Abstract
A 77‐year‐old‐man was admitted to hospital for treatment of a huge hepatocellular carcinoma by transarterial chemoembolization. After treatment, the patient developed acute tumor lysis syndrome with hyperkalemia, hyperuricemia, hyperphosphatemia, hypocalcemia, metabolic acidosis and acute renal failure, which was successfully treated. In the treatments of solid organ tumors, acute tumor lysis syndrome is an extremely rare complication. To the best of the authors’ knowledge, this patient is the third case of such a complication after transarterial chemoembolization for a hepatocellular carcinoma in the English literature. (Cancer Sci 2008; 99: 2104–2105)
Case report
A 77‐year‐old man was admitted to our hospital for treatment of a huge hepatocellular carcinoma (HCC), which was detected by physical examination. The patient had no past history of serious illnesses, including any hepatitis virus infections, diabetes mellitus, operations or any hospitalization. On physical examination, a huge tumor was palpable in the upper abdomen. On admission, laboratory investigations found hemoglobin of 9.8 g/dL, platelet count of 288 000/mm3, alanine aminotransferase (ALT) of 46 IU/L, lactate dehydrogenase (LDH) of 233 IU/L, alkaline phosphatase (Al‐p) of 525 IU/L, total bilirubin of 1.0 mg/dL, creatinine of 1.0 mg/dL, uric acid of 8.6 mg/dL and alpha‐fetoprotein of 140 700 ng/mL. Before transarterial chemoembolization (TACE), computed tomography during arterial portography revealed a huge HCC occupying both hepatic lobes (Fig. 1). A transcutaneous arterial catheter was into the right hepatic artery and 70 mg of hydrochloric acid epirubicin, 20 mL of iodized oil esters and 160 mg of porous gelatine grains with a diameter of 1 mm were infused. The patient received TACE from only the right hepatic artery because of the large size of tumor (Fig. 2). One day after TACE, the patient complained of general fatigue and ran a low‐grade fever. In addition, he became oliguric. Laboratory investigations found ALT of 480 IU/L, LDH of 10 260 IU/L, Al‐p of 730 IU/L, creatinine of 1.4 mg/dL and potassium of 5.7 mmol/L. On day 3 after TACE, laboratory data included ALT of 181 IU/L, LDH of 2668 IU/L, Al‐p of 595 IU/L, creatinine of 5.2 mg/dL, uric acid of 16.3 mg/dL, potassium of 5.7 mmol/L, calcium of 7.7 mg/dL, phosphorous of 10.3 mg/dL, pH of 7.352 and anion gap of 20.3 mmol/L (Fig. 3a,b). Since the patient developed hyperkalemia, hyperuricemia, hyperphosphatemia, hypocalcemia, metabolic acidosis and acute renal failure, the diagnosis of acute tumor lysis syndrome was entertained, which was treated by allopurinol and intravenous hydration with sodium bicarbonate. The patient made a satisfactory recovery and was discharged on day 31 after TACE.
Figure 1.
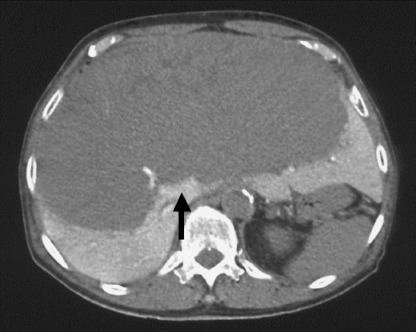
Computed tomography during arterial portography before transarterial chemoembolization revealed a huge hepatocellular carcinoma (arrow) occupying both hepatic lobes.
Figure 2.
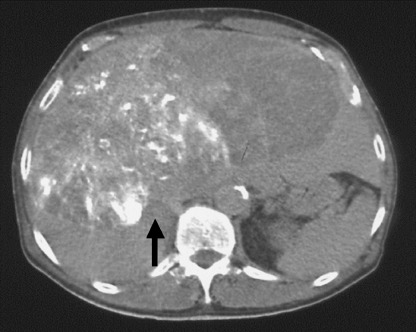
Plain computed tomography after transarterial chemoembolization revealed iodized oil esters accumulation (arrow) in the huge hepatocellular carcinoma of the right hepatic lobe.
Figure 3.
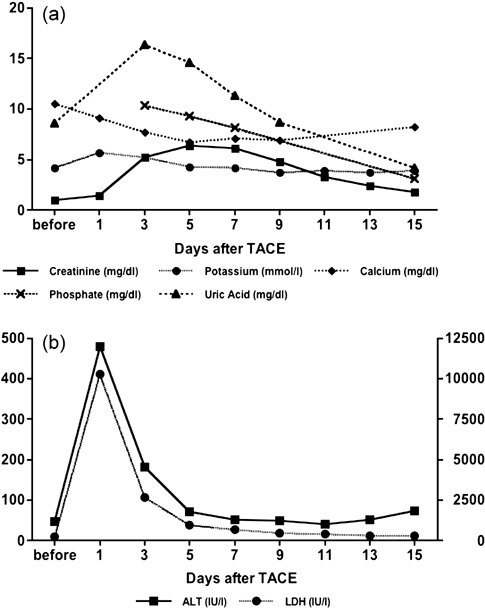
Changes in laboratory data of metabolic profiles and liver function after transarterial chemoembolization (TACE). (a) Y‐axis shows values of creatinine (mg/dL), potassium (mmol/L), calcium (mg/dL), phosphate (mg/dL) and uric acid (mg/dL). (b) Y‐axis shows values of alanine aminotransferase (IU/L) and YY‐axis shows values of lactate dehydrogenase (IU/L). All laboratory data was improved by intravenous hydration with sodium bicarbonate and administration of allopurinol.
Discussion
Acute tumor lysis syndrome is a common complication after treatments of blood‐borne malignancies such as Burkkit's lymphoma,( 1 , 2 ) acute lymphocytic leukemia( 3 , 4 ) and high‐grade non‐Hodgkin's lymphoma.( 5 , 6 ) However, in the treatments of solid organ tumors, acute tumor lysis syndrome is an extremely rare complication, because these cancers in general are resistant to cytotoxic therapies.( 7 , 8 , 9 ) Mortality by acute tumor lysis syndrome after chemotherapy of solid organs is higher than those of blood‐borne malignancies. One of the reasons for the high mortality is due to little recognition that acute tumor lysis syndrome can develop following treatment of solid organ tumors.( 7 , 8 ) In the treatment of HCC, only four such cases have been reported in the English literature, consisting of two cases following TACE,( 10 ) a case following systemic chemotherapy( 11 ) and a case following radiofrequency ablation;( 12 ) three of the four patients died of acute tumor lysis syndrome. Paye et al. reported that complications of chemoembolization for HCC, such as fever, nausea, abdominal pain and elevation of serum liver transaminases, do not indicate tumor necrosis, but reflect an injury to the non‐tumorous liver parenchyma.( 13 ) In our patient, although serum ALT increased slightly and transiently on day 1 after TACE, acute tumor lysis syndrome did develop. Therefore, we believe that acute tumor lysis syndrome in our patient developed by tumor necrosis following chemoembolization. The factors that predispose a patient to acute tumor lysis syndrome include large tumor diameter, tumors with high sensitivity to treatment, impaired renal function, dehydration and hyperuricaemia before treatment.( 14 ) For high‐risk cases, the measurements of potassium, calcium, phosphorous, urea nitrogen, creatinine and uric acid every 6 hours after treatment for 3 days are recommended. Important points in the management of acute tumor lysis syndrome depend on prevention, early recognition and prompt treatment of metabolic abnormalities, including administration of allopurinol and sodium bicarbonate as well as adequate hydration.
References
- 1. Brereton HD, Anderson T, Johnson RE, Schein PS. Hyperphosphatemia and hypocalcemia in Burkitt lymophoma: complications of chemotherapy. Arch Intern Med 1975; 135: 307–9. [PubMed] [Google Scholar]
- 2. Cadman EC, Lunberg WB, Bertino JR. Hyperphosphatermia and hypocalcemia accompanying rapid cell lysis in a patient with Burkitt's lymphoma and Burkitt cell leukemia. Am J Med 1977; 62: 283–90. [DOI] [PubMed] [Google Scholar]
- 3. Zusman J, Brown DM, Nesbit ME. Hyperphosphatemia, hyperphosphaturia and hypocalcemia in acute lymphoblastic leukemia. N Engl J Med 1973; 289: 1335–40. [DOI] [PubMed] [Google Scholar]
- 4. Boles JM, Dutel JL, Briere J et al . Acute renal failure caused by extreme hyperphosphatemia after chemotherapy of an acute lymphoblastic leukemia. Cancer 1984; 53: 2425–9. [DOI] [PubMed] [Google Scholar]
- 5. Tsokos GC, Balow JE, Spiegel RJ, Magrath IT. Renal and metabolic complications of undifferentiated and lymphoblastic lymphomas. Medicine 1981; 60: 218–29. [DOI] [PubMed] [Google Scholar]
- 6. Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high‐grade non‐Hodgkin's lymphoma. Am J Med 1993; 94: 133–9. [DOI] [PubMed] [Google Scholar]
- 7. Kalemkerian GP, Darwish B, Varterasian ML. Tumor lysis syndrome in small cell carcinoma and other solid tumors. Am J Med 1997; 103: 363–7. [DOI] [PubMed] [Google Scholar]
- 8. Baeksgaard L, Sorensen JB. Acute tumor lysis syndrome in solid tumors: a case report and review of the literature. Cancer Chemother Pharmacol 2003; 51: 187–92. [DOI] [PubMed] [Google Scholar]
- 9. Castro MP, VanAuken J, Spencer‐Cisek P, Legha S, Sponzo RW. Acute tumor lysis syndrome associated with concurrent biochemotherapy of metastatic melanoma: a case report and review of the literature. Cancer 1999; 85: 1055–9. [PubMed] [Google Scholar]
- 10. Burney IA. Acute tumor lysis syndrome after transcatheter chemoembolization of hepatocellular carcinoma. South Med J 1998; 91: 467–70. [DOI] [PubMed] [Google Scholar]
- 11. Lee CC, Wu YH, Chung SH, Chen WJ. Acute tumor lysis syndrome after thalidomide therapy in advanced hepatocellular carcinoma. Oncologist 2006; 11: 87–8. [DOI] [PubMed] [Google Scholar]
- 12. Lehner SG, Gould JE, Saad WE, Brown DB. Tumor lysis syndrome after radiofrequency ablation of hepatocellular carcinoma. Am J Roentgenol 2005; 185: 1307–9. [DOI] [PubMed] [Google Scholar]
- 13. Paye F, Farges O, Dahmane M, Vilgrain V, Flejou JF, Belghiti J. Cytolysis following chemoembolization for hepatocellular carcinoma. Br J Surg 1999; 86: 176–80. [DOI] [PubMed] [Google Scholar]
- 14. Chasty RC, Liu‐Yin JA. Acute tumour lysis syndrome. Br J Hosp Med 1993; 49: 488–92. [PubMed] [Google Scholar]


