Abstract
Dose regimens of anticancer agents are usually designed on the basis of the maximum tolerable drug doses, and toxicity prevents drug usage at higher doses, even though the drugs may be more effective at the higher doses. We previously studied macromolecular anticancer drugs, i.e. those larger than 40 kDa, and observed their accelerated accumulation in tumors. Their concentration in tumors was more than 5–100‐fold their blood concentration because of the enhanced permeability and retention (EPR) effect. Here, we report that the EPR effect was enhanced by applying nitroglycerin (NG) ointment on the skin of tumor‐bearing animals. Tumors studied included breast cancer, which was induced in Sprague–Dawley rats by the chemical carcinogen 7,12‐dimethylbenz[a]anthracene, and three different transplanted tumor models in mice. NG was applied on tumor or nontumorous normal skin as well. Two to three times more putative macromolecular drug (an Evans blue/albumin complex) was delivered to solid tumors with NG than without NG. We also demonstrated that NG enhanced tumor delivery with another macromolecular drug candidate, PZP, i.e. polyethylene glycol‐conjugated zinc protoporphyrin IX, which inhibits heme oxygenase‐1. In addition, we investigated the therapeutic effect of NG using a combination with low molecular weight anthracycline or high molecular weight PZP in mouse tumor models. NG had no apparent toxicity at the doses used, and showed significantly increased therapeutic effects in both cases. Regardless of its site of application, NG thus enhanced the delivery of the drug to tumors, and enhanced therapeutic effects. (Cancer Sci 2009; 100: 2426–2430)
Nitroglycerin (NG) has been used as medication for angina for more than a century. It is applied topically or orally, and the active molecular species generated is nitric oxide (NO), which occurs via conversion to nitrite then to NO.( 1 , 2 , 3 ) We previously demonstrated that extravasation of macromolecules is considerably enhanced in tumor tissues, and we named the phenomenon the enhanced permeability and retention (EPR) effect of macromolecules in solid tumors.( 4 ) We later demonstrated that the EPR effect is mediated by NO and other vascular mediators including bradykinin, prostaglandins, and vascular endothelial growth factor.( 5 , 6 )
The EPR effect is now known to play a major role in the tumor‐selective delivery of macromolecular or polymeric drugs, or of so‐called nanomedicines including antibodies.( 7 , 8 , 9 ) In experimental tumor models, those nanomedicines lead to 5–100 times greater intratumor drug delivery compared with the drug delivery to blood or normal tissues, though the actual drug level depending on time, type of drug, and other experimental conditions.( 8 , 9 ) Such tumor‐selective delivery, which depends on the EPR effect, was further augmented two‐ to three‐fold by applying higher hydrodynamic pressure via induced hypertension. Namely, slow intravenous (i.v.) infusion of minute doses of angiotensin ΙΙ was used to increase normal systolic blood pressure from 100–120 mm Hg to 150–160 mm Hg,( 10 , 11 ) and clinical results under such protocol are remarkable.( 12 )
Here, we report that topical application of small doses of NG enhanced tumor‐targeted delivery of anticancer drugs to a significant extent. At the same time, the therapeutic effect of these anticancer drugs in various solid tumor models was significantly improved. The method did not seem to affect drug delivery to normal tissues or organs, as discussed below, and thus the drug toxicity appeared to be unchanged.
Materials and Methods
Materials.
Evans blue, 7,12‐dimethylbenz[a]anthracene (DMBA), and corn oil were purchased from Wako Pure Chemical Industries (Osaka, Japan). Protoporphyrin ΙΧ was obtained from Sigma‐Aldrich (St. Louis, MO, USA). The succinimidyl derivative of polyethyleneglycol (PEG) was from NOF (Tokyo, Japan). NG ointment (Vasolator®) was from Sanwa Kagaku Kenkyusho (Nagoya, Japan), contained 20 mg/g of Vaseline, and was used as is or after 10 or 100 times dilution with Vaseline.
Synthesis of PEG‐conjugated zinc protoporphyrin IX (ZnPP) (PZP).
The synthesis, purification, and characterization of PZP have been previously described. (13) Briefly, PZP consisted of two chains of PEG, each of about 2500 Da, conjugated to ZnPP to form micelles of about 180 nm in diameter, which consisted of subunit PZP with a mean molecular mass of about 110 kDa. The heme oxygenase (HO‐1; also called heat shock protein [Hsp]‐32) inhibitory activity of PZP was comparable to that of free ZnPP.
Animals and tumors.
Female Sprague–Dawley rats (age, 5 weeks), male ddY mice (age, 6 weeks), female BALB/c mice (age, 6 weeks), and male C57BL/6J mice (age, 6 weeks) were purchased from SLC (Shizuoka, Japan). Three rats were housed per cage, and four or five mice were housed per cage. All animals were maintained at 22 ± 1°C and 55 ± 5% relative humidity with a 12‐h light/dark cycle. All experiments were carried out according to the Laboratory Protocol for Animal Handling of Sojo University and Kumamoto University.
Measurement of blood flow.
Blood flow in both tumor tissue (S‐180) and normal tissue (muscle, ddY mouse) was measured by a laser flow meter (Model ALF‐21, Advance Co. Ltd., Tokyo, Japan), as described in the Figure 2 legend, in which a needle‐type probe (type NSS, 5 × 802, Advance Co., Ltd.) was installed. Mice were anesthetized by isoflurane gas using an anesthesia chamber system (SF‐B01; DS Pharm, BioMed, Osaka, Japan). Five to 10 min was allowed for stabilization before NG application.
Figure 2.
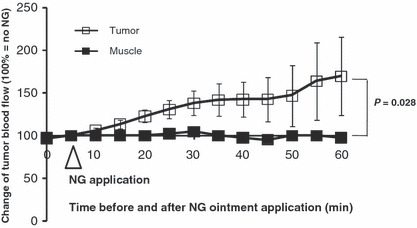
Nitroglycerin (NG) enhanced blood flow in tumor tissue and normal tissue. Blood flow was measured with a laser flowmeter (ALF‐21; Advance Co., Ltd., Tokyo, Japan). S‐180 tumor‐bearing mice with tumor diameters of 6–7 mm were anesthetized and placed on a warm pad (30–35°C). The blood flow was monitored at first for 5 min to confirm it was stable, and then NG at dose of 1.0 mg/tumor was applied on the tumor in the dorsal skin. Blood flow in the thigh muscle was measured by application of NG at a dose of 1.0 mg/site over the skin. Error bars show 95% confidence intervals. The concentration of NG was 20 mg/g ointment (Vaseline). Differences between muscle and tumor were compared with the extract two‐sided Wilcoxon tests.
Delivery of a putative macromolecular drug (Evans blue/albumin complex) to mouse S‐180 tumors in combination with NG.
S‐180 cells (2 × 106), obtained from mouse ascites maintained by weekly passage in ascetic form, were implanted subcutaneously (s.c.) in the dorsal skin of ddY mice. When the diameters of S‐180 tumors were 5–8 mm, NG was applied as an ointment at doses of 0.001–1.0 mg/tumor to the skin overlying the tumors, and within 5 min, 10 mg/kg Evans blue in 0.1 mL of PBS was injected i.v. At scheduled times thereafter, mice were killed, the tumors were removed and weighed, and the dye (Evans blue) in the tumor was extracted with formamide, as described previously.( 5 ) Controls in all experiments consisted of ointment without NG.
EPR effect‐based drug delivery to other solid tumors.
Mouse fibrosarcoma Meth‐A cells (2 × 106), maintained by intraperitoneal (i.p.) passage in BALB/c mice, were implanted s.c. in the dorsal skin of BALB/c mice. In addition, colon adenocarcinoma C38 cells were implanted s.c. in the dorsal skin of C57BL/6 mice. When Meth‐A and C38 tumors reached diameters of 5–8 mm, NG ointment was applied, then Evans blue at 10 mg/mL in 0.1 mL of PBS was injected i.v. within 5 min, and the amount of Evans blue in the tumor after 4 h was quantified as described above. In addition, breast tumors were induced in rats by oral administration of the carcinogen DMBA in corn oil. When the breast tumor diameters became 5–10 mm, NG ointment was applied to the skin overlying the tumors at NG doses of 0.02, 0.2, and 2.0 mg/tumor before Evans blue administration, as described above.
Macromolecular drug delivery to solid tumors as measured by radioactive 65Zn‐labeled PZP.
When the diameters of S‐180 tumors in ddY mice became 5–8 mm, NG ointment at 0.1 mg/tumor was applied to the skin overlying the tumors or to the nontumorous abdominal skin, opposite side of the tumors on the dorsal skin (distance to tumors: about 5 cm). Mice then immediately received i.v. injections of 65Zn‐labeled PZP via the tail vein (about 12 000 cpm/mouse). After 4 and 24 h, mice were killed, blood was collected from the inferior vena cava, and then mice were subjected to reperfusion with 10 mL of saline containing 5 units/mL heparin to remove blood components in the blood vessels of various organs and tissues. Tumor tissues and normal organs and tissues, including the liver, spleen, kidney, intestine, stomach, colon, heart, brain, lung, skin, muscle, and bone marrow, were collected and weighed. Radioactivity of the samples was measured by using a gamma counter (1480 Wizard 3″; PerkinElmer Life Sciences, Boston, MA, USA).
Investigation of the therapeutic effect of macromolecular and low‐molecular‐weight antitumor drugs in combination with NG.
One week after injection of S‐180 tumor cells in ddY mice, when tumor diameters became 5–8 mm, NG ointment (at an NG dose of 0.1 mg/tumor) was rubbed on the skin overlying the tumors, followed by i.v. injections of PZP at doses of 1 or 5 mg/kg. This therapeutic protocol was carried out daily for 3 days.
Similarly, the combination therapy of NG with PZP was investigated in the DMBA‐induced breast cancer model (i.e. NG ointment [0.1 mg/tumor] application followed by i.v. injection of PZP 24 mg/kg daily for 3 days). In this experiment, a second treatment (NG + PZP) was carried out 7 days after the first treatment.
In addition, by using a similar protocol to that described above, we investigated the therapeutic effect of the conventional low‐molecular‐weight anthracycline antitumor drug aclarubicin against C38 solid tumors with or without NG ointment. Briefly, when tumors became 5–8 mm in diameter, NG (0.01 mg/tumor) was applied to the skin overlying the tumors. Then anthracycline (5 mg/kg) was administrated via i.v. injection. The treatments continued for 3 days.
Tumor volume (V) was calculated by using the following formula: V = (L × W 2)/2, where L is the longitudinal cross‐section and W is the transverse section of the solid tumor, in mm.
Statistical analysis.
All data are expressed as means ± SEM. Student’s t‐test and extract two‐side Wilcoxon tests were used to determine the significance of differences between each experimental group; P < 0.05 was considered significant. Controls in all experiments consisted of ointment without NG.
Results
NG‐enhanced macromolecular drug delivery (EPR effect) to S‐180 tumors.
We investigated whether NG altered the EPR effect, that is, the delivery of macromolecular drugs (Evans blue/albumin complex) to tumors and inflammatory sites. Figure 1 shows the theoretical mechanism of NO generation from nitrite in hypoxic tumor tissues, which is analogous to the situation in a cardiac infarct site, i.e. both tissues become relatively hypoxic and acidic. Under these conditions, NO2 − is released from NG, and NO2 − conversion to NO may occur and NO will promote the permeability of the blood vessels (the EPR effect).( 5 ) Consistent with this hypothesis, NG significantly facilitated increased blood flow in the tumor (Fig. 2, P = 0.028) whereas no change of blood flow was seen in the normal muscle tissue.
Figure 1.
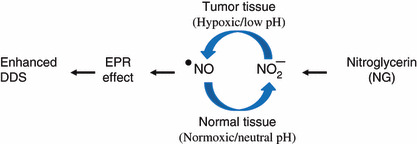
Hypothetical mechanism of nitric oxide (NO) generation. NO was generated from nitrite, predominantly in hypoxic tumor tissues rather than normal tissues. DDS, drug delivery system; EPR, enhanced permeability and retention.
The present results showed a time‐dependent increase in accumulation of a putative macromolecular drug, Evans blue/albumin complex, in S‐180 solid tumors (Fig. 3a, P = 0.006). Namely, NG resulted in two‐ to three‐fold greater drug delivery to solid tumors at 4 h after drug injection than without NG (Fig. 3a, P = 0.002). Further, the tumors retained the higher drug concentration for at least 24 h after NG treatments. Similar results were observed with other solid tumors (Meth‐A, C38, and DMBA‐induced breast cancer) and in PZP experiment below: all of which showed a two‐ to three‐fold increased drug delivery compared with delivery without NG.
Figure 3.
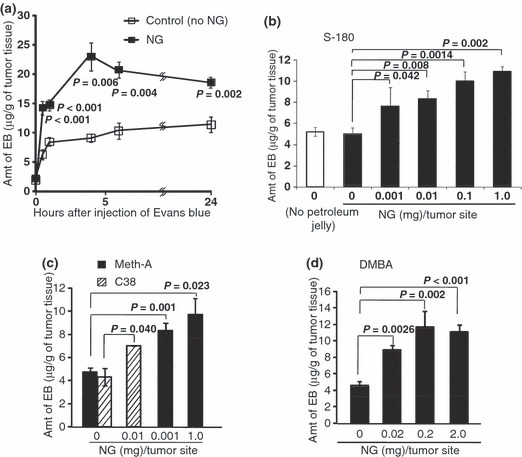
Time‐ and nitroglycerin (NG) dose‐dependent enhancement of macromolecular drug delivery to various solid tumors. (a) Time course of enhancement of delivery of Evans blue (EB) to S‐180 solid tumors by NG ointment (1 mg/tumor) (as shown in Fig. 2) applied to the skin overlying tumors. (b–d) Dose‐dependent enhancement of macromolecular drug delivery to S‐180, Meth‐A, C38, and 7,12‐dimethylbenz[a]anthracene (DMBA)‐induced breast tumors, respectively. In all tumor models, when tumor diameters were 5–8 mm, 10 mg/mL Evans blue in 0.1 mL of PBS was injected intravenously, and 4 h later the concentration of Evans blue in tumors was quantified (see Materials and Methods and text for details). Error bars show 95% confidence intervals. Differences between control (no NG) and NG groups were compared with the Student’s t‐test. Statistical tests were two‐sided. ns, non‐significant.
Further, NG‐dose dependent increase of drug delivery or permeability (EPR effect) was examined in different tumor models (S‐180, MethA, Colon38, DMBA tumors), as shown in Figure 3(b–d). NG as low as 0.001 mg/tumor was effective up to 2 mg/tumor. This was confirmed also in other settings as discussed later.
Enhancement of delivery of radioactive polymeric macromolecular drug to solid tumors.
To reconfirm the effect of NG, we investigated another macromolecular drug, the PEGylated candidate drug PZP (Fig. 4).( 13 , 14 , 15 ) Use of NG resulted in two times more delivery of PZP to tumors than that without use of NG at both 4 and 24 h after drug injection (Fig. 4a,b). This effect was also found when NG was applied to mice at a distance of 5 cm from the tumor (Fig. 4c). The effect of NG in enhancing macromolecular delivery was significant (P = 0.002 and 0.004 at 4 h and 24 h, respectively) in tumors, but in most normal organs, it was insignificant, expect in the spleen and liver (P = 0.05 and 0.1) where about 15–30% increased delivery was seen at 24 h (Fig. 4a,b). Also important findings of the effect of NO on the enhanced tumor delivery (EPR effect) were that its effect was both dose‐ and time‐dependent (Fig. 3). It was interesting that application of NG to abdominal skin – when the tumor was located on the dorsal skin – also produced significantly increased delivery of PZP to the tumor (Fig. 4b and c).
Figure 4.
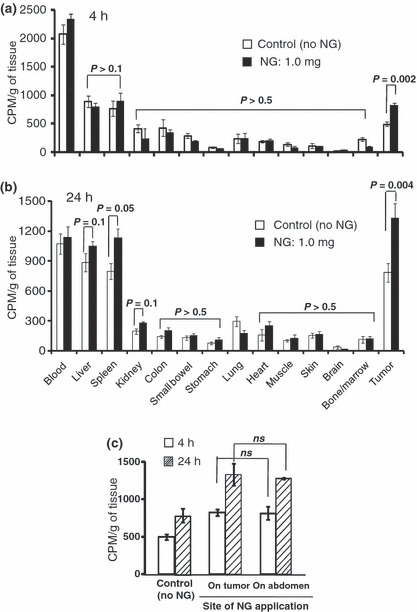
Nitroglycerin (NG)‐enhanced radioactive polymeric drug delivery to S‐180 solid tumors. NG was applied as an ointment at a dose of 1.0 mg/tumor either to the skin overlying tumors in (a,b) or to nontumorous abdominal skin, opposite side of the tumor on the dorsal skin (distance to tumor: about 5 cm) in (c). 65Zn‐labeled PZP (12 000 cpm/mouse) was then injected intravenously into the tumor‐bearing mice via the tail vein. After 4 h (a) and 24 h (b), the mice were killed; samples of blood, tumor, and normal tissues and organs were collected; and radioactivity of these tissues and organs was measured. Error bars show 95% confidence intervals. Differences between control (no NG) and NG groups were compared with the Student t‐test. Statistical tests were two‐sided. CPM, count per minute.
Augmentation of therapeutic effects of anticancer drugs in combination with NG.
To demonstrate whether NG also enhances the therapeutic effect of anticancer drugs, we first used PZP against S‐180 solid tumors. As Figure 5(a) shows, NG in combination with PZP exhibited significantly greater suppression of tumor growth when compared with PZP alone, in which the tumors regressed completely in three of five mice on day 25 after the treatment. In addition, NG in combination with the low‐molecular‐weight anthracycline antitumor agent aclarubicin significantly suppressed the growth of C38 tumors compared with aclarubicin alone (Fig. 5b). These results indicated that the beneficial effect of NG appears applicable to both low‐ and high‐molecular‐weight drugs.
Figure 5.
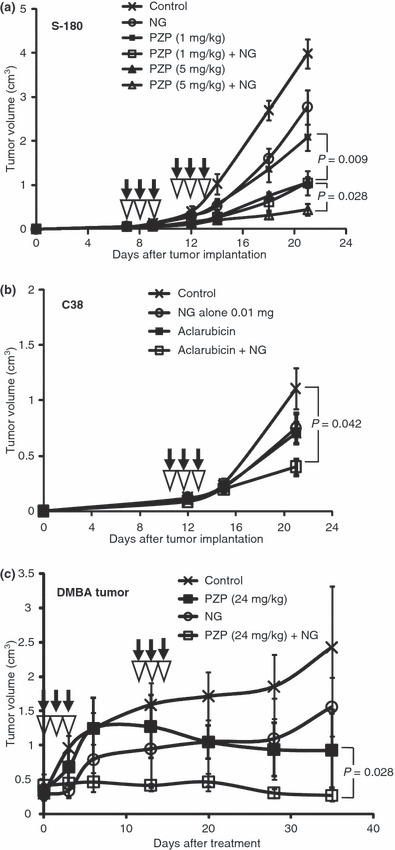
Enhancement of the therapeutic effect of antitumor drugs by nitroglycerin (NG) treatment. (a) The effect of the combination of NG (0.1 mg/tumor) and the macromolecular drug polyethylene glycol‐conjugated zinc protoporphyrin IX (PZP) against S‐180 solid tumors. (b) The effect of NG (0.01 mg/tumor) in combination with the low‐molecular‐weight drug aclarubicin (5 mg/kg) against C38 solid tumors. (c) Therapeutic effect of PZP plus NG (0.1 mg/tumor) in 7,12‐dimethylbenz[a]anthracene (DMBA)‐induced tumor. Black arrows indicate the times of administration of the drugs; opened inverted triangles, NG treatment. Error bars show 95% confidence intervals. Differences between control (antitumor drug alone) and the combination of NG groups were compared with extract two‐side Wilcoxon tests. Statistical tests were two‐sided.
Next, we extended the study further to DMBA‐induced rat tumors. Many anticancer agents have little effect against chemically induced tumors, and our DMBA‐induced breast cancer was a more realistic tumor model than the transplanted tumors. In this tumor model, we also observed an additive therapeutic effect of NG with PZP in which three tumor out of 10 regressed completely in the case of 24 mg PZP/kg with NG (Fig. 5c, P = 0.028).
Discussion
The present results reveal the effect of NG as facilitating vascular blood flow and also vascular permeability of drugs in solid tumors, regardless of the site of NG application. The effect is analogous to the cardiac infarct, where vascular dilatation has been primarily observed in hypoxic cardiac tissue. NG conversion to NO in the relatively hypoxic tumor may be pathobiochemically similar to that in infarct tissue.( 1 , 2 ) Jordan et al. also have reported that isosorbite dinitrate (ISDN) increased partial O2 (pO2) in tumors due to increased blood flow.( 16 ) Further NO converted from nitrite sensitized tumor cell radiotherapy, as previously shown by Mitchell et al. ( 17 ) However, there has been no previous report concerning the enhancement of the EPR‐effect or cancer drug delivery by NG‐derived NO. Consistent with our results, Frérart et al. recently reported( 18 ) that in tumors, production of NO from nitrite observed under acidic/hypoxic conditions increased the partial O2 pressure of the transplantable liver tumor (carcinoma).
The significantly increased macromolecular drug delivery induced by NG was a dose‐dependent phenomenon that was observed even at a concentration as low as 0.001 mg NG/mouse (Fig. 3a). More importantly, the amount of drug delivered to the chemical (DMBA)‐induced breast tumors in rats, analogous to natural breast cancer, also showed this increased EPR effect (Fig. 3d).
Lastly, it should also be emphasized that we always observed that NG per se has a tumor suppressive effect, which suggests a mechanism other than just enhancing drug delivery. NG significantly increased blood flow into tumors (Fig. 2), and not only did it induce increase of drug delivery, it might have also down‐regulated some gene expression, which may have contributed to tumor growth, as well as normalizing hypoxic conditions. Consistent with the present findings, Yasuda et al. reported that NG increased the sensitivity of tumors to chemotherapy by increasing the blood flow of hypoxic tumors. These authors further observed suppression in tumors of hypoxia‐inducible factor (HIF)‐1α, vascular endothelial growth factor, and P‐glycoprotein expression,( 19 ) all of which are known to play important roles in the resistance of cancer cells to chemotherapy. More recently they confirmed remarkable clinical benefit from nitro‐compounds.( 20 ) Therefore, combination therapy with NG may have a significantly additive chemotherapeutic effect via multiple mechanisms.
In conclusion, the therapeutic strategy of using NG appears to have important benefits, and further efforts toward clinical applications are warranted.
Acknowledgments
This work was supported by Grants‐in‐Aid for Scientific Research on Cancer Priority Areas (no. 20015045), and Scientific Research (C) (nos. 20590049, S0801085) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, to H.M.
References
- 1. Fukuto JM, Cho JY, Switzer CH. The chemical properties of nitric oxide and related nitrogen oxides. In: Ignarro LJ, ed. Nitric Oxide: Biology and Pathobiology. San Diego, CA: Academic Press, 2000; 23–39. [Google Scholar]
- 2. Feelisch M, Noack EA. Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol 1987; 139: 19–30. [DOI] [PubMed] [Google Scholar]
- 3. Chen Z, Stamler JS. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends Cardiovasc Med 2006; 16: 259–65. [DOI] [PubMed] [Google Scholar]
- 4. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986; 46: 6387–92. [PubMed] [Google Scholar]
- 5. Maeda H, Noguchi Y, Sato K, Akaike T. Enhanced vascular permeability in solid tumor is mediated by nitric oxide and inhibited by both new nitric oxide scavenger and nitric oxide synthase inhibitor. Jpn J Cancer Res 1994; 85: 331–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu J, Akaike T, Maeda H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and nitric oxide scavenger. Cancer Res 1998; 58: 159–65. [PubMed] [Google Scholar]
- 7. Seki T, Fang J, Maeda H. Tumor targeted macromolecular drug delivery based on the enhanced permeability and retention effect in solid tumor. In: Lu Y, Mahato RI, eds. Pharmaceutical Perspectives of Cancer Therapeutics. New York: AAPS‐Springer, 2009; 93–12. [Google Scholar]
- 8. Maeda H, Sawa T, Konno T. Mechanism of tumor‐targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J Control Release 2001; 74: 47–61. [DOI] [PubMed] [Google Scholar]
- 9. Maeda H. SMANCS and polymer‐conjugated macromolecular drugs: advantages in cancer chemotherapy. Adv Drug Deliv Rev 2001; 46: 169–85. [DOI] [PubMed] [Google Scholar]
- 10. Greish K, Fang J, Inutsuka T, Nagamitsu A, Maeda H. Macromolecular therapeutics: advantages and prospects with special emphasis on solid tumour targeting. Clin Pharmacokinet 2003; 42: 1089–105. [DOI] [PubMed] [Google Scholar]
- 11. Li CJ, Miyamoto Y, Kojima Y, Maeda H. Augmentation of tumour delivery of macromolecular drugs with reduced bone marrow delivery by elevating blood pressure. Br J Cancer 1993; 67: 975–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagamitsu A, Greish K, Maeda H. Elevating blood pressure as a strategy to increase tumor targeted delivery of macromolecular drug SMANCS: Cases of advanced solid tumors. Jpn J Clin Oncol 2009; doi: 10.1093/jjco/hyp074. [DOI] [PubMed] [Google Scholar]
- 13. Sahoo SK, Sawa T, Fang J et al. Pegylated zinc protoporphyrin: a water‐soluble heme oxygenase inhibitor with tumor‐targeting capacity. Bioconjug Chem 2002; 13: 1031–8. [DOI] [PubMed] [Google Scholar]
- 14. Fang J, Sawa T, Akaike T et al. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res 2003; 63: 3567–74. [PubMed] [Google Scholar]
- 15. Fang J, Sawa T, Akaike T, Greish K, Maeda H. Enhancement of chemotherapeutic response of tumor cells by a heme oxygenase inhibitor, pegylated zinc protoporphyrin. Int J Cancer 2004; 109: 1–8. [DOI] [PubMed] [Google Scholar]
- 16. Jordan BF, Misson P‐D, Demeure R et al. Changes in tumor oxygenation/perfusion induced by the NO donor, isosorbide dinitrate, in comparison with carbogen: monitoring by EPR and MRI. Int J Radiat Oncol Biol Phys 2000; 48 (2): 565–70. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell JB, Wink DA, DeGraff W et al. Hypoxic mammalian cell radiosensitization by nitric oxide. Cancer Res 1993; 53 (24): 5845–8. [PubMed] [Google Scholar]
- 18. Frérart F, Sonveaux P, Rath G et al. The acidic tumor microenvironment promotes the reconversion of nitrite into nitric oxide: towards a new and safe radiosensitizing strategy. Clin Cancer Res 2008; 14: 2768–74. [DOI] [PubMed] [Google Scholar]
- 19. Yasuda H, Nakayama K, Watanabe M et al. Nitroglycerin treatment may enhance chemosensitivity to docetaxel and carboplatin in patients with lung adenocarcinoma. Clin Cancer Res 2006; 12: 6748–57. [DOI] [PubMed] [Google Scholar]
- 20. Yasuda H, Yanagihara K, Nakayama K et al. Therapeutic applications of nitric oxide for malignant tumor in animal models and human studies. In: Bonavida B, ed. Nitric Oxide and Cancer. New York: Springer Science, 2009. (in press). [Google Scholar]


