Abstract
Chronic hepatitis C virus (HCV) infection often results in hepatocellular carcinoma (HCC). Previous studies have shown that there might be some characteristic mutations in the core region of HCV related to HCC. Thus, we downloaded and analyzed HCV genotype 1b core gene sequences from HCV databases online to identify them. Based on the information of the sequences, 63 from patients with HCC and 188 from non‐HCC were enrolled into our analysis. Then, the nucleotides at each position were compared by χ2‐test between the two groups, and 24 polymorphisms were found to be associated with HCC. Further analysis of these 24 polymorphisms by logistic regression indicated that eight were significantly related to the increased HCC risk: A028C, G209A, C219U/A, U264C, A271C/U, C378U/A, G435A/C, and G481A. Moreover, U303C/A was associated with the decreased HCC risk. These mutations could bring about four amino acid substitutions: K10Q, R70Q, M91L, and G161S. In conclusion, eight characteristic mutations in the HCV‐1b core gene related to the occurrence of HCC were identified. The structural and functional alterations of core protein due to these mutations and the relationship with the occurrence of HCC need to be further studied. (Cancer Sci 2009; 100: 2465–2468)
Hepatitis C virus (HCV) is a major cause of chronic hepatitis worldwide and a major risk factor for hepatocellular carcinoma (HCC). More than 70% of patients with HCC in Japan are infected with HCV, and HCC has become the major cause of death in patients chronically infected with HCV.( 1 , 2 , 3 ) In Asia, especially in Japan, HCV‐1b is the most prevalent genotype and is more likely to develop into HCC than other genotypes.( 4 , 5 ) Although there are many published data about the correlation between HCV infection and HCC development, details of hepatocarcinogenesis by HCV remain unclear. HCV core protein is implicated in hepatocarcinogenesis for its ability to modulate cellular gene transcription and protein expression, intracellular signal transduction, cell proliferation and apoptosis.( 6 , 7 , 8 , 9 )
Being an RNA virus, the HCV genome exhibits a considerable degree of sequence variation. Studies have proved that certain kinds of mutations may lead to functional changes of the virus, such as the resistance to antiviral treatments and the association with HCC.( 10 , 11 ) Furthermore, there are more mutations in the core region in patients with HCC than that in those without HCC. Moreover, the rate of nucleotide substitutions in the core gene is significantly greater for isolates from HCC patients compared to those from individuals with chronic hepatitis.( 12 ) It was also found that there was a significantly higher variability within the core region of tumor tissue isolates than that of isolates from non‐tumor tissue. Mutant sequence diversity ranged from silent mutations, as well as amino acid substitutions, to appearance of in‐frame stop codons and deletions leading to frame‐shifts. In contrast, the variability of the NS5 region sequences between isolates from tumor and non‐tumor tissue was not significantly different.( 13 ) Recently, a report indicated that amino acid substitutions in the HCV core region are important predictor of hepatocarcinogenesis.( 11 ) Even in patients without HCC, the substitution of amino acid 70 in the hepatitis C virus core region of genotype 1b is an important predictor of elevated alpha‐fetoprotein.( 14 )
Based on the data above, we suppose that there might be some characteristic mutations in the HCV‐1b core gene related to the occurrence of HCC. Because we can find thousands of HCV sequences from databases online, we have tried to identify these mutations by analyzing such sequences in this study.
Materials and Methods
Collection of HCV core gene sequences HCV core gene sequences were downloaded from the following databases: European HCV database (euHCVdb), HCV Databases from Los Alamos National Laboratory, HCV Database (HCVdb) from the Viral Bioinformatics Resource Center (VBRC), and National Center for Biotechnology Information (NCBI).
Inclusion criteria All the sequences enrolled into our analysis needed to comply with the following inclusion criteria: (i) the diagnosis of patients could be confirmed; (ii) the genotype of HCV could be confirmed and samples were isolated from sera of patients infected with HCV‐1b; and (iii) the core gene sequence was of full length.
Exclusion criteria Sequences were excluded if: (i) the diagnosis of patients or the genotype of HCV was unknown; (ii) samples came from liver, other tissues, ascites, or pooled sera; (iii) patients were co‐infected with hepatitis B virus or human immunodeficiency virus; (iv) patients had ever been treated with interferon (IFN); (v) patients had ever undergone orthotopic liver transplantation (OLT); (vi) samples came from cell culture, animal experiments, chimeric DNA, or fusion protein; (vii) sequences came from repeated samples; and (viii) existence of deletion mutations or insertion mutations.
Statistical analysis According to the diagnosis of the cases, sequences enrolled were divided into two groups: group HCC and group non‐HCC. The first base of the start codon (AUG) was designated position 1. All the 573 nucleotides of the core region were compared respectively between group HCC and non‐HCC. All the 573 nucleotides of the core region were compared respectively between group HCC and non‐HCC by Pearson χ2‐test to find out significantly different mutations (when there were cells that the expected count was less than 5 or 1, we chose different χ2‐test methods – Continuity correction or Fisher's exact test). Then, the significant bases were further analyzed by logistic regression to identify the mutations significantly related to the occurrence of HCC. A P‐value of <0.05 was considered statistically significant.
Results and Discussion
Enrollment of sequences We downloaded sequences of HCV core region from HCV databases online, mainly from euHCVdb. A total of 2841 sequences of HCV‐RNA with full‐length core region were downloaded. Then, we checked the information provided by the databases and the published papers so that we could get the details about these sequences such as the diagnoses of patients, genotypes of HCV, the origin of samples, and so on. Finally, we picked out the sequences that complied with the inclusion and exclusion criteria and enrolled them into our analysis. As a result, from 2841 sequences, we found 1336 sequences of genotype 1b. Of these, 547 sequences were from identified samples and the others lacked information that identified the types of the samples. Among the 547 sequences with definite origin, 292 were from sera and the others (228 from livers, 20 from cultured cells, seven from plasmids) were ruled out. Of the 292 sequences derived from sera, seven were from animal experiments, 24 were from repeated cases at different time points, and four and six were from cases that had undergone OLT and IFN treatments, respectively. Finally, 251 sequences complied with the inclusion and exclusion criteria and were enrolled into our analysis. Sixty‐three were from patients with HCC and 188 were from HCV carriers or patients with acute or chronic hepatitis without HCC. For the 251 sequences, 73.7% (185/251) were directly sequenced and 26.3% (66/251) represented one clone of available clones. Thus, it is possible that about a quarter of our data could contain mutations due to PCR error. However, the sequences were collected from 42 different studies and the statistical analysis may have overcome this problem.
Identification of the wild‐type nucleotides For the reason that there are frequent mutations of the HCV sequence, it is impossible to know the real wild‐type nucleotides. It might be reasonable to identify the wild‐type nucleotide based on the consensus of a large number of sequences. In our analysis, we defined the consensus of the 1336 HCV‐1b sequences as the wild type, except the position of nucleotide (nt) 209. Because 209G was treated as wild type in almost all the published papers and it was also the consensus type of 2841 all genotypes sequences, we still defined “G” as the wild type even if “A” was the consensus.
Significantly different mutations between group HCC and non‐HCC By performing the χ2‐test or Fisher’s exact test, we compared all the 573 nucleotides of the core gene between group HCC and non‐HCC. A total of 24 nucleotide mutations were found to be associated with occurrence of HCC. Of the 24 nucleotide mutations, 21 increased the risk of HCC while three decreased the risk of HCC (Table 1).
Table 1.
Significant nucleotide mutations found by χ2‐tests
| Gene position | Types of Nucleotide | Percentage of mutant | Amino acid | P‐values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild | Mutant | Non‐HCC | HCC | Wild type | Mutant | ||||||
| nt 009 | G | → | A/U | 20.2 | → | 68.3 | Thr | Thr | <0.001* | ||
| nt 028 | A | → | C | 1.1 | → | 7.9 | Lys | → | Gln | 0.015† | |
| nt 039 | U | → | G/A | 0 | → | 4.8 | Arg | Arg | 0.015‡ | ||
| nt 048 | C | → | G/U | 0 | → | 4.8 | Asn | → | Lys/Asn | 0.015‡ | |
| nt 066 | C | → | U | 14.4 | → | 30.2 | Val | Val | 0.005* | ||
| nt 078 | C | → | U | 28.7 | → | 3.2 | ↓ | Gly | Gly | <0.001* | |
| nt 116 | G | → | C | 0 | → | 7.9 | Arg | → | Pro | 0.001† | |
| nt 209 | G | → | A | 28.7 | → | 52.4 | Arg | → | Gln | 0.001* | |
| nt 219 | C | → | U/A | 7.4 | → | 23.8 | Gly | Gly | <0.001* | ||
| nt 264 | U | → | C | 12.2 | → | 36.5 | Asn | Asn | <0.001* | ||
| nt 270 | C | → | U | 8 | → | 33.3 | Gly | Gly | <0.001* | ||
| nt 271 | A | → | C/U | 21.8 | → | 65.1 | Met | → | Leu | <0.001* | |
| nt 303 | U | → | C/A | 64.9 | → | 47.6 | ↓ | Arg | Arg | 0.015* | |
| nt 309 | U | → | C | 22.3 | → | 39.7 | Ser | Ser | 0.007* | ||
| nt 378 | C | → | U/A | 16.5 | → | 34.9 | Leu | Leu | 0.002* | ||
| nt 417 | C | → | U | 5.9 | → | 20.6 | Leu | Leu | 0.001* | ||
| nt 435 | G | → | A/C | 10.1 | → | 34.9 | Gly | Gly | <0.001* | ||
| nt 446 | G | → | A | 0.5 | → | 7.9 | Arg | → | Lys | 0.004† | |
| nt 456 | G | → | A | 24.5 | → | 47.6 | Ala | Ala | 0.001* | ||
| nt 462 | C | → | U | 25 | → | 49.2 | Gly | Gly | <0.001* | ||
| nt 471 | U | → | C/G | 38.3 | → | 20.6 | ↓ | Val | Val | 0.01* | |
| nt 472 | C | → | G/U | 2.1 | → | 9.5 | Leu | → | Val/Leu | 0.026† | |
| nt 481 | G | → | A | 2.7 | → | 20.6 | Gly | → | Ser | <0.001† | |
| nt 549 | C | → | U | 14.4 | → | 30.2 | Ser | Ser | 0.005* | ||
*Pearson χ2‐tests; †continuity correction; ‡Fisher’s exact test.
Gene polymorphisms and amino acid substitutions associated with HCC The 24 significant nucleotide mutations found by χ2‐tests were further analyzed by logistic regression. Eight polymorphisms were significantly related to the increased HCC risk: A028C, G209A, C219U/A, U264C, A271C/U, C378U/A, G435A/C, and G481A; and U303C/A was significantly associated with decreased HCC risk (Table 2). Furthermore, four polymorphisms brought about amino acid substitutions which might change the structure and functions of core protein and lead to HCC. For the other five synonymous mutations, it is possible that they might lead to amino acid substitutions of ARFP (alternative ribosomal frame shift protein) which has been reported to be able to affect cell proliferation and apoptosis( 15 ) (Table 3). However, HCC is thought to be a consequence of long‐term infection of HCV and chronic hepatitis. Although the gene polymorphisms and amino acid substitutions in the HCV core region identified in this analysis were proven to be significantly associated with HCC, whether they are “causes” or “effects” of HCC remains to be determined.
Table 2.
Significant nucleotide mutations identified in logistic regression
| Gene position | Mutation types | Odds ratio | 95% CI | P‐values | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| nt 028 | A→C | 14.65 | 1.31 | 163.51 | 0.029 |
| nt 209 | G→A | 3.30 | 1.40 | 7.78 | 0.006 |
| nt 219 | C→U/A | 4.37 | 1.49 | 12.77 | 0.007 |
| nt 264 | U→C | 4.13 | 1.62 | 10.52 | 0.003 |
| nt 271 | A→C/U | 5.50 | 2.26 | 13.37 | <0.001 |
| nt 303 | U→C/A | 0.42 | 0.18 | 0.98 | 0.045 |
| nt 378 | C→U/A | 2.69 | 1.08 | 6.73 | 0.034 |
| nt 435 | G→A/C | 4.16 | 1.61 | 10.72 | 0.003 |
| nt 481 | G→A | 10.484 | 2.77 | 39.62 | 0.001 |
Nagelkerke R 2 = 0.572.
Table 3.
Amino acid substitutions of core protein and ARFP due to nucleotide mutations
| Codon | Gene position | Amino acid position | Nucleotide | Amino acid | ||||
|---|---|---|---|---|---|---|---|---|
| Wild type | Mutant | Wild type | Mutant | |||||
| Normal | nt 028 | aa 010 | AAA | → | CAA | Lys | → | Gln |
| nt 209 | aa 070 | CGG | → | CAG | Arg | → | Gln | |
| nt 219 | aa 073 | GGC | → | GGU/A | Gly | Gly | ||
| nt 264 | aa 088 | AAU | → | AAC | Asn | Asn | ||
| nt 271 | aa 091 | AUG | → | C/UUG | Met | → | Leu | |
| nt 303 | aa 101 | CGU | → | CGC/A | Arg | Arg | ||
| nt 378 | aa 126 | CUC | → | CUU/A | Leu | Leu | ||
| nt 435 | aa 145 | GGG | → | GGA/C | Gly | Gly | ||
| nt 481 | aa 161 | GGC | → | AGC | Gly | → | Ser | |
| +1 Codon | nt 028 | aa 010 | GAA | → | GAC | Glu | → | Asp |
| nt 209 | aa 070 | GGC | → | AGC | Gly | → | Ser | |
| nt 219 | aa 073 | GCA | → | GU/AA | Ala | → | Val/Glu | |
| nt 264 | aa 088 | AUG | → | ACG | Met | → | Thr | |
| nt 271 | aa 091 | GCA | → | GCC/U | Ala | Ala | ||
| nt 303 | aa 101 | GUG | → | GC/AG | Val | → | Ala/Glu | |
| nt 378 | aa 126 | UCA | → | UU/AA | Ser | → | Leu/Stop | |
| nt 435 | aa 145 | GGG | → | GA/CG | Gly | → | Glu/Ala | |
| nt 481 | aa 161 | ACG | → | ACA | Thr | Thr | ||
| −1 Codon | nt 028 | aa 010 | AAA | → | ACA | Lys | → | Thr |
| nt 209 | aa 070 | CCG | → | CCA | Pro | Pro | ||
| nt 219 | aa 073 | CAG | → | T/AAG | Gln | → | Stop/Lys | |
| nt 264 | aa 088 | UGA | → | CGA | Stop | → | Arg | |
| nt 271 | aa 091 | CAU | → | CC/UU | His | → | Pro/Leu | |
| nt 303 | aa 101 | UGG | → | C/AGG | Trp | → | Arg | |
| nt 378 | aa 126 | CAC | → | U/AAC | His | → | Tyr/Asn | |
| nt 435 | aa 145 | GGG | → | A/CGG | Gly | → | Arg | |
| nt 481 | aa 161 | CGG | → | CAG | Arg | → | Gln | |
ARFP, alternative ribosomal frame shift protein.
Recently, a similar study( 16 ) determined four amino acid substitutions associated with increased HCC risk, and three of them were the same as our results (G209A, A271C/U, and G481A). Moreover, up to now, the G209A polymorphism has been thought to be related with both IFN/Ribavirin (RBV) treatment resistance and HCC.( 10 , 11 ) The relationship between them is an interesting question. We guess that apoptosis might make an important function in this process. Apoptosis is considered to be a common pathway of virus clearance. HCV core protein could suppress apoptosis and escape this clearance mechanism.( 9 ) On the other hand, inhibition of apoptosis might be able to make the genetically damaged hepatocytes survive and lead to neoplastic transformation. Whether the amino acid substitution of R70Q could enhance these effects is worth studying.
Alteration of the RNA and protein secondary structures of the HCV‐1b core gene Gene mutations of A271C/U, G435A/C, and G481A, were found to be able to cause the changes of the RNA secondary structure of HCV‐1b core gene using RNAdraw software( 17 ) (Karolinska Institute, Stockholm, Sweden). Furthermore, Chou–Fasman protein secondary prediction with the gene analysis software Genetyx (Genetyx, Tokyo, Japan) showed that A028C might turn the coil structure of AA8‐12 into β‐sheet and G209A might turn the coil stucture of AA71 into α‐helix (Fig. 1). The results indicated that the point‐mutations of the core gene might change the secondary structure of not only RNA but also protein. As a result, the functions of both RNA and protein of the core region, such as interaction with other DNA/RNA or proteins (lymphotoxin B receptor, heterogeneous‐nuclear ribonucleoprotein, tumor necrosis factor receptor 1, etc.),( 18 , 19 , 20 ) might change and lead to HCC.
Figure 1.
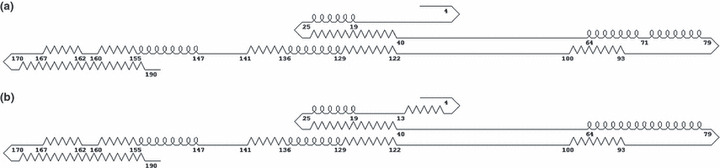
Secondary structure of wild‐type (a) and mutant (b) hepatitis C virus (HCV)‐1b core protein predicted by Chou–Fasman analysis with Genetyx. The straight, bent, looped, and zigzag lines represent coil, β‐sheet, α‐helix, and turn structures, respectively. The numbers indicate the amino acid positions. A028C might turn the coil stucture of AA8‐12 into β‐sheet and G209A might turn the coil stucture of AA71 into α‐helix. (The secondary structure of the wild type was predicted based on the following consensus amino acid sequence of 1336 HCV‐1b core, except 70R: MSTNPKPQRKTKRNTNRRPQDVKFPGGGQIVGGVYLLPRRGPRLGVRATRKTSERSQPRGRRQPIPKARRPEGRAWAQPGYPWPLYGNEGMGWAGWLLSPRGSRPSWGPTDPRRRSRNLGKVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRVLEDGVNYATGNLPGCSFSIFLLALLSCLTIPASA.)
In conclusion, eight characteristic mutations in the HCV‐1b core gene related to the occurrence of HCC were identified: A028C, G209A, C219U/A, U264C, A271C/U, C378U/A, G435A/C, and G481A. Meanwhile, U303C/A was identified to be significantly associated with the decreased HCC risk. These mutations could bring about four amino acid substitutions: K10Q, R70Q, M91L, and G161S. The structural and functional alterations of core protein due to these mutations and the relationship with the occurrence of HCC need to be further studied.
Acknowledgments
The study was supported by the Global COE Program “Center of Education and Research for Advanced Genome‐Based Medicine: For personalized medicine and the control of worldwide infectious diseases”, the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; by Health and Labor Sciences Research Grants for Research on Hepatitis from the Ministry of Health, Labor and Welfare, Japan; and by a Japan–China Sasakawa Medical Fellowship.
References
- 1. Saito I, Miyamura T, Ohbayashi A et al. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A 1990; 87: 6547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shiratori Y, Shiina S, Imamura M et al. Characteristic difference of hepatocellular carcinoma between hepatitis B‐ and C‐ viral infection in Japan. Hepatology 1995; 22: 1027–33. [DOI] [PubMed] [Google Scholar]
- 3. Kato Y, Hamasaki K, Aritomi T et al. Most of the patients with cirrhosis in Japan die from hepatocellular carcinoma. Oncol Rep 1999; 6: 1273–6. [DOI] [PubMed] [Google Scholar]
- 4. Takada A, Tsutsumi M, Zhang SC et al. Relationship between hepatocellular carcinoma and subtypes of hepatitis C virus: a nationwide analysis. J Gastroenterol Hepatol 1996; 11: 166–9. [DOI] [PubMed] [Google Scholar]
- 5. Lee CM, Hung CH, Lu SN et al. Viral etiology of hepatocellular carcinoma and HCV genotypes in Taiwan. Intervirology 2006; 49: 76–81. [DOI] [PubMed] [Google Scholar]
- 6. Kato N, Yoshida H, Ono‐Nita SK et al. Activation of intracellular signaling by hepatitis B and C viruses: C‐viral core is the most potent signal inducer. Hepatology 2000; 32: 405–12. [DOI] [PubMed] [Google Scholar]
- 7. Otsuka M, Kato N, Lan K‐H et al. Hepatitis C virus core protein enhances P53 function through augmentation of DNA‐binding affinity and transcriptional ability. J Biol Chem 2000; 275: 34122–30. [DOI] [PubMed] [Google Scholar]
- 8. Yoshida H, Kato N, Shiratori Y et al. Hepatitis C virus core protein activates NF‐κB‐dependent signaling through tumor necrosis factor receptor‐associated factor. J Biol Chem 2001; 276: 16399–405. [DOI] [PubMed] [Google Scholar]
- 9. Otsuka M, Kato N, Taniguchi H et al. Hepatitis C virus core protein inhibits apoptosis via enhanced Bcl‐xL expression. Virol 2002; 296: 84–93. [DOI] [PubMed] [Google Scholar]
- 10. Akuta N, Suzuki F, Sezaki H et al. Association of amino acid substitution pattern in core protein of hepatitis C virus genotype 1b high viral load and non‐virological response to interferon‐ribavirin combination therapy. Intervirology 2005; 48: 372–80. [DOI] [PubMed] [Google Scholar]
- 11. Akuta N, Suzuki F, Kawamura Y et al. Amino acid substitutions in the hepatitis C virus core region are the important predictor of hepatocarcinogenesis. Hepatology 2007; 46: 1357–64. [DOI] [PubMed] [Google Scholar]
- 12. Shimizu I, Yao DF, Horie C et al. Mutations in a hydrophilic part of the core gene of hepatitis C virus in patients with hepatocellular carcinoma in China. J Gastroenterol 1997; 32: 47–55. [DOI] [PubMed] [Google Scholar]
- 13. Rüster B, Zeuzem S, Krump‐Konvalinkova V et al. Comparative sequence analysis of the core‐ and NS5‐region of hepatitis C virus from tumor and adjacent non‐tumor tissue. J Med Virol 2001; 63: 128–34. [PubMed] [Google Scholar]
- 14. Akuta N, Suzuki F, Kawamura Y et al. Substitution of amino acid 70 in the hepatitis C virus core region of genotype 1b is an important predictor of elevated alpha‐fetoprotein in patients without hepatocellular carcinoma. J Med Virol 2008; 80: 1354–62. [DOI] [PubMed] [Google Scholar]
- 15. Shao SW, Wu WB, Bian ZQ et al. Hepatitis C virus F protein inhibits cell apoptosis by activation of intracellular NF‐kappaB pathway. Hepatol Res 2009; 39: 282–9. [DOI] [PubMed] [Google Scholar]
- 16. Fishman SL, Factor SH, Balestrieri C et al. Mutations in the hepatitis C virus core gene are associated with advanced liver disease and hepatocellular carcinoma. Clin Cancer Res 2009; 15: 3205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matzura O, Wennborg A. RNAdraw: an integrated program for RNA secondary structure calculation and analysis under 32‐bit Microsoft Windows. Comput Appl Biosci 1996; 12: 247–9. [DOI] [PubMed] [Google Scholar]
- 18. Matsumoto M, Hsieh TY, Zhu N et al. Hepatitis C virus core protein interacts with cytoplasmic tail of lymphotoxin‐beta recepor. J Virol 1997; 71: 1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsieh TY, Matsumoto M, Chou HC et al. Hepatitis C virus core protein interacts with heterogeneous nuclear ribonucleoprotein. J Biol Chem 1998; 273: 17651–9. [DOI] [PubMed] [Google Scholar]
- 20. Zhu N, Khosnan A, Schneider R et al. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF‐induced apoptosis. J Virol 1998; 72: 3691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


