Abstract
Mesothelioma is a type of malignant tumor that most commonly arises from the pleural or peritoneal membrane and is usually associated with previous exposure to asbestos. In humans, ERC/mesothelin is expressed on the normal mesothelium and in some cancers such as mesothelioma or ovarian carcinoma. Recently, several enzyme‐linked immunosorbent assay (ELISA) systems for ERC/mesothelin have been developed, the reported usefulness of which has been assessed and demonstrated as a diagnostic tool. However, the basic roles or physiological functions of, and relationship between, ERC/mesothelin and asbestos exposure–mediated carcinogenesis remain to be resolved. In order to elucidate the precise mechanism, animal models of mesothelioma are desperately needed. In this study, we consider the development of a novel specific ELISA system for not only rat N‐ERC/mesothelin but also C‐ERC/mesothelin, and the first data on the presence of rat ERC/mesothelin in the body fluids of rat malignant mesothelioma–bearing nude mice. The transplanted mice have revealed the higher concentrations of rat N‐ERC/mesothelin in the blood and ascites than C‐ERC/mesothelin. We hope these novel ELISA systems are useful in the rat model system to clarify the mechanism of asbestos‐induced carcinogenesis and to develop new effective drugs for mesothelioma. (Cancer Sci 2008; 99: 666–670)
The number of cases of mesothelioma caused by exposure to asbestos is growing, and as exemplified by the shocking events in 2005 and highlighted in TV and newspaper reports every day in Japan, this condition represents an enormous social problem.
Mesothelioma is a type of malignant tumor that most commonly arises from the pleural or peritoneal membrane. It is believed that mesothelioma is caused by the inhalation of asbestos fibers. However, how the aspirated asbestos in the lung might provoke tumor formation in the pleural membrane that covers the lungs, or the peritoneal membrane, has not yet been clarified.( 1 , 2 )
In regard to human mesothelioma, a group from Australia has reported that soluble mesothelin‐related protein (SMRP), the C‐terminal fragment of mesothelin, may be a tumor marker. Another group from National Institute of Health has reported that the N‐terminal fragment of mesothelin may be a mesothelioma marker.( 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 ) Also, Shiomi et al. reported the usefulness of the N‐ERC/mesothelin as a serum marker for mesothelioma.( 9 )
The mesothelin protein is present in the normal mesothelium, a membrane lining several body cavities including the pleura, peritoneum, and pericardium. It could be speculated that mesothelioma derived from the mesothelium should demonstrate overexpression of mesothelin. This protein, 622 amino acids in length, is expressed as a GPI anchor–type membranous protein of with a molecular weight of about 71 kDa, that is cleaved by a furin‐like protease into the 31 kDa N‐terminal fragment and 40 kDa C‐terminal fragment.
On the other hand, we have conducted research on the Eker model rat, a rat model of renal cell calcinoma, and cloned the Erc gene, which is expressed in abundance in the kidney of Eker rats. The expected amino acid sequence of the rat Erc cDNA exhibits 87.4% similarity to the mouse protein and 56.1% similarity to human MPF/mesothelin. Although the similarity to the human protein is not so high, both the sequences possess two hydrophobic regions, secretory signal sequences at the N terminus, signal sequences characteristic of a GPI anchor‐type protein at the C terminal, secretory recognition sequences for a furin‐like protease, and are located in the same chromosome as the tumor suppresser Tsc2/TSC gene. Based on the above, it is considered that the rat ERC may be a functional orthologue of human mesothelin; therefore, we call this gene product ERC/mesothelin.
We attempted to develop a rat ERC/mesothelin ELISA assay kit following the idea that ERC/mesothelin may be a potentially useful marker of malignant mesothelioma in humans, since the possible existence of a strong relationship between ERC/mesothelin and mesothelioma in humans has been suggested.
Shiomi et al. have developed a human ERC/mesothelin ELISA assay kit and reported that ERC/mesothelin is a useful marker for malignant mesothelioma.( 9 )
Nakaishi et al. established an assay system using mouse monoclonal antibody (MoAb) 30F2 as the solid antibody and a polyclonal antibody of N‐ERC (150–160) as the enzyme‐labeled antibody, for detecting the N‐terminal fragment, N‐ERC, of ERC/mesothelin. They measured the blood concentrations of N‐ERC/mesothelin in the Eker rat, a model of renal cell carcinoma using this assay system.( 12 )
In this study, to investigate whether ERC/mesothelin could be a mesothelioma marker in the rat and to investigate the functions of ERC/mesothelin in relation to the development and progression of mesothelioma, we developed a novel highly specific and sensitive sandwich ELISA system for not only rat N‐ERC/mesothelin but also C‐ERC/mesothelin. Moreover, a Xenograft mouse model for mesothelioma has been developed.
Using these novel ELISA systems, we determined the presence of ERC/mesothelin in the body fluids of tumor‐bearing nude mice, in order to explore the potential usefulness of this protein as a marker of mesothelioma.
Materials and Methods
Production of antibodies. Production of rabbit PoAb. We synthesized synthetic peptides corresponding to the peptide sequences 150–166, 259–280, 306–327, and 549–566 of full‐length rat ERC/mesothelin and immunized rabbits. Each antiserum was purified by affinity column chromatography using an antigen peptide‐coupled solid‐phase column.
Production of mouse MoAb. MoAb 30F2( 12 ) was purified from the resultant ascitic fluid of transplanted mice with hybridoma cells with a protein A column.
Western blotting. The full‐length rat ERC/mesothelin cDNA was transfected into CHO‐K1 cells and the cell culture supernatant was collected. The supernatant was subjected to Western blot analysis with N‐ERC (150–166) and N‐ERC (259–280) PoAb, and rat C‐ERC (549–566) and rat C‐ERC (306–327) PoAb. Secondary antibodies in the form of goat antirabbit IgG conjugated to peroxidase (No. 17502; Immuno‐Biological Laboratories Co. Ltd. (IBL), Gumna, Japan) were then added and allowed to react with the membranes at room temperature for 1 h. ERC/mesothelin on the membranes was visualized by the ECL detection system (Amersham Biosciences, GE Healthcare UK Ltd., Buckinghamshire, England).
Epitope Mapping of MoAb. We produced four different molecular sizes of the GST‐fusion protein by cutting the rat ERC/mesothelin gene into four 0.2 kb fragments.
The samples were electrophoresed on 12.5% Laemmli gel, and transferred to a polyvinylidene difluoride memblane.
The membrane was incubated with 1 µg/mL of the first group of antibodies (30F2 MoAb; N‐ERC [259–280] PoAb) in phosphate‐buffered saline (PBS) for 1 h, incubated with the second group of antibodies, and then detected as described in the section above.
Establishment of the ELISA assay system
Production of standard rat ERC/mesothelin protein for the ELISA system. The full‐length cDNA of the rat ERC/mesothelin coding region was inserted into the pcDNA3.1(+) vector (Invitrogen, Carlsbad, CA, USA) and transfected into CHO‐K1 cells. The culture supernatant of the stable transfectant was collected and applied on affinity column chromatography using 30F2 MoAb. The eluate was additionally purified with affinity column chromatography using N‐ERC (259–280) PoAb. The concentration of purified protein was measured by protein assay (Bio‐Rad, Tokyo, Japan). The purity of the protein was demonstrated densitometrically by using a densitograph (ATTO, Tokyo, Japan) and gel‐filtration chromatography (data not shown). This affinity‐purified rat N‐ERC/mesothelin was used as the ELISA standard.
Establishment of the ELISA system. An Immuno Module Plate (Nalge Nunc, Rochester, NY, USA) was coated with the 30F2 MoAb (in 0.1 mol carbonate buffer, pH 9.5) and incubated at 4°C overnight, then blocked with 1% bovine serum albumin in PBS. The sample and standard N‐ERC/mesothelin proteins were diluted with 0.05% Tween 20 in PBS and added to each well, followed by incubation of the well at 37°C for 1 h. After nine washes with the washing buffer, 100 µL of horseradish peroxidase‐labeled N‐ERC (259–280) PoAb was added to each well, followed by incubation for 30 min at 4°C. After nine washes with the washing buffer, 100 µL of tetramethyl benzidine buffer was added as the substrate to each well, followed by incubation for 30 min at room temperature in the dark. Color development was stopped by the addition of 100 µL of stop solution (1 N H2SO4). The optical density of each sample at 450 nm was then measured.
The same protocol was employed for the assay of rat C‐ERC/mesothelin.
For this assay, the Immuno Module Plate was coated with rat C‐ERC (549–566) PoAb, and rat C‐ERC (306–327) PoAb was used as a horseradish peroxidase‐labeled antibody.
Immunohistochemistry of rat ERC/mesothelin in Wistar rats. Four‐micrometer‐thick tissue sections were prepared from the formalin‐fixed, paraffin‐embedded tissue of Wistar rats. After deparaffinization, the tissue sections were heated in 10 mmol citrate buffer (pH 6.0) for antigen retrieval, and treated with 3% hydrogen peroxide. Then, the sections were incubated with 1 µg/mL of a primary rat C‐ERC (549–566) PoAb in PBS‐T at room temperature overnight. Envision K4002 (Dako Japan Co. Ltd., Kyoto, Japan) was applied to the tissue sections, without dilution, as the secondary antibody. Diaminobenzidine (DAB) was used as the substrate for peroxidase.
FACS analysis. We confirmed the expressions of rat ERC/mesothelin in the cultured cells of the rat mesothelioma cell line (MeET‐4)( 13 ) and Yoshida salcoma (YS),( 14 ) using rat C‐ERC/mesothelin antibodies.
Cells were cultured in TIL medium (No. 33612; IBL, Gunma, Japan) containing 10% FCS, washed, and susupended to obtain 2 × 106 cells/mL/50 µL/tube.
The cells were incubated with 5 µg/mL of the primary antibody (Rat C‐ERC [549–566] and [306–327] PoAb) for 1 h at 4°C.
Goat antirabbit IgG conjugated to fluorescein‐isothiocyanate (No. 17522, IBL) was used as the secondary antibody; it was applied at 50 µg/mL or 20 µL to each well, followed by incubation at 4°C for 20 min. FACS analysis was then conducted.
Intraperitoneal transplantation of mesothelioma cells in nude mice and measurement of rat ERC/mesothelin in the body fluids. MeET‐4 and YS cells were cultured in TIL medium containing 10% FCS, washed, and suspended with physiological saline solution to obtain 1 × 107/0.5 mL.
The cell suspensions were injected hypodermically into the abdominal cavity of nude mice (Balb/c 6–8 week old females; Charles River Laboratories, Kanagawa, Japan).
The ascitic fluid and serum specimens were collected 8 and 12 days after transplantation and the concentration of N‐ and C‐ERC/mesothelin was measured using the specific ELISA systems.
Results
Specificity of the antibodies. The specificities of the antibodies were confirmed by Western blot analysis of the culture supernatant.
The 30‐kDa N‐ERC/mesothelin fragment was detected using rat N‐ERC (150–166) or N‐ERC (259–280) antibodies, and the 40‐kDa C‐ERC/mesothelin fragment was detected using rat C‐ERC (549–566) and rat C‐ERC (306–327) antibodies (Fig. 1a).
Figure 1.
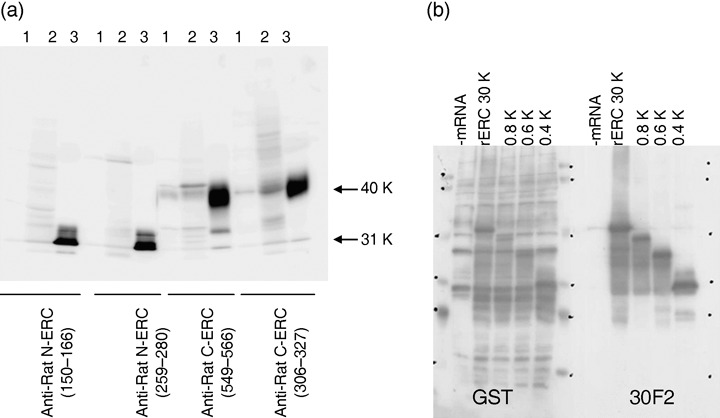
Characterization of anti‐ERC/mesothelin antibodies. (a) Western blot analysis lane 1, MeET‐4 cell lysate; lane 2, lysates of MeET‐4 subcutaneously transplanted into nude mouse; and lane 3, culture supernatant of rat ERC/CHO transfectant. (b) The Epitope mapping of antirat ERC/mesothelin MoAb (30F2). Four different molecular sizes of the rat ERC/mesothelin fused with GST protein were produced and underwent Western blot analysis by 30F2 MoAb. 30F2MoAb reacted with all the GST‐fusion proteins.
Epitope mapping of 30F2 MoAb. Antirat ERC/mesothelin (30F2) mouse MoAb reacted with all the GST‐fusion proteins prepared with peptide fragments cut from the rat ERC/mesothelin.
We confirmed that the epitope of 30F2 MoAb was on the N‐terminal portion of ERC/mesothelin (Fig. 1b).
Establishment of the ELISA system. We used mouse 30F2 MoAb as the solid antibody and the HRP‐conjugated rat N‐ERC (259–280) rabbit PoAb as the enzyme‐labeled antibody in the novel N‐ERC/mesothelin assay kit (No. 27765; IBL, Gunma, Japan) (Fig. 2).
Figure 2.
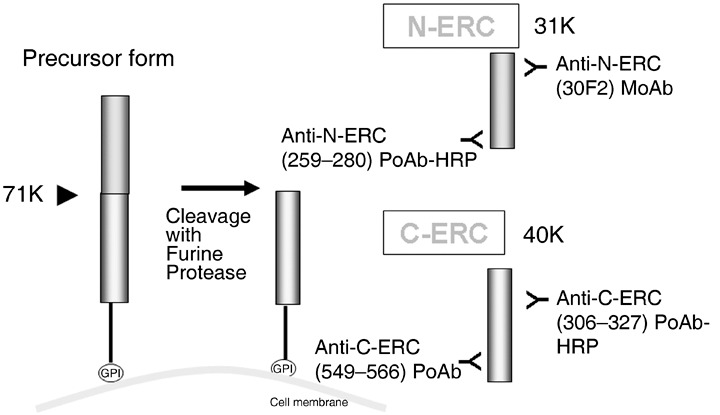
Products of ERC/mesothelin gene and enzyme‐linked immunosorbent assay (ELISA) system. We used mouse 30F2 MoAb as the solid antibody and the HRP‐conjugated rat N‐ERC (259–280) rabbit PoAb as the enzyme‐labeled antibody in the novel N‐ERC/mesothelin assay kit. For the Rat C‐ERC/mesothelin assay kit, we used rat C‐ERC (549–566) rabbit PoAb as the solid antibody and HRP‐conjugated rat C‐ERC (306–327) rabbit PoAb as the enzyme‐labeled antibody.
For the rat C‐ERC/mesothelin assay kit, we used rat C‐ERC (549–566) rabbit PoAb as the solid antibody and HRP‐conjugated rat C‐ERC (306–327) rabbit PoAb as the enzyme‐labeled antibody (No. 27761; IBL). The results exhibited a linear log/log plot for the recombinant rat N‐ERC/mesothelin concentration range between 0.078 ng/mL and 5.0 ng/mL by N‐ERC/mesothelin assay kit, and the recombinant rat C‐ERC/mesothelin range between 0.1 ng/mL and 7.0 ng/mL by C‐ERC/mesothelin assay kit (Fig. 3).
Figure 3.
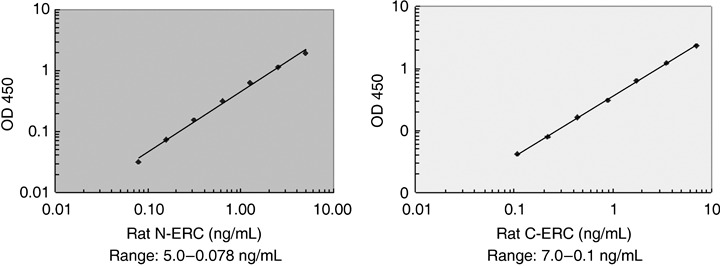
Typical standard curves of rat ERC/mesothelin enzyme‐linked immunosorbent assay (ELISA) systems. The typical standard curves for rat N‐ERC/mesothelin and C‐ERC/mesothelin are shown.
Determination of the presense of rat ERC/mesothelin in the cell culture supernatant by ELISA system. We determined the presence of rat ERC/mesothelin in the cell culture supernatant using the following method. MeET‐4, YS, and rat ERC/mesothelin/CHO cells (5 × 104) were seeded into 24‐well culture plates. Culture supernatants were then collected from four wells at various time‐points.
The concentration of rat ERC/mesothelin was increased with the increasing proliferative activity of the MeET‐4 cells. However, N‐ and C‐ERC/mesothelin were not detected in the YS cells (Fig. 4). These results corresponded with the mRNA expression of ERC/mesothelin by reverse transcription–polymerase chain reaction method (data not shown).
Figure 4.
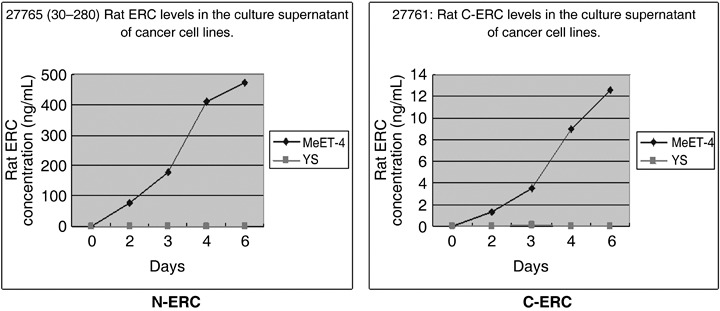
Rat ERC levels in the culture supernatant of cancer cell lines. On day 0, rat mesothelioma cells (MeET‐4) ( ) or Yoshida sarcoma (YS) (
) or Yoshida sarcoma (YS) ( ) cells (5 × 104) were seeded in 24‐well culture plates. At each time point, culture supernatants were taken from four wells. The concentration of rat ERC/mesothelin was determined using sandwich enzyme‐linked immunosorbent assay (ELISA).
) cells (5 × 104) were seeded in 24‐well culture plates. At each time point, culture supernatants were taken from four wells. The concentration of rat ERC/mesothelin was determined using sandwich enzyme‐linked immunosorbent assay (ELISA).
Intraperitoneal transplantation of mesothelioma cells in nude mice and measurement of rat ERC/mesothelin in the body fluids. The concentrations of rat N‐ERC and C‐ERC/mesothelin in the blood and ascitic fluid of nude mice transplanted intraperitoneally with MeET‐4 and YS cells were measured by the specific ELISA systems.
As a result, the ascites with the transplantation of MeET‐4 into abdominal cavity showed quite high concentrations of N‐ and C‐ERC/mesothelin (727.5 ng/mL to 2680.5 ng/mL of N‐ERC/mesothelin, 108 ng/mL to 251.5 ng/mL of C‐ERC/mesothelin). The serum harvested showed a relatively high concentration (511.2 ng/mL to 1075.8 ng/mL of N‐ERC/mesothelin, 32.7 ng/mL to 60.8 ng/mL of C‐ERC/mesothelin). The serum harvested from mice that underwent subcutaneous transplantation of MeET‐4 showed a low concentration of N‐ and C‐ERC/mesothelin (357.6 ng/mL to 605.8 ng/mL of N‐ERC/mesothelin, 6.3 ng/mL to 10.4 ng/mL of C‐ERC/mesothelin). (Fig. 5). The ERC/mesothelin in mouse body fluids detected by each ELISA system should be of rat MeET‐4 origin, because of tjhe absence of detectable amounts of rat ERC/mesothelin concentrations without transplantation.
Figure 5.
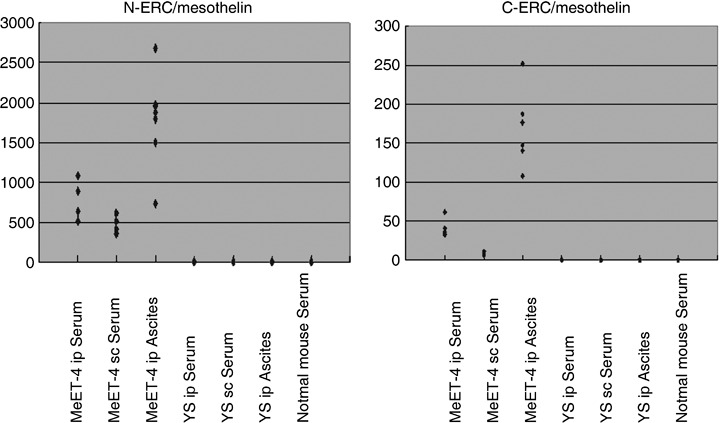
Rat N‐ and C‐ERC/mesothelin concentrations in body fluids of tumor‐bearing nude mouse. The ascites with the transplantation of rat mesothelioma cells (MeET‐4) or Yoshida sarcoma (YS) into the abdominal cavity (MeET‐4 or YS ip ascites), serum harvested as same manner (MeET‐4 or YS ip Serum), and serum harvested from mice with subcutaneous transplantations of MeET‐4 or YS (MeET‐4 or YS sc Serum) were measured for N‐ and C‐ERC/mesothelin concentrations by specific enzyme‐linked immunosorbent assay (ELISA) systems.
The N‐ or C‐ERC/mesothelin was not detected in the ascites and serum with the transplantation of YS cells.
In almost all cases when ERC/mesothelin was detected, the concentrations of N‐ERC/mesothelin were higher than the concentrations of C‐ERC/mesothelin. N‐ERC/mesothelin might thus be considered a good marker compared to C‐ERC/mesothelin.
Immunohistochemistry. The results of immunohistochemistry confirmed the expression of rat ERC/mesothelin in the lung (Fig. 6a).
Figure 6.
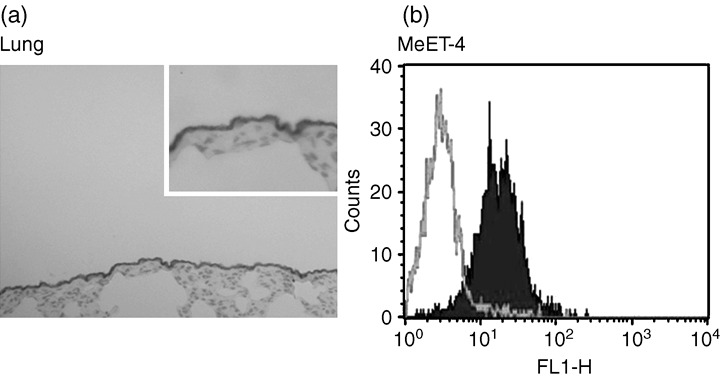
The cell surface expression of rat C‐ERC/mesothelin using anti‐C‐ERC/mesothelin antibody (306). (a) Immunohistochemical analysis in a normal rat lung. An enlarged view of a portion of stained epithelial cells was presented in upper right. (b) FACS analysis of MeET‐4 mesothelioma cells.
FACS analysis. Cell surface expression of the ERC/mesothelin was confirmed using the specific antibody against rat C‐ERC/mesothelin, in both MeET‐4 and YS1567 cells (Fig. 6b).
C‐ERC/mesothelin was detected on the cell surface, but N‐ERC/mesothelin was not detected. This results showed that N‐ERC/mesothelin fragments should be secreted into the blood or body fluid immediately after protease digestion. The mechanism of releasing C‐ERC/mesothelin from the cell surface has not yet been fully elucidated. However, C‐ERC/mesothelin seems to be attached to the cell surface by phosphatidylinositol. It is released when cells are treated with phosphatidylinositol‐specific phospholipase C.( 15 )
Discussion
As mentioned earlier, there appears to be a strong relationship between ERC/mesothelin and mesothelioma caused by asbestos exposure in humans; however, the nature of the relationship or the functions of the protein have not yet been clarified at present. The rat ERC/mesothelin was detected in the blood and ascitic fluid of the nude mice transplanted with MeET‐4 cells, using a specific ELISA system. This results demonstrated that the rat ERC/mesothelin is of malignant MeET‐4 cell origin. In the future, we would like to clarify the relationship between the rat ERC/mesothelin found in the body fluids of MeET‐4 tumor–bearing mice and mesothelioma.
The measurement range of the ELISA system for N‐ERC/mesothel described by Nakaishi et al.( 12 ) was between 1.25 ng/mL and 80 ng/mL. However, in this study, the novel ELISA system indicated an improved measurement range between 0.078 ng/mL and 5.0 ng/mL by using new antibodies. This improvement could achieve precise measurements in the blood and body fluids of mice.
This system should be a useful tool for elucidating the functions of ERC/mesothelin and its role in the development of mesothelioma, as well as screening and the evaluation of antimesothelioma drugs. Also, we hope these ELISA and mouse model systems will be valuable in evaluating new antimesothelioma therapies.
Acknowledgments
We thank Aki Arita, Mizuka Segawa, Shunsuke Miyai, and Kazuya Miyashita for helping in the management of this study and the critical reading of this manuscript. This work is supported by a Grant‐in‐Aid for Cancer Research and Grants‐in Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Ministry of Health, Labor, and Welfare of Japan. This study was partially supported by a consignment expense for the Molecular Imaging Program on ‘Research Base for PET Diagnosis’ from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1. Hino O, Kobayashi E, Nishizawa M et al . Renal carcinogenesis in the Eker rat. J Cancer Res Clin Oncol 1995; 121: 602–5. [DOI] [PubMed] [Google Scholar]
- 2. Yamashita Y, Yokoyama M, Kobayashi E, Takai S, Hino O. Mapping and determination of the cDNA sequence of the Erc gene preferentially expressed in renal cell carcinoma in the Tsc2 gene mutant (Eker) rat model. Biochem Biophys Res Commun 2000; 275: 134–40. [DOI] [PubMed] [Google Scholar]
- 3. Robinson BW, Creaney J, Lake R et al . Mesothelin‐family proteins and diagnosis of mesothelioma. Lancet 2003; 362: 1612–6. [DOI] [PubMed] [Google Scholar]
- 4. Scholler N, Fu N, Yang Y et al . Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci 1999; 96: 11531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson BW, Creaney J, Lake R et al . Soluble mesothelin‐related protein‐A blood test for mesothelioma. Lung Cancer 2005; 49: S109–11. [DOI] [PubMed] [Google Scholar]
- 6. Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res 2004; 10: 3937–42. [DOI] [PubMed] [Google Scholar]
- 7. Onda M, Willingham M, Nagata S et al . New monoclonal antibodies to mesothelin useful for immunohistochemistry, fluorescence‐activated cell sorting, Western blotting, and ELISA. Clin Cancer Res 2005; 11: 5840–6. [DOI] [PubMed] [Google Scholar]
- 8. Hassan R, Remaley AT, Sampson ML et al . Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 2006; 12: 447–53. [DOI] [PubMed] [Google Scholar]
- 9. Shiomi K, Miyamoto H, Segawa T. et al . A novel ELISA system for detection of a ‘N‐ERC/Mesothelin’ in the sera of mesothelioma patients. Cancer Sci 2006; 97: 928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maeda M, Hino O. Molecular tumor markers for asbestos‐related mesothelioma: serum diagnostic markers. Pathol Int 2006; 56: 649–54. [DOI] [PubMed] [Google Scholar]
- 11. Hino O, Shiomi K, Maeda M. Diagnostic biomarker of asbestos‐related mesothelioma: Example of translational research. Cancer Sci 2007; 98: 1147–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakaishi M, Kajino K, Ikesue M et al . Establishment of the enzyme‐linked immunosorbent assay system to detect the amino terminal secretory form of rat Erc/Mesothelin. Cancer Sci 2007; 98: 659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuwahara M, Takeda M, Takeuchi Y, Kuwahara M, Harada T, Maita K. Transforming growth factor beta production by spontaneous malignant mesothelioma cell lines derived from Fisher 344 rats. Virchows Arch 2001; 438: 492–7. [DOI] [PubMed] [Google Scholar]
- 14. Tsuchiya H, Tsuchiya Y, Kobayashi T, Kikuchi Y, Hino O. Isolation of genes differentially expressed between the Yoshida sarcoma and long‐survival Yoshida sarcoma variants: origin of Yoshida sarcoma revisited. Jpn J Cancer Res 1994; 85: 1099–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci 1996; 93: 136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


