Abstract
CD109 is a glycosylphosphatidylinositol (GPI)–anchored glycoprotein whose expression is up‐regulated in squamous cell carcinomas (SCCs) of the lung, esophagus, and uterus. The purpose of this study was to evaluate CD109 expression in oral tumors, including premalignant lesions, and to assess the clinical application of CD109 in oral cancer. CD109 expression in oral normal and tumor tissues from 124 patients was examined by immunohistochemical staining with anti‐CD109 antibody, and significant relations between clinical features and CD109 expression were statistically assessed. We found that high levels of CD109 expression were frequently detected in SCCs and premalignant lesions of the oral cavity, but not in normal squamous epithelia. The CD109 expression level was higher in well‐differentiated SCCs than in poorly differentiated SCCs. Furthermore, premalignant lesions highly expressing CD109 showed higher risk to progress to SCCs. Oral SCC cell lines overexpressing CD109 exhibited accelerated cell growth in vitro compared with control cell lines. In addition, overexpression of CD109 impaired the transforming growth factor (TGF)–β1‐mediated suppression of cell growth. These findings suggest that CD109 plays a role in the development of oral cancers, and is a useful prognostic marker to predict malignant transformation of premalignant lesions. (Cancer Sci 2008; 99: 1916–1923)
Up‐regulation or down‐regulation of the expression of some genes, including oncogenes and tumor suppressor genes, trigger the development of human tumors; the products of these genes are potentially good molecular targets for cancer diagnosis and treatment. In oral cancers, despite the ease of clinical examination, most cancer patients are diagnosed with advanced‐stage cancer and are difficult to treat because of the anatomic location of the lesions or because the treatment would impact on the patients’ quality of life. Therefore, early diagnosis of high‐risk premalignant lesions is most important for good prognosis.
CD109 is a glycosylphosphatidylinositol (GPI)–anchored cell‐surface glycoprotein and is a member of the α2‐macroglobulin‐C3, C4, and C5 family of thioester‐containing proteins.( 1 , 2 , 3 ) The CD109 protein was first identified as a cell‐surface antigen detected by a monoclonal antibody raised against the primitive lymphoid/myeloid cell line KG1a.( 1 ) It has been reported that CD109 is expressed on a subset of fetal and adult CD34+ bone marrow mononuclear cells, mesenchymal stem cell subsets, phytohemagglutinin (PHA)–activated T lymphoblasts, thrombin‐activated platelets, leukemic megakaryoblasts, endothelial cells, and some human tumor cell lines, but not on fresh peripheral leukocytes and normal bone marrow leukocytes.( 1 , 2 , 4 , 5 ) It was also shown that CD109 carries the biallelic platelet‐specific alloantigen Gov, which is implicated in refractoriness to platelet transfusion, post‐transfusion purpura, and neonatal alloimmune thrombocytopenia.( 6 , 7 , 8 ) A Tyr703Ser polymorphism of CD109 is associated with Gova and Govb alloantigenic determination.( 9 )
We identified CD109 as a gene up‐regulated in cells overexpressing oncogenic RET tyrosine kinase with a multiple endocrine neoplasia (MEN) type 2B mutation.( 10 , 11 , 12 , 13 ) Analysis of its gene expression pattern in human tissues and tumor cell lines showed that the CD109 transcript is expressed mainly in testis and some tumor cell lines, suggesting that CD109 may be a cancer‐testis antigen.( 13 ) It was also revealed that the expression level of the CD109 transcript assessed by quantitative real‐time polymerase chain reaction (PCR) is increased in squamous cell carcinomas (SCC) of the esophagus, lung, and uterus.( 13 , 14 ) In addition, high expression levels of CD109 protein were frequently detected in lung SCC tissues in an immunohistochemical staining study using an anti‐CD109 antibody.( 15 ) On the other hand, CD109 expression in normal tissues appears to be restricted to limited cells, including myoepithelial cells of the breast, salivary, lacrimal, and bronchial secretary glands, and basal cells of the prostate and bronchial epithelia.( 15 , 16 ) The high expression in SCCs and the limited expression in normal tissues suggests that CD109 may have a role in the development of SCC, although the physiological functions of CD109 and the clinical significance of CD109 expression remain unclear.
In the present study, we analyzed the clinical significance of CD109 expression using clinical specimens of human oral cancer as well as cultured cells derived from oral cancer tissue. Our immunohistochemical study detected CD109 in oral neoplasms, including premalignant lesions, in which a high expression level of CD109 was preferentially detected in well‐differentiated SCCs. Statistical analysis revealed that high expression of CD109 was associated with malignant transformation of premalignant lesions. Furthermore, CD109 overexpression in cells accelerated cell proliferation and impaired an antiproliferative effect mediated by TGF‐β1 in vitro. These findings suggest that CD109 expression plays a role in the development of oral cancer.
Materials and Methods
Cells and cell‐culture conditions. SAS cells (derived from human oral poorly differentiated SCC), A431 cells (derived from human skin SCC), and their derivative transfectants were maintained in Dulbecco's modified Eagle's medium supplemented with 8% fetal bovine serum at 37°C in 5% CO2.
Vector construction and generation of stable transfectants. Construction of pcDNA3.1(+)/FLAG‐CD109 possessing FLAG‐tagged full‐length human CD109 cDNA has been described previously.( 13 ) To generate the stable cell lines overexpressing FLAG‐tagged CD109 (FLAG‐CD109) or control cell lines (SAS‐CD109 and SAS‐VC, respectively), SAS cells were transfected with pcDNA3.1(+)/FLAG‐CD109 or empty plasmid using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA), and 500‐µg/mL Geneticin (Invitrogen) was added to the medium 48 h after transfection. Several clones of stable transfectants were obtained after Geneticin selection, and the FLAG‐CD109 expression levels were assessed by western blotting with an anti‐FLAG M2 antibody.
Antibodies. Rabbit polyclonal anti‐CD109 antibody was produced by immunization with a synthetic peptide of 17 amino acids of human CD109 (amino acids 1383–1399) as described previously (Fig. 1a).( 13 ) Anti‐FLAG M2 and anti‐β‐actin monoclonal antibodies were purchased from Sigma (St Louis, MO, USA).
Figure 1.
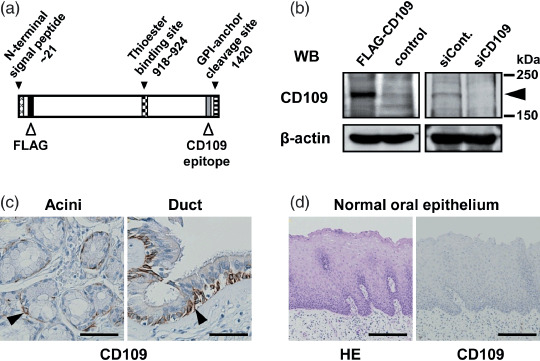
CD109 expression in normal tissues of the oral cavity. (a) Schematic illustration of CD109 protein. A FLAG sequence was inserted after the signal peptide sequence to generate the FLAG‐CD109 recombinant protein. (b) Western blotting for validation of anti‐CD109 antibody specificity. Lysates form SAS cells transfected with pcDNA3.1(+)/FLAG‐CD109, pcDNA3.1(+), control siRNA, and CD109 siRNA (FLAG‐CD109, Control, siCont. and siCD109, respectively) were subjected to western blotting with anti‐CD109 and anti‐β‐actin antibodies. The arrowhead indicates CD109 protein. (c,d) Immunohistochemical staining (hematoxylin–eosin [HE]) of normal oral tissues with the anti‐CD109 antibody. Scale bars: c, 50 µm; d, 200 µm.
Anti‐Smad2/3 monoclonal antibody and antiphospho‐Smad2 polyclonal antibody were purchased from BD Bioscience (Mississauga, ON, Canada) and Cell Signaling Technologies (Beverly, MA, USA), respectively.
Western blotting. Culture cells were lyzed in sodium dodecyl sulfate (SDS) sample buffer (62.5 mM Tris‐HCl [pH 6.8] 2% SDS, 25% glycerol, 20 µg/mL bromophenol blue, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate, 2%β‐mercaptoethanol) with sonication. After boiling for 2 min, the lysates were applied to polyacrylamide gels and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% bovine serum albumin in TBST buffer (20 mM Tris‐HCl [pH 7.6] 137 mM NaCl, 0.1% Tween 20) for 60 min, and then incubated with primary antibodies for 60 min at room temperature (RT) followed by an incubation with secondary antibodies conjugated to horseradish peroxidase (Dako, Kyoto, Japan) for 60 min at RT. After washing the membranes, antigen‐antibody complexes were visualized using an ECL Detection Reagent (GE Healthcare UK, Buckinghamshire, England). To prepare lysates from normal and tumor tissues, about 1 mg of each tissue was lyzed in SDS sample buffer by sonication, and cleared by centrifugation.
RNA interference. siRNA‐mediated knockdown of CD109 was performed using a siGENOME SMART pool specific to human CD109, purchased from Dharmacon (Lafayette, CO, USA). SAS cells were transfected with siRNA (50 nM) using Lipofectamine RNAiMAX reagent (Invitrogen). After 48 h incubation, cells were subjected to western blotting or cell proliferation assay.
Patients and tissues. Formalin‐fixed, paraffin‐embedded specimens were prepared from biopsy tissues or surgically resected tissues of the oral cavity from non‐pretreated patients clinically diagnosed with oral neoplasms at the Department of Oral and Maxillofacial Surgery of Nagoya University Hospital from 1994 to 2006. The pathological diagnosis of each lesion was confirmed by two pathologists. This study was approved by the Ethical Committee of Nagoya University School of Medicine. The clinical features of the patients are summarized in Table 1. The clinical stages of malignant tumors were determined according to the International Union Against Cancer (UICC) classification of head and neck tumors, 2002.
Table 1.
Number of patients in each clinical feature and histopathological group
| Clinical feature | Total | Histopathology † | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal | Dysplasia | CIS | Well | Moderately | Poorly | Others ‡ | ||
| Total | 124 | 8 | 22 | 8 | 39 | 18 | 11 | 18 |
| Gender | ||||||||
| Male | 62 | 1 | 12 | 5 | 23 | 7 | 6 | 8 |
| Female | 62 | 7 | 10 | 3 | 16 | 11 | 5 | 10 |
| Mean age (years) | 61.1 | 52.5 | 63.2 | 70.0 | 61.5 | 66.4 | 60.4 | 52.9 |
| (Range) | (22–90) | (38–65) | (48–78) | (53–83) | (33–87) | (34–90) | (42–78) | (22–84) |
| Locus | ||||||||
| Tongue | 57 | 4 | 9 | 5 | 22 | 12 | 5 | 0 |
| Gingiva | 31 | 2 | 7 | 1 | 8 | 5 | 1 | 7 |
| Buccal mucosa | 13 | 1 | 3 | 2 | 7 | 0 | 0 | 0 |
| Oral floor | 12 | 1 | 2 | 0 | 2 | 1 | 3 | 3 |
| Palate | 8 | 0 | 1 | 0 | 0 | 0 | 2 | 5 |
| Others§ | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Clinical stage¶ | ||||||||
| 0 | 8 | 8 | 0 | 0 | 0 | 0 | ||
| I | 15 | 0 | 8 | 3 | 0 | 4 | ||
| II | 18 | 0 | 9 | 4 | 0 | 5 | ||
| III | 19 | 0 | 12 | 2 | 2 | 3 | ||
| IVA | 26 | 0 | 10 | 8 | 6 | 2 | ||
| IVB | 5 | 0 | 0 | 1 | 3 | 1 | ||
| IVC | 1 | 0 | 0 | 0 | 0 | 1 | ||
CIS, Carcinoma in situ; Moderately, moderately differentiated SCC; Normal, normal epithelium; Others, other malignant tumors; Poorly, poorly differentiated SCC; SCC, squamous cell carcinoma; Well, well‐differentiated SCC.
Adenoid cystic carcinoma, 6; ameloblastic fibrosarcoma, 1; basal cell adenocarcinoma, 1; malignant melanoma, 3; mucoepidermoid carcinoma, 3; myoepithelial carcinoma, 1; polymorphous adenocarcinoma, 1.
Metastatic carcinoma from other organs (lung small cell c. 1; thyroid papillary c. 1).
Parotid gland, two cases; mandible, one case.
Two metastatic carcinomas were excluded.
Immunohistochmical analysis. Immunohistochemical staining was performed using the UltraTech HRP Streptavidin‐Biotin Detection System (Beckman Coulter, CA, USA). Formalin‐fixed paraffin‐embedded sections (3 µm) were deparaffinized in xylene, and dehydrated through a series of graded alcohols. For antigen retrieval, they were pretreated with boiling in 0.01 M citrate buffer (pH 6.0) for 10 min using a microwave. Endogenous peroxidase was blocked with 3% hydrogen peroxide (H2O2) in methanol for 15 min. After the blocking step, sections were incubated with anti‐CD109 antibody for 60 min at RT, and the biotinylated secondary antibody was applied for 10 min at RT. The sections were then reacted with peroxidase‐conjugated streptavidin for 10 min, and reaction products were visualized using 3,3′‐diaminobenzidine tetrahydrochloride and H2O2. Nuclear counterstaining was performed using hematoxylin. As a negative control, staining was also performed without the anti‐CD109 antibody. Positive expression of CD109 protein in tumor tissues was judged when more than 10% of the tumor region was stained by the anti‐CD109 antibody. The expression scores were defined as 0, 1, 2, and 3 when the CD109‐positive areas were less than 10%, 10–30%, 30–60%, or more than 60% of the lesions, respectively.
Cell proliferation assay. Cells were seeded in 96‐well plates (1 × 103 cells per well) with 100 µL of Dulbecco's modified Eagle's medium supplemented with 4% fetal bovine serum. Twenty‐four h after seeding, the cell proliferation assay was commenced using WST‐1 Reagent (Roche, Basel, Switzerland) according to the manufacturer's protocol. Absorbance was measured at a wavelength of 450 nm with a reference wavelength of 620 nm using a microplate reader (Tecan, Palm Springs, CA, USA).
Statistical analysis. Statistical analyses for significant relations between clinical features and CD109 expression were carried out using the χ2‐test (on positive ratios) or the Mann–Whitney U‐test (on average scores). Statistical significance of the measured values was analyzed using the two‐tailed Student's t‐test (in cell proliferation). P < 0.05 was considered to be significant.
Results
CD109 expression in normal tissues in the oral cavity. A high level of CD109 was detected in SCCs of the lung in our previous immunohistochemical staining study using an anti‐CD109 antibody;( 15 ) however, the clinical significance of CD109 expression was unclear. To address this question, we analyzed CD109 expression precisely in human oral tissues by immunohistochemical staining with the anti‐CD109 antibody which we previously developed.( 15 , 16 )
Western blot analysis showed that the anti‐CD109 antibody clearly recognized endogeneous and exogeneously expressed CD109 proteins in SAS human oral SCC cells, with a molecular mass of about 180–190 kDa (Fig. 1a,b) as previously reported.( 15 , 16 ) Exogenously expressed CD109 was also detected using an anti‐FLAG antibody (data not shown). When the endogeneous CD109 in SAS cells was depleted by transfection with CD109 siRNA, the band representing CD109 became undetectable, indicating the specificity of the CD109 antibody (Fig. 1b).
Patients’ clinical features are summarized in Table 1. In normal tissues of the oral cavity, CD109 expression was detected in myoepithelial cells of the minor salivary acini and basal cells of salivary ducts (Fig. 1c, arrowheads). No positive expression was observed in the normal squamous epithelia (Fig. 1d, Table 2).
Table 2.
Summary of CD109 expression, positive ratios and scores in each pathological group
| Histopathology | Number of positive/total | % of positive | Expression scores † | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| Normal | 0/8 | 0 | 8 | 0 | 0 | 0 | |
| Dysplasia | 18/22 | 81.8** | 4 | 6 | 8 | 4 | |
| CIS | 8/8 | 100** | 0 | 2 | 3 | 3 | |
| SCC | (Well) | 39/39 | 100** | 0 | 5 | 11 | 23 |
| (Moderately) | 16/18 | 88.9** | 2 | 4 | 5 | 7 | |
| (Poorly) | 7/11 | 63.6* | 4 | 4 | 2 | 1 | |
| Others | 4/18 | 22.2 | 14 | 3 | 1 | 0 | |
Scores 0, 1, 2 and 3 represent the CD109 positive areas; less than 10%, 10–30%, 30–60% or more than 60% of the lesions, respectively.
P < 0.05,
P < 0.001, compared with the groups of other malignant tumors and normal epithelium using the χ2 test, respectively.
CIS, Carcinoma in situ; SCC, squamous cell carcinoma.
CD109 expression in oral tumor tissues. Next, we analyzed CD109 expression in oral neoplastic lesions, including dysplasia, SCCs, and other malignant tumors (Table 1). As shown in Table 2 (% of positive), all the cases of carcinoma in situ (CIS) and well‐differentiated SCC, including verrucous carcinoma, were positive for CD109 expression (Fig. 2a,b). Keratinizing regions were also highly positive (Fig. 2c). By contrast, the positive ratios in SCCs decreased as the grade of differentiation became poor (88.9% and 63.6% in moderately and poorly differentiated SCCs, respectively) (Table 2, Fig. 2d). CD109 expression was confirmed by western blotting with the anti‐CD109 antibody using well‐differentiated SCC tissues immunohistochemically positive for CD109 and their adjacent normal squamous epithelia, in which high levels of CD109 were detected in tumor tissues, indicating the specific immunoreactivity of anti‐CD109 antibody (Fig. 2h).
Figure 2.
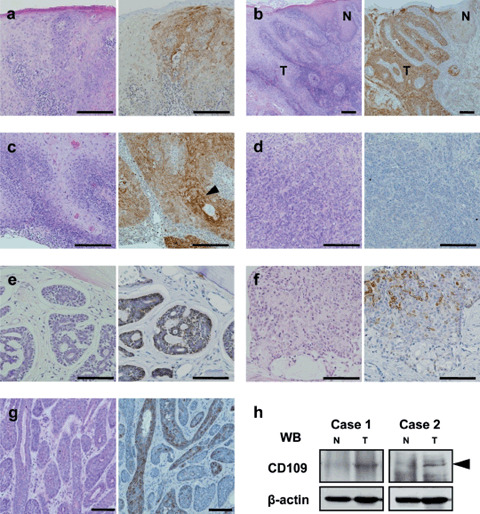
CD109 expression in oral tumor tissues. (a) Carcinoma in situ. (b) Well‐differentiated squamous cell carcinoma (SCC). (c) Well‐differentiated SCC with keratinizing regions (arrowhead). (d) Poorly differentiated SCC (negative case). (e) Adenoid cystic carcinoma. (f) Mucoepidermoid carcinoma. (g) Basal cell adenocarcinoma. Left panels, hematoxylin–eosin stain; right panels, CD109 immunostaining. Scale bars: a,b,c,d, 200 µm; e,f,g, 100 µm. (h) Western blotting for CD109 expression in SCC and adjacent normal tissues. Lysates were prepared from well‐differentiated SCC and adjacent normal tissues of two patients. N, normal tissue; T, tumor tissue.
On the other hand, only a few cases of other malignant tumors, including adenoid cystic carcinomas, mucoepidermoid carcinomas, and basal cell adenocarcinomas, were revealed to be CD109‐positive (Table 2). The myoepithelial components of adenoid cystic carcinomas (two cases), the squamous cell component of mucoepidermoid carcinoma (one case), and basal cell adenocarcinoma (one case) were positive for CD109 (Fig. 2e–g); however, the other cases were negative for CD109 (data not shown).
To evaluate the expression level of CD109 in tumor tissues, we established a scoring system based on the percentages of the tumor regions that were positive for CD109; scores were defined as 0, 1, 2, and 3 when the CD109 positive areas were less than 10%, 10–30%, 30–60%, or more than 60% of the lesions, respectively. The numbers of the cases with each score across all the pathological groups are summarized in Table 2. As shown in Fig. 3(a), the average score of each pathological group revealed that CD109 expression level was highest in well‐differentiated SCCs (the average score was 2.46) followed by CIS (the average score was 2.13). Interestingly, the levels of CD109 expression in SCCs decreased as the grade of differentiation became poor (the average scores were 1.94 and 1.00 in moderately and poorly differentiated SCCs, respectively). Although the relation between CD109 expression and other clinical features such as age, gender, clinical stage, and 5‐year survival without recurrence were assessed in the group of well‐differentiated SCCs, no significant relation was detected (Fig. 3b).
Figure 3.
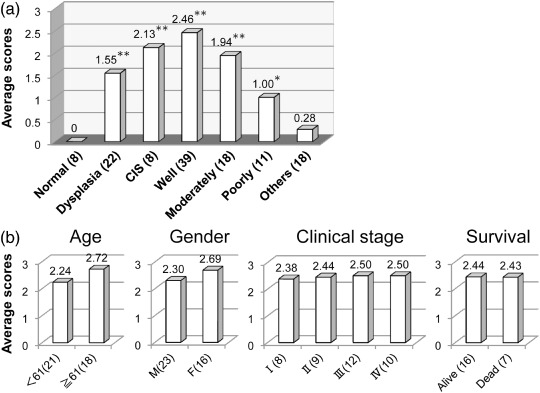
CD109 expression levels associated with histopathological (a) and clinical features (b). Average expression scores in each group are shown. (n), number of cases; CIS, Carcinoma in situ; Moderately, moderately differentiated SCC; Normal, normal epithelium; Others, other malignant tumors; Poorly, poorly differentiated SCC; Well, well‐differentiated SCC. *P < 0.05, **P < 0.001 compared with groups of Others and Normal using the Mann–Whitney U‐test.
CD109 expression is associated with malignant transformation of premalignant lesions. To assess the contribution of CD109 to the carcinogenic process of oral cancer, CD109 expression was also analyzed in dysplasias by immunohistochemical staining. The percentage of cases positive for CD109 was 81.8% (Table 2). Then, all the cases were divided into two groups on the basis of the clinical course: Group A, dysplasias that did not progress to SCC during a 3‐year follow‐up period; and Group B, dysplasias that progressed to SCCs during this follow‐up period. The percentages of CD109‐positive cases in Group A and B were 55.6% and 100%, respectively (Fig. 4a, Table 3). The CD109 expression levels of the two groups were also assessed using our scoring system. The average scores in Group A and B were 0.89 and 2.11, respectively. The average score in Group B was comparable to that in the CIS group (Fig. 4b). It is interesting to note that two Group B cases with a score of 1 developed moderate‐ or poorly differentiated SCCs, whereas all seven Group B cases with scores of 2 and 3 developed well‐differentiated SCCs. These data suggest that CD109 expression may contribute to the malignant transformation of premalignant lesions, and that its expression level may be associated with the differentiation of SCCs.
Figure 4.
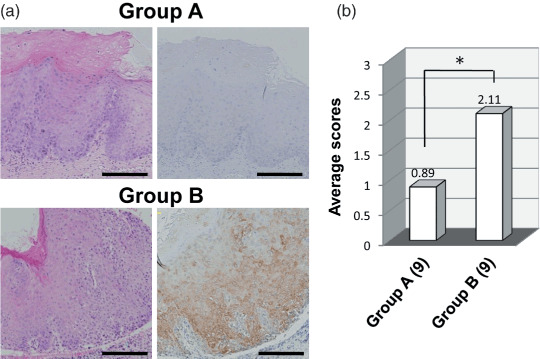
CD109 expression is associated with malignant transformation of dysplasia. Group A, dysplasias that did not progress to squamous cell carcinoma (SCC) in the follow‐up periods; Group B, dysplasias that progressed to SCCs during the follow‐up period. (a) Representative examples of CD109 staining in the cases of Group A and B. Left panels, hematoxylin–eosin stain; right panels, CD109 immunostaining. Scale bars, 200 µm (b) Average expression scores in Group A and B. (n), number of cases. *P = 0.014, using the Mann–Whitney U‐test.
Table 3.
CD109 expression in two groups of dysplasia
| Malignant change | n | Expression scores † | Average period ‡ (months) | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| Group A | (–) | 9 | 4 | 2 | 3 | 0 | – |
| Group B | (+) | 9 | 0 | 2 | 4 | 3 | 20.2 (6–35) |
Scores 0, 1, 2, and 3 represent the CD109 positive areas; less than 10%, 10–30%, 30–60%, or more than 60% of the lesions, respectively.
Average of periods from diagnosis of dysplasia to the diagnosis of squamous cell carcinoma.
CD109 overexpression accelerates cell proliferation in vitro. To investigate the effect of CD109 expression on the biological properties of cancer cells, we generated three stable transfectants overexpressing FLAG‐CD109 and control transfectants using the oral SCC cell line SAS (SAS‐CD109 and SAS‐VC). The expression level of CD109 in SAS‐CD109 cells was approximately five‐times as high as that in control cells when assessed by western blotting with the anti‐CD109 antibody (Fig. 5a). Then, the cell growth of each transfectant was analyzed in a cell proliferation assay using WST‐1 reagent. As shown in Fig. 5(b), SAS‐CD109 cells grew significantly faster than SAS‐VC cells. In contrast, CD109 knockdown cells with siRNA exhibited slower cell growth than control cells (Fig. 5c). Western blot analysis showed that the effect of CD109 knockdown continued for 5 days after siRNA transfection (data not shown). In addition, we generated a transfectant of the skin SCC cell line A431 overexpressing CD109. A431‐CD109 cells also grew significantly faster than A431‐VC cells (Suppl. Fig. S1). These results suggest that CD109 overexpression accelerates cell proliferation and contributes to tumor growth.
Figure 5.
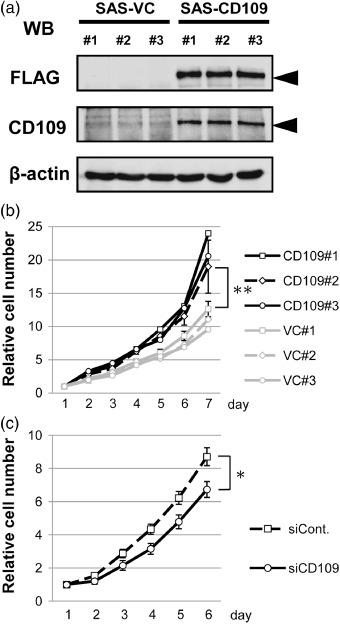
CD109 overexpression accelerates cell proliferation. (a) Western blotting for assessment of FLAG‐CD109 expression in SAS‐VC and SAS‐CD109 cells. Lysates of three SAS‐VC and three SAS‐CD109 cell lines were subjected to western blotting with anti‐FLAG, anti‐CD109, and anti‐β‐actin antibodies. (b) Proliferation analyses of SAS‐VC and SAS‐CD109 cell lines. (c) Proliferation analyses of SAS transfected with control or CD109 siRNA. The absorbance values at day 1 were defined as 1.0. The means ± SD of three independent experiments are shown (bars). *P < 0.01, **P < 0.001, using the Student's t‐test.
CD109 attenuates TGF‐β1/Smad2 signaling and impairs TGF‐β1‐mediated suppression of cell growth. It was recently reported that CD109 interacts with the type I TGF‐β receptor and inhibits TGF‐β1 signaling by direct modulation of receptor activity in human keratinocytes.( 17 , 18 ) Thus, we performed the TGF‐β1 stimulation assay to assess the levels of Smad2 phosphorylation in CD109‐overexpressing or CD109‐knockdown cells. As shown in Fig. 6(a), when SAS transfectants were stimulated with the recombinant human TGF‐β1 (50 pM), Smad2 phosphorylation in SAS‐CD109 cells significantly decreased compared to that in SAS‐VC cells. In contrast, CD109 knockdown increased Smad2 phosphorylation by TGF‐β1 stimulation. These results demonstrate that CD109 negatively regulates TGF‐β1/Smad2 signaling in SAS as observed in human keratinocytes.( 18 )
Figure 6.
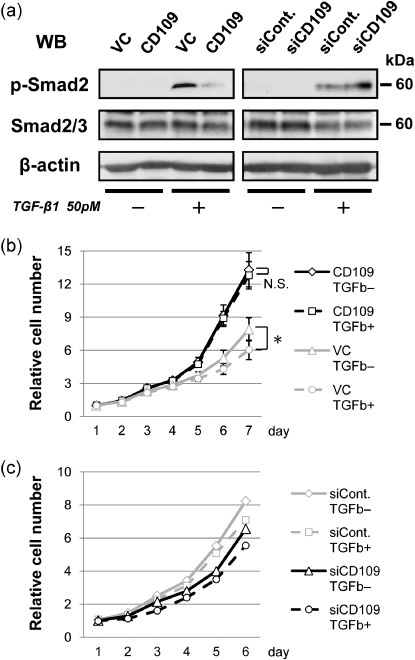
CD109 attenuates TGF‐β/Smad2 signaling. (a) Western blotting for assessment of Smad2 phosphorylation induced by TGF‐β1 (50 pM) in CD109‐overexpressing cells (left panel), and CD109‐knockdown cells (right panel). After 1 h stimulation with TGF‐β1, lysates of SAS‐VC and SAS‐CD109 cells, or SAS cells transfected with control and CD109 siRNA (siCont. and siCD109), were subjected to western blotting with antiphospho‐Smad2 (p‐Smad2), antitotal Smad2/3, and anti‐β‐actin antibodies. (b) Proliferation analyses of SAS‐VC and SAS‐CD109 cell lines in the presence or absence of TGF‐β1 (TGF‐β+ or TGF‐β–). The means ± SD are shown (bars). *P < 0.01, using the Student's t‐test. N.S., not significant. (c) Proliferation analyses of knockdown cells in the presence or absence of TGF‐β1.
To further investigate whether TGF‐β1 affects the growth of CD109‐overexpressing cells, a cell‐proliferation assay was performed in the absence or presence of TGF‐β1. As shown in Fig. 6(b), TGF‐β1 significantly suppressed the growth of SAS‐VC cells, whereas it did not affect the growth of SAS‐CD109 cells. This is consistent with the finding that a high level of CD109 expression inhibits Smad2 phpsphorylation. In contrast, the degrees of cell‐growth suppression by TGF‐β1 were not significantly different between control and CD109 siRNA‐transfected cells (Fig. 6c). This is probably due to a low level of endogenous CD109 expression in SAS cells.
Discussion
CD109 is a cell‐surface glycoprotein whose function is obscure. We have been analyzing the CD109 expression in human normal and tumor tissues by immunohistochemical staining using an anti‐CD109 antibody.( 15 , 16 ) In lung tumor tissues, high levels of CD109 were frequently detected in SCCs compared with other histological types of lung carcinoma, including adenocarcinomas, large cell carcinomas, and small cell carcinomas, whereas in normal lung tissues, CD109 expression was confined to myoepithelial cells of the bronchial secretary glands and basal cells of the bronchial and bronchiolar epithelia.( 15 ) CD109‐positive immunoreactivity in normal tissues was also detected in restricted cells, such as myoepithelial cells of the mammary, salivary and lacrimal glands, and basal cells of the prostate.( 16 ) Our findings, together with previous reports showing CD109 expression on hematopoietic and mesenchymal stem cell subsets, activated T lymphoblasts and platelets, and some human tumor cell lines,( 1 , 2 , 4 , 5 ) demonstrate that CD109 plays a role in cell proliferation and tumor development, especially that of SCC, and that CD109 expression in normal tissues may be strictly controlled.
In the present study, we assessed the significance of CD109 expression in tumor development and cell proliferation using human oral tumor tissues and cancer cell lines. We found that CD109 is highly expressed in well‐differentiated SCCs rather than in moderately or poorly differentiated SCCs, and that its expression level is associated with the malignant transformation of premalignant lesions. Furthermore, we found that CD109 overexpression accelerates cell proliferation in vitro. Although an association of CD109 with other clinical features, including patients’ survival, was not demonstrated, our findings suggest that CD109 expression is associated with oral cancer development, in part due to an acceleration of cell proliferation. It was also demonstrated that CD109 is a possible molecular marker for high‐risk premalignant lesions. However, further investigation is necessary to elucidate the role of CD109 in tumor development.
It was recently reported that CD109 protein is a component of the TGF‐β1 receptor system in human keratinocytes.( 17 , 18 ) It was also shown that CD109 overexpression decreases TGF‐β1‐induced phosphorylation of Smad2 and Smad3, and TGF‐β1‐induced growth inhibition.( 18 ) It is well known that TGF‐β signaling is involved in diverse cellular activities, such as cell proliferation, differentiation, migration, and extracellular matrix deposition via Smad and mitogen‐activated protein kinase (MAPK) pathways.( 19 , 20 ) During carcinogenesis, it possesses dual tumor‐suppressive and ‐promoting effects, depending on the stage.( 20 , 21 , 22 ) TGF‐β shows antiproliferative effects on normal epithelial cells and during the early tumor stage, but the antiproliferative effects decrease and the tumor‐promoting effects become prominent during the advanced tumor stage.( 20 , 21 ) Notably, our in vitro data suggests that CD109 impairs the TGF‐β1‐mediated growth suppression of oral cancer cells. Considering our results showing the association of high level CD109 expression with malignant transformation of premalignant lesion in the oral cavity, it is possible that CD109 expression in premalignant lesions inhibits the antiproliferative effects of the TGF‐β signal and contributes to tumor development. It is also interesting to note that the involvement of CD109 in the TGF‐β signaling pathway was shown in human keratinocytes, the origin of SCCs.( 17 , 18 )
The molecular mechanisms underlying the malignant transformation of a premalignant lesion in the oral cavity largely remain unknown. It was reported that the high level of nuclear factor‐κB expression and the enhanced activities of gelatinases are associated with malignant transformation in the oral mucosa.( 23 ) In addition, gene expression profiling showed that the transcriptional changes occurring during high‐risk dysplasia more closely resemble those in SCCs than non‐progressing dysplasias.( 24 ) Moreover, amplification of TAOS1 and EMS1 in the chromosomal region 11q13 was reported to be one of the potential mechanisms underlying oral carcinogenesis.( 25 ) Recently, Järvinen et al. identified amplification of the CD109 gene by gene expression microarray analysis using 18 oral tongue SCC cell lines.( 26 ) Many oral SCC patients are diagnosed at an advanced stage, resulting in poor prognosis. Thus, early diagnosis of high‐risk premalignant lesions and minimal cancers is important. Because lesions in the oral cavity are easy to examine for pathological diagnosis, and because the clinical risk factors for oral cancer are well investigated, there is a chance to improve patients’ outcomes through proper diagnosis of high‐risk premalignant lesions before the development of invasive oral carcinoma. The majority of cases are well‐differentiated SCCs; thus, it will be a great advantage to apply CD109 as a molecular maker for high‐risk premalignant lesions and minimal SCC. Further investigation of the clinical applications of CD109 may facilitate the early diagnosis of high‐risk premalignant lesions and improve the outcome of treatments for cancer in the oral cavity.
Supporting information
Fig. S1. CD109 overexpression accelerates proliferation of A431 squamous cell carcinoma (SCC) cells. (a) Western blotting for assessment of FLAG‐CD109 expression in A431‐VC and A431‐CD109 cells. Lysates of the transfectants were subjected to western blotting with anti‐FLAG, anti‐CD109, and anti‐β‐actin antibodies. (b) Proliferation analyses of A431‐VC and A431‐CD109 cell lines. The absorbance values at day 1 were defined as 1.0. The means ± SD are shown (bars). *P < 0.001, using the Student’s t‐test.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Acknowledgments
This work was supported by Grants‐in‐Aid for the 21st Century Center of Excellence (COE) Research, Scientific Research (A), and Scientific Research on Priority Area ‘Cancer’ (to M. T.), and Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to Y. M. and K. M.). We thank Dr S. Nakamura, Nagoya University Hospital, for providing paraffin‐embedded specimens of the oral tissue, and Mr Y. Imaizumi, Mr K. Uchiyama, and Ms S. Kawai for their technical assistance.
References
- 1. Sutherland DR, Yeo E, Ryan A, Mills GB, Bailey D, Baker MA. Identification of a cell‐surface antigen associated with activated T lymphoblasts and activated platelets. Blood 1991; 77: 84–93. [PubMed] [Google Scholar]
- 2. Haregewoin A, Solomon K, Hom RC et al . Cellular expression of a GPI‐linked T cell activation protein. Cell Immunol 1994; 156: 357–70. [DOI] [PubMed] [Google Scholar]
- 3. Lin M, Sutherland DR, Horsfall W et al . Cell surface antigen CD109 is a novel member of the α2 macroglobulin/C3, C4, C5 family of thioester‐containing proteins. Blood 2002; 99: 1683–91. [DOI] [PubMed] [Google Scholar]
- 4. Murray LJ, Bruno E, Uchida N et al . CD109 is expressed on a subpopulation of CD34+ cells enriched in hematopoietic stem and progenitor cells. Exp Hematol 1999; 27: 1282–94. [DOI] [PubMed] [Google Scholar]
- 5. Giesert C, Marxer A, Sutherland DR, Schuh AC, Kanz L, Bürring H‐J. Antibody W7C5 defines a CD109 epitope expressed on CD34+ and CD34− hematopoietic and mesenchymal stem cell subsets. Ann NY Acad Sci 2003; 996: 227–30. [DOI] [PubMed] [Google Scholar]
- 6. Kelton JG, Smith JW, Horsewood P, Humbert JR, Hayward CP, Warkentin TE. Gova/b alloantigen system on human platelets. Blood 1990; 75: 2172–6. [PubMed] [Google Scholar]
- 7. Smith JW, Hayward CP, Horsewood P, Warkentin TE, Denomme GA, Kelton JG. Characterization and localization of the Gova/b alloantigens to the glycosylphosphatidylinositol‐anchored protein CDw109 on human platelets. Blood 1995; 86: 2807–14. [PubMed] [Google Scholar]
- 8. Kelton JG, Smith JW, Horsewood P et al . ABH antigens on human platelets: expression on the glycosylphosphatidylinositol‐anchored protein CD109. J Laboratory Clin Med 1998; 132: 142–8. [DOI] [PubMed] [Google Scholar]
- 9. Schuh AC, Watkins NA, Nguyen Q et al . A tyrosine703serine polymorphism of CD109 defines the Gov platelet alloantigens. Blood 2002; 99: 1692–8. [DOI] [PubMed] [Google Scholar]
- 10. Asai N, Jijiwa M, Enomoto A et al . RET receptor signaling: Dysfunction in thyroid cancer and Hirschsprung's disease. Pathol Int 2006; 56: 164–72. [DOI] [PubMed] [Google Scholar]
- 11. Hofstra RMW, Landsvater RM, Ceccherini I et al . A mutation in the RET proto‐oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 1994; 367: 375–6. [DOI] [PubMed] [Google Scholar]
- 12. Carlson KM, Dou S, Chi D et al . Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci USA 1994; 91: 1579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hashimoto M, Ichihara M, Watanabe T et al . Expression of CD109 in human cancer. Oncogene 2004; 23: 3716–20. [DOI] [PubMed] [Google Scholar]
- 14. Zhang JM, Hashimoto M, Kawai K et al . CD109 expression in squamous cell carcinoma of the uterine cervix. Pathol Int 2005; 55: 165–9. [DOI] [PubMed] [Google Scholar]
- 15. Sato T, Murakumo Y, Hagiwara S et al . High‐level expression of CD109 is frequently detected in lung squamous cell carcinomas. Pathol Int 2007; 57: 719–24. [DOI] [PubMed] [Google Scholar]
- 16. Hasegawa M, Hagiwara S, Sato T et al . CD109, a new marker for myoepithelial cells of mammary, salivary, and lacrimal glands and prostate basal cells. Pathol Int 2007; 57: 245–50. [DOI] [PubMed] [Google Scholar]
- 17. Tam BYY, Finnson KW, Philip A. Glycosylphosphatidylinositol‐anchored proteins regulate transforming growth factor‐β signaling in human keratinocytes. J Biol Chem 2003; 278: 49 610–17. [DOI] [PubMed] [Google Scholar]
- 18. Finnson KW, Tam BYY, Liu K et al . Identification of CD109 as part of the TGF‐β receptor system in human keratinocytes. FASEB J 2006; 20: 1525–7. [DOI] [PubMed] [Google Scholar]
- 19. Massague J, Blain SW, Lo RS. TGF‐β signaling in growth control, cancer, and heritable disorders. Cell 2000; 103: 295–309. [DOI] [PubMed] [Google Scholar]
- 20. Leivonen S‐K, Kähäri V‐M. Transforming growth factor‐β signaling in cancer invasion and metastasis. Int J Cancer 2007; 121: 2119–24. [DOI] [PubMed] [Google Scholar]
- 21. Wakefield LM, Roberts AB. TGF‐β signaling. positive and negative effects on tumorigenesis. Curr Opin Genet Dev 2002; 12: 22–9. [DOI] [PubMed] [Google Scholar]
- 22. Bierie B, Moses HL. Tumour microenvironment: TGFβ: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 2006; 6: 506–20. [DOI] [PubMed] [Google Scholar]
- 23. Bindhu OS, Ramadas K, Sebastian P, Pillai MR. High expression levels of nuclear factor kappa B and gelatinases in the tumorigenesis of oral squamous cell carcinoma. Head Neck 2006; 28: 916–25. [DOI] [PubMed] [Google Scholar]
- 24. Hunter KD, Thorlow JK, Fleming J et al . Divergent routes to oral cancer. Cancer Res 2006; 66: 7405–13. [DOI] [PubMed] [Google Scholar]
- 25. Xia J, Chen Q, Li B, Zeng X. Amplifications of TAOS1 and EMS1 genes in oral carcinogenesis: association with clinicopathological features. Oral Oncol 2007; 43: 508–14. [DOI] [PubMed] [Google Scholar]
- 26. Järvinen AK, Autio R, Kilpinen S et al . High‐resolution copy number and gene expression microarray analyses of head and neck squamous cell carcinoma cell lines of tongue and larynx. Genes Chromosomes Cancer 2008; 47: 500–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. CD109 overexpression accelerates proliferation of A431 squamous cell carcinoma (SCC) cells. (a) Western blotting for assessment of FLAG‐CD109 expression in A431‐VC and A431‐CD109 cells. Lysates of the transfectants were subjected to western blotting with anti‐FLAG, anti‐CD109, and anti‐β‐actin antibodies. (b) Proliferation analyses of A431‐VC and A431‐CD109 cell lines. The absorbance values at day 1 were defined as 1.0. The means ± SD are shown (bars). *P < 0.001, using the Student’s t‐test.
Please note: Wiley‐Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item


