Abstract
A transgenic mouse model expressing Simian virus 40 T‐antigen (SV40Tag) under the control of a tetracycline system was generated. In this model, a cerebellar tumor was developed after doxycycline hydrochloride treatment. Real time‐polymerase chain reaction and immunohistochemistry results indicated that the SV40Tag gene was expressed in the tumor. Pathological analysis showed that the tumor belonged to medulloblastoma. Further molecular characterization of the tumor demonstrated that the insulin‐like growth factor (IGF) signaling pathway was activated. We also found that the SV40Tag could bind and translocate insulin receptor substrate 1 into the nucleus in primary cultured tumor cells. The interaction between the IGF pathway and SV40Tag may contribute to the process of malignant transformation in medulloblastoma. This transgenic animal model provides an important tool for studies on the signal pathways involved in the preneoplastic process in medulloblastoma and could help to identify therapeutic targets for brain tumors. (Cancer Sci 2008; 99: 234–240)
Medulloblastoma is an invasive embryonic tumor of the cerebellum with predominant neuronal differentiation. It is the most common malignant brain tumor of childhood, which mainly occurs between 5 and 10 years of age.( 1 ) Despite recent advances in understanding the pathogenesis of medulloblastoma, the specific genetic alterations and molecular mechanisms involved in the brain tumor are not well defined.( 2 ) It is generally accepted that medulloblastoma, like other cancers, represents a genetic disease of somatic cell alterations, such as gene mutations, deletions, translocations, or amplifications. The etiology and risk factors that contribute to the genetic aberrations for the tumors remain to be elucidated. It has been reported that aberrant activations of several cell signal pathways, such as Shh( 3 , 4 ) Wnt( 5 , 6 ) ErbB2( 7 , 8 ) and insulin‐like growth factor 1‐R (IGF1‐R) pathways( 9 , 10 ) were implicated in the pathological processes of brain tumors.
Simian virus 40 (SV40) has been detected in medulloblastoma.( 11 , 12 ) Several lines of evidence indicated that the viral early protein T antigen could be involved in the tumorigenesis process. SV40 T antigen (SV40Tag) is a multifunctional regulatory protein that binds to the tumor suppressor genes, including p53( 13 ) and the retinoblastoma (Rb) family of proteins.( 14 ) Furthermore, the antigen could interact with insulin receptor substrate I (IRS‐1), which plays a critical role in transforming R‐cells.( 15 , 16 ) It is known that the interactions between SV40 T‐antigen (SV40Tag) and tumor suppressor proteins stimulate DNA duplication, facilitate cell proliferation and induce cell transformation.( 17 , 18 ) However, the molecular mechanism of the SV40 induction of tumors is not completely clear.
Transgenic technology has provided a way to determine whether a particular gene product could contribute to the neoplastic process. Furthermore, the transgenic animal models could be used for studies on tumor development, progression and therapeutic targets identification and evaluation. Recently, a number of SV40Tag transgenic animal models have been reported, in which the transgene was constitutively expressed.( 19 , 20 , 21 , 22 , 23 , 24 ) These animal models were used for the studies of neoplastic development, as well as the equilibrium between proliferation and cell death.( 25 , 26 , 27 ) However, the constitutive expression of SV40Tag may affect the development of the animal and therefore, hamper the analysis of critical steps of tumorigenesis. In an attempt to circumvent these limitations, we generated inducible transgenic mice expressing SV40Tag under the control of tetracycline. In this report, we have described the generation and molecular characterization of the tetracycline‐responsive SV40Tag transgenic mouse model for medulloblastoma.
Materials and Methods
Generation of the inducible SV40Tag transgenic mice. A 2.5‐kb fragment containing the SV40Tag gene from pBC‐SV40Tag( 28 ) was generated with Xho I digestion and cloned into the Sal I site of pTRE‐2 DNA vector (pTRE‐SV40Tag). Linearized pTRE‐SV40Tag and pTet‐on (clontech, K1621‐A) vectors were microinjected into the male pronucleus of fertilized zygotes of FVB mice. The eggs were implanted into a pseudopregnant ICR foster mother as described.( 29 ) Founder (G0) mice were identified by polymerase chain reaction (PCR) and Southern blot analysis. Briefly, genomic DNA was isolated from the tails of transgenic mice using a standard method. For PCR of SV40Tag, a pair of spanning SV40Tag intron primers was designed and synthesized (Forward: 5′‐ACT TTG GAG GCT TCT GGG AT‐3′ and Reverse: 5′‐GGT GTA AAT AGC AAA GCA AGC A‐3′). PCR reaction was carried out under the following conditions: 35 cycles of 94°C for 1 min, 60°C for 45 s, and 72°C for 50 s. The primer sequences of rtTA were as follows: Forward: 5′‐TAG ATA GGC ACC ATA CTC ACT T‐3′ and Reverse: 5′‐CCT CGA TGG TAG ACC CGT AA‐3′. PCR reaction was carried out with 35 cycles of 94°C for 1 min, 53°C for 45 s, and 72°C for 50 s. PCR products were detected using electrophoresis on 1.0% agarose gel. Transgenic mice were further confirmed by Southern blot analysis. Genomic DNA was digested overnight with Nde I and subjected to electrophoresis on 0.8% agarose gel. The 1‐kb fragment of plasmid pTRE‐SV40Tag digested with Nde I was used as a probe. Plasmid pTet‐on and genomic DNA were digested with BamH I and run on 0.8% agarose gel. DNA was transferred onto nylon membrane (Millipore Ltd). Southern blot analysis was carried out according to the protocol of the ECLTM Direct Nucleic Acid Labeling and Detection System (Amersham).
Administration of doxycycline hydrochloride and RNA analysis. Twenty‐four SV40Tag and rtTA double transgenic mice (12 male and 12 female) at 6 weeks of age were exposed to 0.05 mg/mL of doxycycline hydrochloride (Dox) (Sigma) in drinking water. Twenty‐four pTet‐on/pTRE‐SV40Tag double transgenic mice without administration of Dox were used as controls. Total RNA was isolated from the tumor and other tissues with TRIzol reagent (Invitrogen) according to the manufacturer's instruction. First strand cDNA was synthesized using M‐MLV Reverse Transcriptase (Invitrogen). Reverse transcription (RT)‐PCR reaction was carried out using the SV40Tag primers described above. PCR reaction was carried out for 35 cycles: 94°C for 1 min, 60°C for 45 s, and 72°C for 50 s. The PCR products were analyzed on 1.0% agarose gel.
Histological and immunohistochemical analysis. Tumor and other tissues were cut into 0.5‐cm3 pieces, fixed in 4% paraformaldehyde and embedded in paraffin. Tissue sections of 3 µm were cut using freezing microtome (Leica) and stained with hematoxylin and eosin. Immunohistochemical analysis of tissues was carried out using the following antibodies: anti‐SV40Tag polyclonal antibody (1:400, Santa Cruz Biotechnology Inc.); anti‐GFAP (1:400, Zymed); anti‐NSE (1:200, Zymed); and anti‐S100.β (1:500, Zymed). Tissues were incubated with normal goat serum at room temperature for 20 min and treated with antibody overnight after quenching endogenous peroxidase with 0.6% hydrogen peroxide. The tissues were then incubated with biotinylated secondary antibody and avidin DH‐biotinylated houseadish peroxidase‐H complex. The immunoreactivity was visualized with 3,3′‐diaminobenzidine tetrachloride (DAB) of strept avidin biotin complex kit (Boster) according to the manufacturer's protocol. The nuclei were counterstained with hematoxylin. Slices were observed under microscopy and photographed (Olympus).
Fluorescence real‐time quantitative PCR. Tumors of transgenic mice with Dox treatment and normal cerebella of transgenic mice without Dox treatment were used for gene expression analysis by fluorescence real‐time quantitative RT‐PCR. The primers were designed using PrimerExpress software and synthesized by Sangon. The primers were: Gli1 (Forward: 5′CGT TTG AAG GCT GTC GGA AGT3′; Reverse: 5′GGA CCT GCG GCT GAC TGT GT3′), Gli2 (Forward: 5′CAC AGG GCG GGC ACA AGA T3′; Reverse: 5′GGA GGG CAG TGT CAA GGA A3′), Ptc1 (Forward: 5′GGA GGA GAA CAA GCA ACT3′; Reverse: 5′AAA GGG AAC TGA GCG TAC3′), β‐cantenin (Forward: 5′TCT ACG CCA TCA CGA CAC3′; Reverse: 5′CAG ACA GAC AGC ACC TTC3′), IGF‐1R (Forward: 5′GCC TTG GTC TCC TTG TCC TT3′; Reverse: 5′CTC TGG CGT CCC TTG GTT3′),IRS‐1£¨Forward: 5′CCT GGA GTA TTA TGA GAA CGA G3′; Reverse: 5′CGG CAA TGG CAA AGT GT3′) Math1 (Forward: 5′CAG GGT GAG CTG GTA AGG AGA3′; Reverse: 5′CGT TGT TGA AGG ACG GGA TAA3′). Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer's instruction. First strand cDNA was synthesized using M‐MLV Reverse Transcriptase (Invitrogen). glyceraldehyde‐3‐phosphate dehydrogenase (Forward: 5′TCA ACG ACC CCT TCA TTG AC3′; Reverse: 5′ATG CAG GGA TGA TGT TCT GG3′) was used as a reference gene. We used three mice with tumors and three normal mice for each experiment and repeated the experiment three times. The relative gene expression levels were calculated and presented with 2−ΔΔCt values.( 30 )
Double immunoflurescence staining. Primary cultured cells from tumor tissue were incubated on glass slides without Dox treatment. After being rinsed with phosphate‐buffered saline (PBS), the cells were fixed with cold acetone for 10 min. The slides were blocked with goat serum for 15 min. The slides were incubated with anti‐SV40 T antigen monoclonal antibody (Santa Cruz Biotechnology Inc.) at a dilution of 1:500 for 3 h at 37°C. The slides were washed three times with PBS, incubated with secondary antibody conjugated with CY3 anti‐mouse immunoglobulin G (IgG) (Chemicon) at a dilution of 1:1000 for 1 h at 37°C in a dark and humidified atmosphere. The slides were washed three times with PBS and treated with anti‐IRS‐1 monoclonal antibody (Upstate) at a dilution of 1:500 for 2 h at 37°C. The slides were washed three times with PBS, and then incubated with secondary antibody conjugated with fluorescein isothiocyanate (FITC) anti‐rabbit IgG (Santa Cruz Biotechnology Inc.) for 1 h at 37°C in a dark and humidified atmosphere. The stained cells were washed three times with PBS and observed under a Leica DMIL fluorescence microscope.
Immunoprecipitation and western blotting analyses. The brain tumors of transgenic mice with Dox treatment and the normal cerebella of transgenic mice without Dox treatment were lysed in the RIPA buffer (20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X‐100, 10 mM sodium pyrophosphate, 3 mM β‐glcerophosphate, 5 mM ethylenediaminetetraacetic acid (EDTA), 1 mM NaVO4, 10 µg/mL leupeptin and 1 mM phenylmethanesulfonyl fluoride). After centrifugation at 12 000g for 10 min at 4°C, the protein in the upper lysate was transferred to a 1.5‐mL microcentrifuge tube on ice. One hundred microliters of rabbit serum and 20 µL of protein A‐Agarose (Santa Cruz Biotechnology Inc.) were added to each mL of the lysate. The mixture was incubated at 4°C overnight. After centrifugation at 2500g for 5 min at 4°C, the upper lysate was transferred to a fresh 1.5‐mL microcentrifuge tube. Ten microliters of anti‐SV40 T antigen monoclonal antibody (Santa Cruz Biotechnology Inc.), anti‐IRS‐1 monoclonal antibody (Upstate) and 20 µL of protein A‐Agarose were added to the mixture and incubated overnight at 4°C. The immunoprecipitates were collected by centrifugation at 2500g for 5 min at 4°C. After being washed four times with RIPA buffer, the immunoprecipitates were boiled in a water bath for 5 min with sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) gel loading buffer. The proteins were separated on 7.5% PAGE and electro‐transferred onto nitrocellulose membrane. After blocking with TBST (10 mM Tris‐HCl, pH 7.5, 100 mM NaCl, 0.1% Tween‐20) containing 5% non‐fat milk, the membrane was incubated with a primary antibody at 37°C for 2 h. The membrane was washed three times with TBST, and incubated with secondary antibody (1:2000 diluted in TBST containing 5% bovine serum albumin) at 37°C for 1 h. After three washes with TBST, the membrane was developed using the ECL chemiluminiscene kit (Amersham). A pre‐stained molecular weight marker was used to show the size of the protein (Fermentas).
Results
Generation of double transgenic mice. Polymerase chain reaction and Southern blot analysis showed that three founder mice carrying the pTRE‐SV40Tag and pTet‐on double transgenes were generated (Fig. 1). The double‐transgenic founder mice were bred with normal FVB mice, and the pups were genotyped for transmission of the transgene. Two mice, one male and one female, were bred successfully and used to establish the lineages designated pTRE‐SV40Tag/pTet‐on 123 (line 123) and pTRE‐SV40Tag/pTet‐on 144 (line 144).
Figure 1.

Genotype of the inducible Simian virus 40 T‐antigen (SV40Tag) transgenic mice. (a) Polymerase chain reaction (PCR) analysis of the transgenic mice. PCR was carried out as described in Materials and Methods. M, DL 2000 DNA marker; 74, Founder mice 74 genomic DNA showed two PCR bands for SV40Tag and Tet‐on. 80, Founder mice 80 genomic DNA showed a single PCR band for Tet‐on; 123, Founder mice 123 genomic DNA showed two PCR bands for SV40Tag and Tet‐on; 144, Founder mice 144 genomic DNA showed two PCR bands for SV40Tag and Tet‐on. (b) Southern blot analysis of the transgenic mice. 74, Founder mice 74 genomic DNA showed two bands for SV40Tag and Tet‐on; 80, Founder mice 80 genomic DNA showed a single band for Tet‐on; 123, Founder mice 123 genomic DNA showed two bands for SV40Tag and Tet‐on; 144, Founder mice 144 genomic DNA showed two bands for SV40Tag and Tet‐on.
Doxycycline hydrochloride induction of brain tumor in the transgenic mice. Twenty‐four transgenic pups, 12 male and 12 female, from lineage 123 were generated. After 2 months of induction with Dox, 22 (10 male and 12 female) of these mice became moribund and showed clear evidence of ridgy cranium. Complete necropsies were carried out on the founder and six moribund progenies. Each mouse had a brain tumor as shown in Fig. 2(a). The tumors tended to spread along the surface of the cerebellar cortex and often grew outward as exophytic masses invading the brain (Fig. 2b). To examine whether the transgene has been expressed in the tumor, we carried out RT‐PCR for SV40Tag. Our result showed that a SV40Tag specific 272 bp PCR product was generated from the tumor cDNA (Fig. 3). Furthermore, immunohistochemical analysis demonstrated that SV40Tag protein was expressed in the tumor of the transgenic mice (Fig. 4). Without the administration of Dox, few of the transgenic mice developed brain tumor after 12 months of age.
Figure 2.
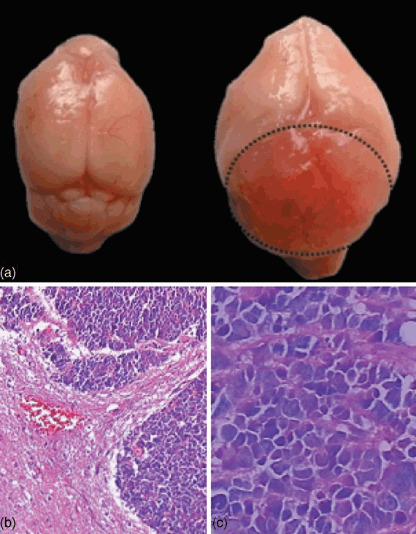
Morphology analysis of the brain tumor and tumor cells. Brains from 6 month old male normal or transgenic mice were dissected. Cells were stained with the hematoxylin and eosin staining method as described in the Materials and Methods. (a) Normal brain (left) and brain with the tumor from the transgenic mouse (right). (b) Brain slice shows both normal and tumor cells after HE staining (100 ×). (c) Brain slice shows tumor cells after HE staining (200 ×).
Figure 3.
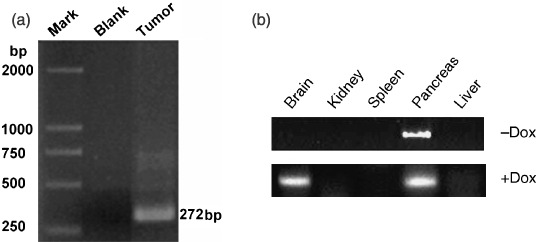
Analysis of the Simian virus 40 T‐antigen (SV40Tag) mRNA in the brain tumor. Reverse transcription‐polymerase chain reaction (RT‐PCR) was carried out to detect the expression of SV40Tag in the brain tumor (a) and other tissues (b). Mark, DL‐2000 DNA Marker; Blank, PCR reaction without RNA template; Tumor, PCR reaction with RNA from the tumor tissue; –Dox, without doxycycline hydrochloride (Dox) administration; +Dox, with Dox administration.
Figure 4.
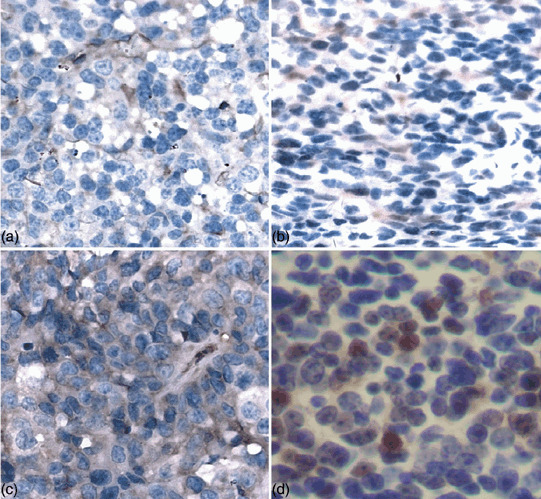
Immunohistochemistry analysis of Simian virus 40 T‐antigen (SV40Tag) and tumor markers in the brain tumor. Immunohistochemistry analysis was carried out as described in Materials and Methods. Antibodies against GFAP, S100‐β, NSE and SV40Tag were used for the experiment. a, GFAP; b, S100‐β; c, NSE; d, SV40Tag.
Histological and immunohistochemistry analysis of the brain tumor. The brain tumor exhibited closely packed, round to oval embryonal cells with scant cytoplasm that closely resembled human medulloblastoma (Fig. 2c). Histological and immunohistochemistry analyses were carried out to further characterize the brain tumor. The tumor sections were stained with markers of glia (GFAP; Fig. 4a), neuronal cells (S100.β; Fig. 4b), primitive neuroepithelial cells and embryonal tumors (intermediate filament nestin; Fig. 4c). Our results demonstrated that all the molecular markers were positive in the brain tumor, suggesting that the tumor maintained the characterizations of divergent neuroectodermal differentiation and medulloblasoma.
Involvement of insulin and IGF pathways in the brain tumor. It has been reported that granule cells could be the cells of origin for medullublastoma.( 2 ) The external germinal layer of the cerebellum is missing in Math1‐null embryos, implying that Math1 expression is required for granule cell genesis.( 31 ) Therefore, we carried out quantitative real‐time RT‐PCR to examine the expression of the known germinal granule cell marker Math1 in brain tumors. Indeed, our results showed that the expression of Math1 was significantly induced in the brain tumors of transgenic mice (Fig. 5).
Figure 5.
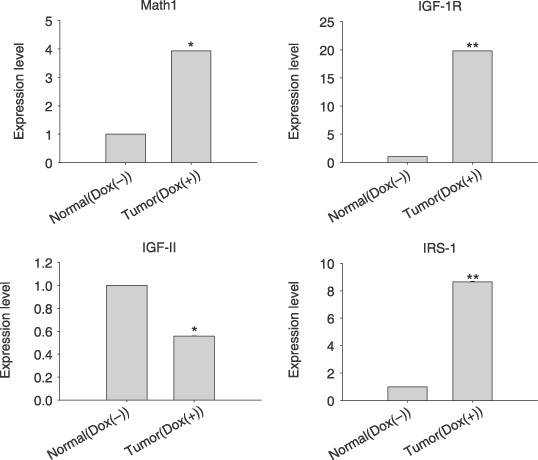
The expression analysis of genes associated with math1 and insulin‐like growth factor (IGF) pathway in the brain tumor. Quantitative real time reverse transcription‐polymerase chain reaction (RT‐PCR) was carried out as described in the Materials and Methods. Normal (Dox–), normal cerebella of transgenic mice without doxycycline hydrochloride (Dox) treatment; Tumor (Dox+), brain tumor of transgenic mice with Dox treatment. IRS‐1, insulin receptor substrate I. Fold changes were calculated (2−ΔΔCt value) and paired Student t‐test are shown *P < 0.05; **P < 0.01.
A number of signal pathways have been implied in the participation of tumor genesis. Therefore, we examined the expression of genes associated with insulin, IGF, sonic hedgehog signaling and wnt pathways. Interestingly, our results demonstrated that the expression levels of IGF‐IR and IRS‐1 were increased in brain tumors (Fig. 5). The expression of genes involved in sonic hedgehog and wnt signaling pathways, such as Shh, Gli1, Gli2 and β‐cantenin, did not show any change in brain tumors (data not shown).
Interaction of SV40Tag and IRS‐1 in the brain tumor. It has been reported that SV40Tag formed a complex with IRS‐1 in R‐cells.( 16 ) Thus, we have asked the question: does the induction of SV40Tag expression participate in the protein–protein interaction with IRS‐1? Immunoprecipitation and Western blot analyses were carried out to examine the possibility. First, we used anti‐SV40Tag antibody for the immunoprecipitaion, followed by blotting with anti‐IRS‐1 antibody. Figure 6 shows that IRS‐1 was coprecipitated with SV40Tag. Next, we carried out the immunoprecipitation assay with IRS‐1 antibody and Western blot with anti‐SV40Tag antibody. The results further confirmed that SV40Tag and IRS‐1 were coimmunoprecipitated, suggesting that they formed a complex in the brain tumor (Fig. 6).
Figure 6.
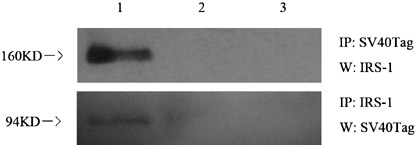
Simian virus 40 T‐antigen (SV40Tag) and insulin receptor substrate I (IRS‐1) interaction analysis in the brain tumor. Immunoprecipitation (IP) and Western blot analysis were carried out as described in the Materials and Methods. Upper image, immunoprecipitation with an anti SV40Tag antibody followed by blotting with IRS‐1 antibody; lower image, immunoprecipitation with an anti IRS‐1 antibody followed by blotting with SV40Tag antibody; lane 1, IP followed by Western blot using tumor tissue from transgenic mice with doxycycline hydrochloride (Dox) treatment; lane 2, IP followed by Western blot using the normal cerebella from transgenic mice without Dox treatment; lane 3, negative control with a normal immunoglobulin G (IgG).
Colocalization of IRS‐1 and SV40Tag in the nucleus. Insulin receptor substrate I is the major cytoplasmic component of the insulin and IGF‐I signaling pathways.( 32 ) We have examined the cellular distribution of IRS‐1 in brain tumors. Double staining of IRS‐1 and SV40Tag was carried out to detect the colocalization of these two proteins in the brain tumor. IRS‐1 antibody with green fluorescence and anti‐SV40 T‐antigen antibody labeled with red fluorescence were used for the experiment. We found that SV40Tag was exclusively distributed in the nucleus (Fig. 7c). Interestingly, the cells showed a predominant nuclear and much less cytoplasmic immunostaining for IRS‐1 (Fig. 7b). Figure 7(d) shows the merged picture of the colocalization of IRS‐1 and SV40Tag.
Figure 7.
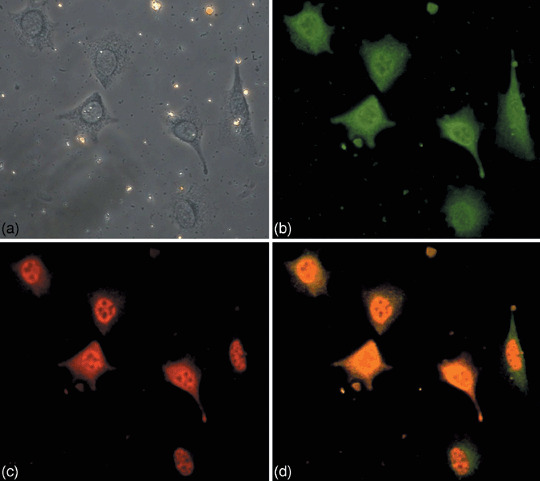
Colocalization of Simian virus 40 T‐antigen (SV40Tag) and insulin receptor substrate I (IRS‐1) in the cells of brain tumor. (a) Primary cultured cells from the brain tumor of transgenic mice were used for the double staining experiment as described in the Materials and Methods. Cells were stained with anti‐IRS‐1 antibody (b) and anti‐SV40‐Tag antibody (c). The overlap staining with (b) and (c) is shown in (d). (a) shows the phase contrast microscopy observation of the same cells.
Discussion
Simian virus 40 is a member of the polyoma family with a double‐stranded DNA genome.( 33 ) It has been reported that SV40 had tumorigenic activity in rodents and could transform many types of cells( 34 , 35 , 36 , 37 ) including human fetal tissues, newborn human kidney cells, and various human tumor cell lines.( 38 , 39 ) The virus has been detected by PCR and DNA sequence analysis in human tumors.( 40 , 41 , 42 , 43 ) SV40Tag is a multifunctional regulatory protein that can bind and inactivate with several tumor suppressor genes, including p53( 33 , 44 ) and the retinoblastoma (Rb) family of proteins.( 14 ) A number of transgenic mouse models expressing SV40Tag have been reported.( 19 , 20 , 21 , 22 , 23 , 24 ) For example, it has been reported that SV40Tag transgenic mice showed hepatocellular carcinoma development from confluent hyperplasia.( 20 ) Mice expressing SV40Tag with the intestinal trefoil factor promoter developed tumor in the colon.( 21 ) Interestingly, ovarian specific promoter (OSP)‐Tag mice developed tumors in a variety of tissues, although the expression of Tag that controlled by the OSP‐1 promoter occurred predominantly in the ovary.( 22 ) The C31/SV40 T‐antigen transgenic mice of mammary cancer provided a useful model for studies on tumor development and apoptosis.( 23 , 25 , 45 , 46 , 47 ) These SV40Tag transgenic mice were generated in such a way that the transgene was under the control of a tissue‐specific promoter. We have generated an inducible SV40Tag transgenic mouse model with a cytomegalovirus promoter. After tetracycline induction, SV40Tag mice developed tumors in a variety of tissues, including the pancreas and brain. In this report, we have focused on the brain tumor, which was located between the cross of the cerebrum and cerebellum. Histological analysis demonstrated that the tumor consisted of densely packed tumor cells, which were round to oval or carrot‐like in shape. It also showed hyperchromatic nucleus with scanty cytoplasm and high mitotic phenotypes. These characteristics strongly suggest that the brain tumor is similar to human medulloblastoma.
Human medulloblastoma is thought to arise from granule cell precursors in the external germinal layer (EGL) of the developing cerebellum.( 48 ) Since Math1 is a known molecular marker for granule cell precursors, we examined the expression of this gene in the brain tumor. Indeed, Math1 was found to be highly expressed in the tumor, suggesting that it might be developed from the granule cell precursors of the cerebellum. Another characteristic of human medulloblastoma is that the brain tumor shows both glial differentiation and neuronal differentiation.( 49 ) Interestingly, our immunohistochemistry experiment showed that the markers of both glial and neuronal cells were highly expressed in the brain tumor. Taken together, these results further confirmed that the brain tumor of the inducible SV40Tag transgenic mouse represented an animal model of human medulloblastoma.
Brain tumors are the second most common cancers in children.( 50 ) Medulloblastoma is one of five embryonic tumors in the central nervous system. Several cell‐signaling pathways have been implicated in tumor formation and development, including WNT, SHH and IGF‐1R pathways.( 10 , 51 ) Therefore, we examined the expression of a number of genes associated with these signal pathways. Surprisingly, the expression levels of Shh, Gli1, and Gli2 in the sonic hedgehog (SHH) signaling pathway as well as β‐cantenin in the WNT pathway did not show any significant changes in the brain tumor. It is possible that different pathways were involved in the brain tumor of the transgenic animal model compared with the human medulloblastoma. On the other hand, the expression levels of a number of genes related to the insulin and IGF pathways, such as IGF‐IR, IGF‐II and IRS‐1, showed significant changes in the brain tumor.
Insulin receptor substrate I is a crucial signaling molecule in both insulin and IGF‐1R signaling pathways. It played essential roles for IGF‐1‐mediated cell proliferation.( 52 , 53 ) R‐cells are 3T3‐like fibroblasts derived from mouse embryos with a targeted disruption of the IGF‐I receptor gene. It has been shown that cotransfection of IRS‐1 and the SV40Tag in R‐cells could induce transformation. Furthermore, SV40Tag formed a complex with IRS‐1, which played a critical role in transforming R‐cells.( 15 , 16 ) These results have prompted us to investigate a possible association between IRS‐1 and SV40Tag in the brain tumor of the transgenic mouse model. Indeed, immunoprecipitation and Western blotting analysis with anti‐IRS‐1 and anti‐SV40Tag antibodies demonstrated that these two proteins formed a complex in the brain tumor.
It is interesting to note that IRS‐1 may carry out the transformation of cells by interacting with nucleolin and other nuclear proteins.( 54 ) Moreover, John Cunningham virus Tag, an antigen from John Cunningham virus, could induce the translocation of IRS‐1 into nucleus.( 55 ) Since SV40Tag interacted with IRS‐1 in the brain tumor, we hypothesized that IRS‐1 may also be translocated into the nucleus. To test this possibility, we carried out double immunofluorescence staining analysis. Indeed, SV40Tag and IRS‐1 were colocalized in the nucleus in the primary cultured tumor cells.
In summary, the brain tumor from the inducible SV40Tag transgenic mice showed the induction of gene expression in insulin and IGF pathways. More importantly, SV40Tag and IRS‐1 formed a complex and was translocated into the nucleus in the brain tumor. The IRS‐1 signaling may lead to the activation of downstream signaling and facilitate the transformation of the cells in the brain. Although the etiology of medulloblastomas has not been clearly elucidated, our results provide clues that the activation of the IGF‐1R signaling pathway could play an important role for tumor genesis and development.
Acknowledgments
This work is supported by the National Key Technologies Research and Development Program of China during the 10th Five‐Year Plan Period, no. 2001BA70113 and grants from the Ministry of Science and Technology of China (973 project), Shanghai Commission for Science and Technology, and Shanghai Commission for Education.
References
- 1. Packer RJ, Sutton LN, Elterman R et al . Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg 1994; 81: 690–8. [DOI] [PubMed] [Google Scholar]
- 2. Marino S. Medulloblastoma: developmental mechanisms out of control. Trends Mol Med 2005; 11: 17–22. [DOI] [PubMed] [Google Scholar]
- 3. Di Marcotullio L, Ferretti E, De Smaele E et al . REN (KCTD11) is a suppressor of Hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci USA 2004; 101: 10 833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor MD, Liu L, Raffel C et al . Mutations in SUFU predispose to medulloblastoma. Nat Genet 2002; 31: 306–10. [DOI] [PubMed] [Google Scholar]
- 5. Zurawel RH, Chiappa SA, Allen C, Raffel C. Sporadic medulloblastomas contain oncogenic beta‐catenin mutations. Cancer Res 1998; 58: 896–9. [PubMed] [Google Scholar]
- 6. Huang H, Mahler‐Araujo BM, Sankila A et al . APC mutations in sporadic medulloblastomas. Am J Pathol 2000; 156: 433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Calabrese C, Frank A, Maclean K, Gilbertson R. Medulloblastoma sensitivity to 17‐allylamino‐17‐demethoxygeldanamycin requires MEK/ERKM. J Biol Chem 2003; 278: 24 951–9. [DOI] [PubMed] [Google Scholar]
- 8. Hernan R, Fasheh R, Calabrese C et al . ERBB2 up‐regulates S100A4 and several other prometastatic genes in medulloblastoma. Cancer Res 2003; 63: 140–8. [PubMed] [Google Scholar]
- 9. Wang JY, Del Valle L, Gordon J et al . Activation of the IGF‐IR system contributes to malignant growth of human and mouse medulloblastomas. Oncogene 2001; 20: 3857–68. [DOI] [PubMed] [Google Scholar]
- 10. Del Valle L, Enam S, Lassak A et al . Insulin‐like growth factor I receptor activity in human medulloblastomas. Clin Cancer Res 2002; 8: 1822–30. [PubMed] [Google Scholar]
- 11. Weggen S, Bayer TA, Von Deimling A et al . Low frequency of SV40, JC and BK polyomavirus sequences in human medulloblastomas, meningiomas and ependymomas. Brain Pathol 2000; 10: 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhen HN, Zhang X, Bu XY et al . Expression of the simian virus 40 large tumor antigen (Tag) and formation of Tag‐p53 and Tag‐pRb complexes in human brain tumors. Cancer 1999; 86: 2124–32. [PubMed] [Google Scholar]
- 13. Montano X. Biology of p53 and SV40 large T association. Meth Mol Biol 2001; 165: 201–11. [DOI] [PubMed] [Google Scholar]
- 14. Dyson N, Bernards R, Friend SH et al . Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol 1990; 64: 1353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. D’Ambrosio C, Keller SR, Morrione A, Lienhard GE, Baserga R, Surmacz E. Transforming potential of the insulin receptor substrate 1. Cell Growth Differ 1995; 6: 557–62. [PubMed] [Google Scholar]
- 16. Fei ZL, D’Ambrosio C, Li S, Surmacz E, Baserga R. Association of insulin receptor substrate 1 with simian virus 40 large T antigen. Mol Cell Biol 1995; 15: 4232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neil JC, Cameron ER, Baxter EW. p53 and tumour viruses: catching the guardian off‐guard. Trends Microbiol 1997; 5: 115–20. [DOI] [PubMed] [Google Scholar]
- 18. Fanning E, Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem 1992; 61: 55–85. [DOI] [PubMed] [Google Scholar]
- 19. Marton I, Johnson SE, Damjanov I, Currier KS, Sundberg JP, Knowles BB. Expression and immune recognition of SV40 Tag in transgenic mice that develop metastatic osteosarcomas. Transgenic Res 2000; 9: 115–25. [DOI] [PubMed] [Google Scholar]
- 20. Schirmacher P, Held WA, Yang D, Biempica L, Rogler CE. Selective amplification of periportal transitional cells precedes formation of hepatocellular carcinoma in SV40 large tag transgenic mice. Am J Pathol 1991; 139: 231–41. [PMC free article] [PubMed] [Google Scholar]
- 21. Gum JR Jr, Hicks JW, Crawley SC et al . Mice expressing SV40 T antigen directed by the intestinal trefoil factor promoter develop tumors resembling human small cell carcinoma of the colon. Mol Cancer Res 2004; 2: 504–13. [PubMed] [Google Scholar]
- 22. Garson K, Macdonald E, Dube M, Bao R, Hamilton TC, Vanderhyden BC. Generation of tumors in transgenic mice expressing the SV40 T antigen under the control of ovarian‐specific promoter 1. J Soc Gynecol Invest 2003; 10: 244–50. [DOI] [PubMed] [Google Scholar]
- 23. Green JE, Shibata MA, Yoshidome K et al . The C3(1)/SV40 T‐antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma. Oncogene 2000; 19: 1020–7. [DOI] [PubMed] [Google Scholar]
- 24. Lee SS, Park WY, Chi JG et al . Thymic epithelial tumor progression in an SV40T transgenic mouse model. Cortical thymoma‐thymic carcinoma sequence. Virchows Arch 1998; 432: 33–42. [DOI] [PubMed] [Google Scholar]
- 25. Liu ML, Von Lintig FC, Liyanage M et al . Amplification of Ki‐ras and elevation of MAP kinase activity during mammary tumor progression in C3(1)/SV40 Tag transgenic mice. Oncogene 1998; 17: 2403–11. [DOI] [PubMed] [Google Scholar]
- 26. Shibata MA, Liu ML, Knudson MC et al . Haploid loss of bax leads to accelerated mammary tumor development in C3(1)/SV40‐TAg transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. Embo J 1999; 18: 2692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomas Ried KD, Zoë W, Danny W, Michael J. Difilippantonio, cristina montagna: molecular cytogenetics of mouse models of breast cancer. Breast Disease 2004; 19: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun Q, Feng J, Wei XL et al . Generation and characterization of a transgenic mouse model for pancreatic cancer. World J Gastroenterol 2006; 12: 2785–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hogan BLM, Constantini F, Lacy E. Manipulating the Mouse Embryo: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1994. [Google Scholar]
- 30. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2 (‐Delta Delta C(T) Method. Methods 2001; 25: 402–8. [DOI] [PubMed] [Google Scholar]
- 31. Ben‐Arie N, Bellen HJ, Armstrong DL et al . Math1 is essential for genesis of cerebellar granule neurons. Nature 1997; 390: 169–72. [DOI] [PubMed] [Google Scholar]
- 32. Sun XJ, Rothenberg P, Kahn CR et al . Structure of the insulin receptor substrate IRS‐1 defines a unique signal transduction protein. Nature 1991; 352: 73–7. [DOI] [PubMed] [Google Scholar]
- 33. Bollag B, Chuke WF, Frisque RJ. Hybrid genomes of the polyomaviruses JC virus, BK virus, and simian virus 40: identification of sequences important for efficient transformation. J Virol 1989; 63: 863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fraumeni JF Jr, Ederer F, Miller RW. An evaluation of the carcinogenicity of simian virus 40 in man. Jama 1963; 185: 713–8. [DOI] [PubMed] [Google Scholar]
- 35. Eddy BE, Borman GS, Grubbs GE, Young RD. Identification of the oncogenic substance in rhesus monkey kidney cell culture as simian virus 40. Virology 1962; 17: 65–75. [DOI] [PubMed] [Google Scholar]
- 36. Girardi AJ, Sweet BH, Slotnick VB, Hilleman MR. Development of tumors in hamsters inoculated in the neonatal period with vacuolating virus, SV‐40. Proc Soc Exp Biol Medical 1962; 109: 649–60. [DOI] [PubMed] [Google Scholar]
- 37. Butel JS, Tevethia SS, Melnick JL. Oncogenicity and cell transformation by papovavirus SV40: the role of the viral genome. Adv Cancer Res 1972; 15: 1–55. [DOI] [PubMed] [Google Scholar]
- 38. Shein HM, Enders JF. Multiplication and cytopathogenicity of Simian vacuolating virus 40 in cultures of human tissues. Proc Soc Exp Biol Med 1962; 109: 495–500. [DOI] [PubMed] [Google Scholar]
- 39. O’Neill FJ, Carroll D. Amplification of papovavirus defectives during serial low multiplicity infections. Virology 1981; 112: 800–3. [DOI] [PubMed] [Google Scholar]
- 40. Pepper C, Jasani B, Navabi H, Wynford‐Thomas D, Gibbs AR. Simian virus 40 large T antigen (SV40LTAg) primer specific DNA amplification in human pleural mesothelioma tissue. Thorax 1996; 51: 1074–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Carbone M, Rizzo P, Procopio A et al . SV40‐like sequences in human bone tumors. Oncogene 1996; 13: 527–35. [PubMed] [Google Scholar]
- 42. Lednicky JA, Stewart AR, Jenkins JJ 3rd, Finegold MJ, Butel JS. SV40 DNA in human osteosarcomas shows sequence variation among T‐antigen genes. Int J Cancer 1997; 72: 791–800. [DOI] [PubMed] [Google Scholar]
- 43. Galateau‐Salle F, Bidet P, Iwatsubo Y et al . SV40‐like DNA sequences in pleural mesothelioma, bronchopulmonary carcinoma, and non‐malignant pulmonary diseases. J Pathol 1998; 184: 252–7. [DOI] [PubMed] [Google Scholar]
- 44. Major EO, Traub RG. JC virus T protein during productive infection in human fetal brain and kidney cells. Virology 1986; 148: 221–5. [DOI] [PubMed] [Google Scholar]
- 45. Shibata MA, Maroulakou IG, Jorcyk CL, Gold LG, Ward JM, Green JE. p53‐independent apoptosis during mammary tumor progression in C3(1)/SV40 large T antigen transgenic mice: suppression of apoptosis during the transition from preneoplasia to carcinoma. Cancer Res 1996; 56: 2998–3003. [PubMed] [Google Scholar]
- 46. Shibata MA, Jorcyk CL, Liu ML, Yoshidome K, Gold LG, Green JE. The C3(1)/SV40 T antigen transgenic mouse model of prostate and mammary cancer. Toxicol Pathol 1998; 26: 177–82. [DOI] [PubMed] [Google Scholar]
- 47. Liu ML, Shibata MA, Von Lintig FC et al . Haploid loss of Ki‐ras delays mammary tumor progression in C3(1)/SV40 Tag transgenic mice. Oncogene 2001; 20: 2044–9. [DOI] [PubMed] [Google Scholar]
- 48. Goussia AC, Bruner JM, Kyritsis AP, Agnantis NJ, Fuller GN. Cytogenetic and molecular genetic abnormalities in primitive neuroectodermal tumors of the central nervous system. Anticancer Res 2000; 20: 65–73. [PubMed] [Google Scholar]
- 49. Wechsler‐Reya R, Scott MP. The developmental biology of brain tumors. Annu Rev Neurosci 2001; 24: 385–428. [DOI] [PubMed] [Google Scholar]
- 50. Legler JM, Ries LA, Smith MA et al . Cancer surveillance series [corrected]: brain and other central nervous system cancers. recent trends in incidence and mortality. J Natl Cancer Inst 1999; 91: 1382–90. [DOI] [PubMed] [Google Scholar]
- 51. Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol 2004; 5: 209–18. [DOI] [PubMed] [Google Scholar]
- 52. Rose DW, Saltiel AR, Majumdar M, Decker SJ, Olefsky JM. Insulin receptor substrate 1 is required for insulin‐mediated mitogenic signal transduction. Proc Natl Acad Sci USA 1994; 91: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Waters SB, Yamauchi K, Pessin JE. Functional expression of insulin receptor substrate‐1 is required for insulin‐stimulated mitogenic signaling. J Biol Chem 1993; 268: 22 231–4. [PubMed] [Google Scholar]
- 54. Burks DJ, Wang J, Towery H et al . IRS pleckstrin homology domains bind to acidic motifs in proteins. J Biol Chem 1998; 273: 31 061–7. [DOI] [PubMed] [Google Scholar]
- 55. Lassak A, Del Valle L, Peruzzi F et al . Insulin receptor substrate 1 translocation to the nucleus by the human JC virus T‐antigen. J Biol Chem 2002; 277: 17 231–8. [DOI] [PubMed] [Google Scholar]


