin B as molecules that modulated tumor–stromal cell interactions in prostate cancer. Because the inhibition of cellulose biosynthesis is not considered to participate in our assay system using mammalian cells, in the present study we investigated the mechanism of action of these molecules on tumor–stromal cell interactions.
Phthoxazolin A and inthomycin B inhibited the growth of DU‐145 cells when in coculture with PrSC without significant effect on the growth of DU‐145 cells or PrSC when cultured alone (Fig. 1a,b). CM prepared from phthoxazolin A‐treated PrSC failed to increase the growth of DU‐145 cells, which suggested that phthoxazolin A inhibited the secretion of tumor growth factors from PrSC (Fig. 1c). As we have recently reported, PrSC consists of both fibroblasts and myofibroblasts.( 22 ) Myofibroblasts are known to be a major cell population of cancer‐associated fibroblasts, or reactive stroma, and secrete many factors favorable for tumor cell growth.( 7 , 11 , 12 ) In testing our hypothesis that phthoxazolin A and inthomycin B affected a subpopulation of PrSC myofibroblasts, our results indicated that phthoxazolin A and inthomycin B inhibited myofibroblast differentiation. The compounds inhibited the expression of SM α‐actin, a myofibroblast marker, without affecting vimentin expression (Fig. 2). It was possible that phthoxazolin A had selective cytotoxicity against myofibroblasts. However, when we tested the effect of phthoxazolin A on myofibroblast‐enriched PrSC, no changes in cell viability were observed (Fig. 2e). Furthermore, phthoxazolin A also decreased the expression of SM α‐actin that was already upregulated in myofibroblast‐enriched PrSC (Supporting Fig. S1). These results demonstrated that phthoxazolin A and inthomycin B inhibited myofibroblast differentiation but maintained PrSC as fibroblasts.
To identify the secreted factors affected by phthoxazolin A treatment we carried out proteomic analysis on PrSC‐CM. Among the identified proteins with altered levels in response to phthoxazolin A treatment, we focused on the phthoxazolin A‐dependent decrease of IGFBP‐5 (Fig. 3), as we and others have found that the IGF axis plays a critical role in prostate cancer progression.( 14 , 15 ) Examination of the effects of phthoxazolin A and inthomycin B on the expression of various molecules of the IGF axis by PrSC showed that the compounds inhibited the expression of other IGF axis molecules, including several other IGFBP, although inhibition of IGF‐I was the strongest (Fig. 4). Based on our previous finding that IGF‐I secreted from PrSC increased DU‐145 cell growth in coculture,( 15 ) it appeared likely that phthoxazolin A inhibited the growth of DU‐145 cells in coculture by decreasing the expression of IGF‐I by PrSC.
We then examined the mechanism by which phthoxazolin A might inhibit myofibroblast differentiation or IGF‐I expression. As we previously reported, TGF‐β1 increases the expression of IGF‐I and IGFBP‐3 by PrSC.( 22 ) Therefore, we evaluated the effect of phthoxazolin A on TGF‐β1 activity. SB431542, a potent TGF‐β inhibitor, significantly inhibited the phosphorylation of smad2 and the expression of IGF‐I and IGFBP‐3 in the presence of TGF‐β1 (Fig. 5). In contrast, phthoxazolin A did not affect smad2 phosphorylation, nor the expression of smad4, a common partner of smad2 signaling (Fig. 5b), but weakly inhibited the expression of IGF‐I and IGFBP‐3 in the presence of TGF‐β1 (Fig. 5a). Thus, our results suggested that phthoxazolin A did not suppress TGF‐β1 signaling via the smad pathway. In addition to secreting several molecules of the IGF axis, DU‐145 cells also secrete TGF‐β1 and TGF‐β2.( 35 ) However, phthoxazolin A did not affect the expression of the IGF axis proteins TGF‐β1 and TGF‐β2 by DU‐145 cells (Fig. 4c). Furthermore, mRNA expression levels of TGF‐β1 and TGF‐β2 in PrSC were not affected by phthoxazolin A (data not shown). Thus, based on these results, it is unlikely that phthoxazolin A decreases TGF‐β levels. It has also been reported that c‐jun upregulates the secretion of IGF‐I from prostate stroma.( 36 ) We examined the effect of phthoxazolin A on the expression of c‐jun in PrSC, but found no change in response to phthoxazolin A (data not shown).
Finally, we examined whether phthoxazolin A inhibited PrSC‐derived IGF‐I activity on DU‐145 cells. When PrSC‐CM was added to DU‐145 cells, phosphorylation of IGF‐IR and Akt increased, which demonstrated that IGF‐I secreted from PrSC acts on DU‐145 cells. However, CM prepared from phthoxazolin A‐treated PrSC failed to increase the phosphorylation of those molecules (Fig. 6b). There was also a possibility that phthoxazolin A inhibited tyrosine kinase activity of IGF‐IR directly, but no significant IGF‐IR kinase inhibition was observed due to phthoxazolin A compared with that observed for AG1024, a potent inhibitor of IGF‐IR kinase used as a control (data not shown). Furthermore, phthoxazolin A inhibited the secretion of IGF‐I from PrSC, and the external addition of IGF‐I recovered the inhibition of DU‐145 cell growth in coculture with PrSC by phthoxazolin A (Fig. 6). Therefore, our results demonstrated that phthoxazolin A inhibited the secretion of IGF‐I from PrSC.
Taken together, our study showed that phthoxazolin A, a small molecule, inhibited the growth of prostate cancer cells when cultured with PrSC through the downregulation of IGF‐I secretion by PrSC. Our results also suggested that phthoxazolin A indirectly inhibited TGF‐β activity. It was recently reported that silibinin suppressed prostate cancer growth in vivo through downregulation of the IGF axis.( 37 ) Although we tried to evaluate the efficacy of phthoxazolin A against prostate cancer cells in vivo, we could not obtain effective plasma concentrations of phthoxazolin A due to its high toxicity in mice (data not shown). We are now preparing various derivatives of phthoxazolin A to evaluate its anticancer effects in vivo.
Supporting information
Fig. S1. Effects of phthoxazolin A (Phx A) on myofibroblast differentiation. Prostate stromal cells were precultured for 3 days and then further cultured with the Phx A for 3 days. Cell lysates were prepared and applied to western blotting.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
The authors wish to thank Ms K. Adachi and Ms E. Satoh for their technical assistance. This work was supported by a Grant‐in‐Aid for Cancer Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1. Stahtea XN, Roussidis AE, Kanakis I et al . Imatinib inhibits colorectal cancer cell growth and suppresses stromal‐induced growth stimulation, MT1‐MMP expression and pro‐MMP2 activation. Int J Cancer 2007; 121: 2808–14. [DOI] [PubMed] [Google Scholar]
- 2. Wiseman BS, Werb Z. Stromal effects on mammary gland development and breast cancer. Science 2002; 296: 1046–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karnoub AE, Dash AB, Vo AP et al . Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007; 449: 557–63. [DOI] [PubMed] [Google Scholar]
- 4. Hwang RF, Moore T, Arumugam T et al . Cancer‐associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res 2008; 68: 918–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grossfeld GD, Hayward SW, Tlsty TD, Cunha GR. The role of stroma in prostatic carcinogenesis. Endocr Relat Cancer 1998; 5: 253–70. [Google Scholar]
- 6. Dvorak HF. Tumors: wounds that do not heal. N Eng J Med 1986; 315: 1650–9. [DOI] [PubMed] [Google Scholar]
- 7. Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 2002; 8: 2912–23. [PubMed] [Google Scholar]
- 8. Delinassios JG. Cytocidal effect of human fibroblasts on HeLa cells in vitro . Biol Cell 1987; 59: 69–77. [DOI] [PubMed] [Google Scholar]
- 9. Picard O, Rolland Y, Poupon MF. Fibroblast‐dependent tumorigenicity of cells in nude mice: implication for implantation of metastases. Cancer Res 1986; 46: 3290–4. [PubMed] [Google Scholar]
- 10. Camps JL, Chang SM, Hsu TC et al . Fibroblast‐mediated acceleration of human epithelial tumor growth in vivo . Proc Natl Acad Sci USA 1990; 87: 75–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Micke P, Ostman A. Tumor–stroma interaction: cancer‐associated fibroblasts as novel targets in anti‐cancer therapy? Lung Cacner 2004; 45: S163–75. [DOI] [PubMed] [Google Scholar]
- 12. Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol 2001; 166: 2472–83. [PubMed] [Google Scholar]
- 13. Wernert N. The multiple roles of tumor stroma. Virchows Arch 1997; 430: 433–43. [DOI] [PubMed] [Google Scholar]
- 14. Pollak M, Beamer W, Zhang JC. Insulin‐like growth factors and prostate cancer. Cancer Metastasis Rev 1999; 17: 383–90. [DOI] [PubMed] [Google Scholar]
- 15. Kawada M, Inoue H, Masuda T, Ikeda D. Insulin‐like growth factor I secreted from prostate stromal cells mediates tumor–stromal cell interactions of prostate cancer. Cancer Res 2006; 66: 4419–25. [DOI] [PubMed] [Google Scholar]
- 16. Peehl DM, Sellers RG. Induction of smooth muscle cell phenotype in cultured human prostatic stromal cells. Exp Cell Res 1997; 232: 208–15. [DOI] [PubMed] [Google Scholar]
- 17. Rønnov‐Jessen L, Petersen OW. Induction of α‐smooth muscle actin by transforming growth factor‐β 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest 1993; 68: 696–707. [PubMed] [Google Scholar]
- 18. Tuxhorn JA, McAlhany SJ, Yang F, Dang TD, Rowley DR. Inhibition of transforming growth factor‐β activity decreases angiogenesis in a human prostate cancer‐reactive stroma xenograft model. Cancer Res 2002; 62: 6021–5. [PubMed] [Google Scholar]
- 19. Steiner MS, Barrack ER. Transforming growth factor‐β 1 overproduction in prostate cancer: effects on growth in vivo and in vitro . Mol Endocrinol 1992; 6: 15–25. [DOI] [PubMed] [Google Scholar]
- 20. Cui W, Fowlis DJ, Bryson S et al . TGF‐β1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell 1996; 86: 531–42. [DOI] [PubMed] [Google Scholar]
- 21. Bhowmick NA, Chytil A, Plieth D et al . TGF‐β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 2004; 303: 848–51. [DOI] [PubMed] [Google Scholar]
- 22. Kawada M, Inoue H, Arakawa M, Ikeda D. Transforming growth factor‐β1 modulates tumor–stromal cell interactions of prostate cancer through insulin‐like growth factor‐I. Anticancer Res 2008; 28: 721–30. [PubMed] [Google Scholar]
- 23. Date K, Matsumoto K, Shimura H, Tanaka M, Nakamura T. HGF/NK4 is a specific antagonist for pleiotrophic actions of hepatocyte growth factor. FEBS Lett 1997; 420: 1–6. [DOI] [PubMed] [Google Scholar]
- 24. Date K, Matsumoto K, Kuba K, Shimura H, Tanaka M, Nakamura T. Inhibition of tumor growth and invasion by a four‐kringle antagonist (HGF/NK4) for hepatocyte growth factor. Oncogene 1998; 17: 3045–54. [DOI] [PubMed] [Google Scholar]
- 25. Kawada M, Ishizuka M, Takeuchi T. Enhancement of antiproliferative effects of interleukin‐1β and tumor necrosis factor‐α on human prostate cancer LNCaP cells by coculture with normal fibroblasts through secreted interleukin‐6. Jpn J Cancer Res 1999; 90: 546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kawada M, Inoue H, Usami I et al . Establishment of a highly tumorigenic LNCaP cell line having inflammatory cytokine resistance. Cancer Lett 2006; 242: 46–52. [DOI] [PubMed] [Google Scholar]
- 27. Kawada M, Yoshimoto Y, Minamiguchi K et al . A microplate assay for selective measurement of growth of epithelial tumor cells in direct coculture with stromal cells. Anticancer Res 2004; 24: 1561–8. [PubMed] [Google Scholar]
- 28. Fukazawa H, Mizuno S, Uehara Y. A microplate assay for quantitation of anchorage‐independent growth of transformed cells. Anal Biochem 1995; 228: 83–90. [DOI] [PubMed] [Google Scholar]
- 29. Kawada M, Masuda T, Ishizuka M, Takeuchi T. 15‐Deoxyspergualin inhibits Akt kinase activation and phosphatidylcholine synthesis. J Biol Chem 2002; 277: 27 765–71. [DOI] [PubMed] [Google Scholar]
- 30. Omura S, Tanaka Y, Kanaya I, Shinose M, Takahasi Y. Phthoxazolin, a specific inhibitor of cellulose biosynthesis, produced by a strain of streptomyces sp. J Antibiot 1990; 43: 1034–6. [DOI] [PubMed] [Google Scholar]
- 31. Tanaka Y, Kanay I, Takahashi Y, Shinose M, Tanaka H, Omura S. Phthoxazolin A, a specific inhibitor of cellulose biosynthesis from microbial origin. I. Discovery, taxonomy of producing microorganism, fermentation, and biological activity. J Antibiot 1993; 46: 1208–13. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka Y, Kanaya I, Shiomi K, Tanaka H, Omura S. Phthoxazolin A, a specific inhibitor of cellulose biosynthesis from microbial origin. II. Isolation, physico‐chemical properties, and structural elucidation. J Antibiot 1993; 46: 1214–18. [DOI] [PubMed] [Google Scholar]
- 33. Matsuyama S, Iwadate M, Kondo M et al . SB‐431542 and Gleevec inhibit transforming growth factor‐β‐induced proliferation of human osteosarcoma cells. Cancer Res 2003; 63: 7791–8. [PubMed] [Google Scholar]
- 34. Hjelmeland MD, Hjelmeland AB, Sathornsumetee S et al . SB‐431542, a small molecule transforming growth factor‐β‐receptor antagonist, inhibits human glioma cell line proliferation and motility. Mol Cancer Ther 2004; 3: 737–45. [PubMed] [Google Scholar]
- 35. Verona EV, Elkahloun AG, Yang J, Bandyopadhyay A, Yeh I, Sun L. Transforming growth factor‐β signaling in prostate stromal cells supports prostate carcinoma growth by up‐regulating stromal genes related to tissue remodeling. Cancer Res 2007; 67: 5737–46. [DOI] [PubMed] [Google Scholar]
- 36. Li W, Wu C, Bebbo PG, Olumi AF. Stromally expressed c‐jun regulates proliferation of prostate epithelial cells. Am J Pathol 2007; 171: 1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Raina K, Blouin M, Singh RP et al . Dietary feeding of silibinin inhibits prostate tumor growth and progression in transgenic adenocarcinoma of the mouse prostate model. Cancer Res 2007; 67: 11 083–91. [DOI] [PubMed] [Google Scholar]
- 38. Gregory C, Kim D, Ye P et al . Androgen receptor up‐regulates insulin‐like growth factor binding protein‐5 (IGFBP‐5) expression in a human prostate cancer xenograft. Endocrinology 1999; 140: 2372–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effects of phthoxazolin A (Phx A) on myofibroblast differentiation. Prostate stromal cells were precultured for 3 days and then further cultured with the Phx A for 3 days. Cell lysates were prepared and applied to western blotting.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


