Abstract
Positron emission tomography (PET) has emerged as a significant molecular imaging technique in clinical oncology and cancer research. PET with 18F‐fluorodeoxyglucose (18F‐FDG) demonstrates elevated glucose consumption by tumor cells, and is used clinically for the accurate staging and restaging of cancer, planning of radiotherapy, and predicting response or lack of response in the early stages of treatment. Combined PET and computed tomography (PET‐CT) provides both functional and morphological information of the disease to allow accurate diagnosis of cancer. PET with new radiotracers such as protein synthesis markers and proliferation markers, as well as hypoxia and receptor‐binding agents, will offer patient‐specific images in order to yield tailored diagnostic and prognostic information. (Cancer Sci 2006; 97: 1291–1297)
Positron emission tomography (PET) is a non‐invasive imaging technique that uses various radiolabeled compounds. PET was developed almost three decades ago and was used initially for evaluating cerebral blood flow and metabolism under physiological conditions and also for assessing pharmacological or neuropsychological intervention.
In the past decade, the clinical applications of PET have expanded with the improvement of PET detectors used for whole‐body imaging, and the development of PET in combination with computed tomography (PET‐CT). PET‐CT offers advantages in accurate diagnosis based on both morphological and metabolic images.
Use of PET has spread particularly in clinical oncology using 18F‐fluorodeoxyglucose (18F‐FDG) as a tracer. 18F‐FDG is the only PET radiotracer for cancer approved by Japanese governmental health insurance. The number of PET cameras installed has increased by geometric progression in developed countries worldwide, including Japan, and the number of 18F‐FDG PET studies has increased in proportion to the prevalence of machines.
The increased use of 18F‐FDG PET has also been facilitated by administrative and economic decisions. In 2002, the Ministry of Health, Labor and Welfare approved reimbursement for lung, colorectal, breast, head and neck, brain and pancreas cancers, as well as metastatic liver tumor, lymphoma, melanoma, and cancer of unknown primary; recently, esophageal, uterine, and ovarian cancers were also approved. In the USA, the Centers for Medicare and Medicaid Services announced Medicare coverage for PET in January 2005 for all cancers not currently covered, under the condition that patients are enrolled in prospective clinical studies that meet the requirements of the Food and Drug Administration and are designed to collect additional information in patient management. Their policy will contribute to the development of evidence of the clinical usefulness of 18F‐FDG PET even in uncommon malignancies.
Positron emission tomography with 18F‐FDG has emerged as a significant diagnostic imaging technique in clinical medicine. PET will aid in the provision of accurate diagnosis, treatment and prognosis in individual patients with cancer. Because the uptake of 18F‐FDG depends on the cellular metabolism of glucose, 18F‐FDG PET can assess tumor malignancy, therapeutic response and recurrence more accurately than conventional morphology‐based imaging methods. PET‐CT will aid in the evolution of accurate assessment based on morphological and functional images, and new PET imaging agents other than 18F‐FDG will allow for patient‐specific evaluation based on molecular and genetic markers of disease.
18F‐FDG PET in oncology
18F‐fluorodeoxyglucose PET is used for the assessment of various types of cancer before therapy for staging and planning treatment. It is also used after therapy for restaging and evaluating treatment response as well as detecting recurrence of tumors. Tumor uptake of 18F‐FDG depends on the expression of glucose transporters and hexokinase of tumor cells.( 1 ) Although 18F‐FDG is transported into the cytoplasm and phosphorylated to become 18F‐FDG‐6‐phosphate, it is not metabolized further and stays inside cells, which allows the imaging. The physical half‐life of 18F is 110 min and 18F‐FDG is excreted into the urine.
The uptake of 18F‐FDG is increased in most types of cancer. However, the diagnostic value of 18F‐FDG PET is limited because the uptake is variable in some types of cancer. These include thyroid, hepatocellular and renal cell cancers. Tumors that are well‐differentiated, hypo‐cellular and mucin producing, such as bronchioloalveolar cancer and intraductal papillary mucinous tumor, demonstrate low uptake of 18F‐FDG. Tumors in the kidneys and bladder are also difficult to detect because of the physiological excretion of 18F‐FDG into the urine.
Moreover, although 18F‐FDG PET is useful for the evaluation of neoplasms, the uptake of 18F‐FDG is not tumor‐specific and a variety of normal organs show increased 18F‐FDG uptake. Brain shows high 18F‐FDG uptake because the brain exclusively uses glucose for energy metabolism. Myocardium is demonstrated occasionally on 18F‐FDG PET in hyperglycemia, shortly after a meal, and in altered myocardial metabolism due mainly to ischemia. Physiological 18F‐FDG uptake is seen in tonsil, salivary glands, vocal cord, reactive lymph nodes, liver, gastrointestinal tract and testis, as well as muscles.( 2 ) The extent of uptake in these organs varies depending on physiological state and individual variability. Although the uptake of 18F‐FDG is increased in most types of cancer in comparison to the uptake in most normal organs, tumors in the aforementioned areas are sometimes incorrectly interpreted.
In addition, many pathological processes other than malignant neoplasms show increased 18F‐FDG uptake. Benign tumors in the head and neck and colonic adenoma often show levels of 18F‐FDG uptake as high as malignant tumors. Infiltrating cells in inflammatory processes or granuloma, and proliferating cells in hyperplastic tissue also demonstrate enhanced uptake of 18F‐FDG.
Other PET tracers
As the uptake of 18F‐FDG is not tumor‐specific, several other radiotracers have been used for the imaging of cancer. Table 1 shows the representative PET tracers in oncology, most of which are more tumor‐specific than 18F‐FDG. PET is now considered to be one of the standard means of molecular imaging using these tracers as probes for cellular metabolism, proliferation, hypoxia, receptor and apoptosis, among others.( 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 )
Table 1.
Representative radiotracers for positron emission tomography in oncology
| Clinical application and tracer | Analog | Mechanism of uptake |
|---|---|---|
| Diagnosis of tumor | ||
| 18F‐fluorodeoxyglucose | Glucose | Glucose transport, phosphorylation |
| 11C‐methionine( 3 ) | Methionine | A‐amino acid transport, protein synthesis |
| 11C‐tyrosine( 4 ) | Tyrosine | L‐amino acid transport |
| 18F‐fluoro‐l‐tyrosine( 5 ) | Tyrosine | L‐amino acid transport |
| 18F‐fluoroethyltyrosine( 6 ) | Tyrosine | L‐amino acid transport |
| 18F‐fluoro‐α‐methyltyrosine( 19 ) | Tyrosine | L‐amino acid transport |
| 18F‐fluorothymidine( 20 ) | Thymidine | Nucleoside transport, phosphorylation |
| 11C‐choline( 9 ) | Choline | Synthesis of phosphatidylcholine |
| 18F‐fluorocholine( 10 ) | Choline | Synthesis of phosphatidylcholine |
| Detection of tumor hypoxia | ||
| 18F‐fluoromisonidazole( 11 ) | Misonidazole | Reduction by low oxygen concentration |
| 60Cu‐, 64Cu‐ATSM( 26 ) | ||
| Detection of estrogen receptor | ||
| 18F‐fluoro‐17β‐estradiol( 12 ) | Estradiol | Estrogen receptor binding |
| Therapeutic drug monitoring | ||
| 18F‐fluorouracil( 13 ) | Uracil | Similar to accumulation of 5‐fluorouracil |
| 18F‐paclitaxel( 15 ) | Paclitaxel | Similar to accumulation of paclitaxel |
| Detection of apoptosis | ||
| 18F‐annexin V( 23 ) | Annexin V | Binding to apoptotic cells |
One of the PET radiotracers that we have used is 11C‐choline (Fig. 1). 11C‐choline penetrates the cell membrane and is phosphorylated within the cell, so it remains in the tumor. Cellular uptake of 11C‐choline is thought to be proportional to the rate of tumor duplication, because biosynthesis of cell membranes is fast. Clinical studies found that 11C‐choline has higher contrast than 18F‐FDG in visualizing various types of cancer and is useful for the differentiation between malignant and benign tumors.( 16 , 17 )
Figure 1.
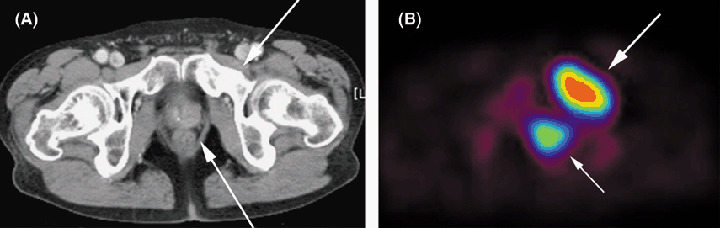
Detection of prostate cancer by positron emission tomography (PET) with 11C‐choline. A man with prostate cancer and bone metastasis underwent PET with 11C‐choline. (A) Computed tomography (CT) shows swelling of the left lobe (arrow) and osteoblastic appearance of the left pubic bone (arrow). (B) PET shows markedly increased uptake of 11C‐choline in both the primary prostate cancer lesion and bone metastasis (arrows). As PET with 18F‐FDG is not suitable for the diagnosis of prostate cancer due to excretion of the 8F‐FDG into urine, PET with 11C‐choline is thought to be useful for the detection of tumors adjacent to the urinary tract.
With further knowledge of the characteristics and clinical effectiveness of these tracers, it will become clear which tracer or combination of tracers is most appropriate for optimal cancer diagnosis.
Protein synthesis markers
A variety of amino acids labeled with radionuclides are used for PET imaging. They are a marker of protein synthesis in a broad sense. Some of them are transported by the specific amino‐acid transporter and metabolized for protein synthesis in the cytoplasm. Others are transported into the cells without further metabolism.
11C‐labeled methionine (MET) has been investigated in the imaging of various types of cancer.( 18 , 19 ) MET is better for imaging neoplasms than FDG due to higher specificity resulting in improved differentiation between cancer and benign processes. MET is transported into cells and is used for protein synthesis. PET with MET shows relatively intense tracer accumulation in the pancreas, liver, bone marrow, and salivary glands as compared with FDG and other amino‐acid tracers not involved in protein synthesis. Therefore, cancers in these organs may be overlooked. The short physical half‐life of 11C is another drawback for the clinical use of MET.
18Fluorine has a longer half‐life than 11C and is advantageous for chemical synthesis and imaging. Several 18F‐based PET tracers have been developed, such as 18F‐fluoro‐L‐tyrosine, 18F‐fluoroethyltyrosine, and 18F‐fluoro‐α‐methyltyrosine (FAMT). We have investigated the clinical utility of FAMT in several tumors including brain tumor, lung cancer, head and neck cancer, and lymphoma.( 20 ) FAMT is transported via L‐type amino‐acid transporter 1 (LAT‐1), which is specific to cancer cells. FAMT is not metabolized inside the cell( 21 ) as FAMT is methylated at its α‐position. Because it does not show intense accumulation in the pancreas and liver, diagnosis of pancreas cancer and liver tumor may be possible. FAMT can provide clear delineation of the tumor in patients with glioma that is not clearly demonstrated on FDG PET, because FDG accumulation in a recurrent tumor is sometimes equivalent to normal gray matter (Fig. 2).
Figure 2.
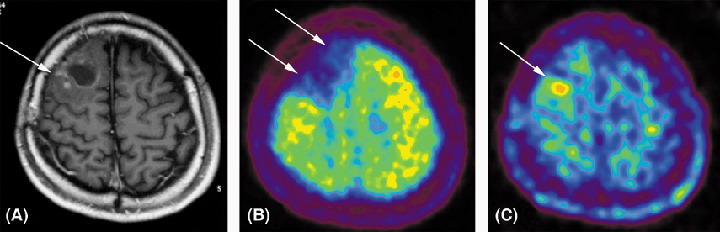
Detection of recurrent glioblastoma by positron emission tomography (PET) with 18F‐fluoro‐α‐methyltyrosine (FAMT). A woman with glioblastoma in the right frontal lobe underwent resection of tumor and adjuvant chemoradiation. (A) After 8 months, magnetic resonance imaging showed gadolinium‐enhancement around the area of resection (arrow). (B) PET with 18F‐FDG showed decreased uptake of 18F‐FDG in the area as compared with normal brain parenchyma (arrows). (C) PET with FAMT shows increased uptake at the posterolateral portion (arrow) indicating recurrence of glioblastoma.
Cellular proliferation markers
As cellular proliferation has a key role in the advancement of cancer, a variety of cellular proliferation markers such as nucleic acids have been radiolabeled as PET tracers. One example is 18F‐fluoro‐3′‐deoxy‐3′‐L‐thymidine (FLT).( 22 ) FLT is phosphorylated by thymidine kinase and enters the salvage pathway without incorporation into the DNA molecule. A stable accumulation of FLT in the cell happens by so‐called metabolic trapping, which is similar to FDG. An early study with FLT demonstrated that the uptake of FLT correlated with the proliferation of cancer cells, as indicated by immunohistological analysis with Ki‐67.( 23 ) Another study indicated its potential use for monitoring tumor response to chemotherapy.( 24 ) Results of previous studies revealed that FLT is more tumor‐specific than FDG, and it is thought to be useful for differential diagnosis and for monitoring tumor response in a wide range of cancers. As FLT accumulates in the bone marrow and the liver, tumors in these organs are not suited for PET diagnosis with FLT. Other agents such as FIAU, FBAU and FMAU are markers of DNA synthesis that reflects cellular proliferation.( 25 , 26 )
Tissue hypoxia markers
Oxygen content in a tumor is lower than in normal tissue. Therefore, hypoxia itself could be a target for tumor imaging. Moreover, localization of tumor hypoxia has radiobiological significance regarding the planning of treatment, because hypoxic cells are resistant to radiation therapy as well as to chemotherapy. Spatial distribution and the extent of hypoxia are crucial data for performing effective radiotherapy.
The uptake level of a tissue hypoxia marker should ideally be proportional to tumor hypoxia. Two compounds, 18F‐fluoromisonidazole (MISO) and 60Cu or 64Cu‐diacetyl‐bis (N‐(4)‐methylthiosemicarbazone) (ATSM), are the principal PET tracers clinically available.( 27 , 28 ) MISO is an analog of a radiosensitizer that has been used for some time. ATSM has a small molecular weight and high membrane permeability, thus it can diffuse; it is retained in hypoxic cells and reduced in viable cells under low cellular oxygen levels.( 29 ) Results of one study showed a significant relationship between ATSM uptake and response to therapy.( 30 ) One or a combination of these agents with FDG will aid in the development of PET‐guided radiotherapy.
Receptor‐binding agents
Quantitative in vivo receptor assay is another advantageous technique using PET. Radiolabeled receptor ligands for imaging neurotransmitters, enzymes, and transporters with high radiochemical purity have been produced for these purposes. For the imaging of tumors with estrogen receptors, 16α‐[18F]‐fluoroestradiol (FES) has been evaluated using PET imaging of breast cancer.( 12 ) FES PET shows functioning estrogen receptors in vivo, and could predict response to anti‐estrogen therapy.( 31 ) Functional imaging allows direct assessment of estrogen receptors specifically expressed in hormone‐refractory tumors that are not accurately evaluated by in vitro estrogen receptor (ER) assay with biopsy specimen. Absence of FES uptake in the tumor implies that the tumor should be treated with chemotherapy other than hormonal therapy. Imaging with 16β‐[18F]‐fluoro‐5α‐dihydrotestosterone (FDHT) has been investigated in patients with prostate cancer.( 32 ) The results show the utility of FDHT in predicting response to androgen ablation therapy.
PET and conventional imaging
Diagnostic imaging, such as CT, magnetic resonance imaging and ultrasonography, has advanced remarkably and has become indispensable for the management of cancer patients. Although clinical oncologists have been using these imaging techniques for many years to evaluate primary tumor and metastases, they have become aware of PET as a new technique to provide additional information. Conventional imaging techniques assess morphological aspects of the tumor, whereas PET assesses functional or metabolic characteristics. Considering these differences, PET is more suitable for the differentiation of malignant tumors from benign processes, assessment of the grade of malignancy, and monitoring tumor response to therapy. Although health insurance coverage is on a disease basis, indication of PET is categorized into diagnosis, staging, restaging, and monitoring response to therapy.
There are many studies that have demonstrated evidence of the usefulness of PET for the diagnosis and staging of various cancers. Recent PET investigations have been more focused on restaging and monitoring tumor response.
Restaging
Positron emission tomography is used for the restaging of various cancers, when recurrence is suspected by clinical findings or by conventional imaging (Fig. 3). Restaging is also performed for accurate assessment of the tumor in order to determine further treatment even when the results of conventional imaging are negative or equivocal (Fig. 4). PET is used for accurate differentiation between viable tumor and necrosis or scar in patients with residual mass. PET is not indicated until 2–3 months after radiation or chemoradiation or until 1–2 months after surgery because post‐therapeutic inflammation causes accumulation of 18F‐FDG and false‐positive findings at the site of radiation or surgery. PET performed during this time should be interpreted with knowledge of the radiation field and type of surgery.
Figure 3.
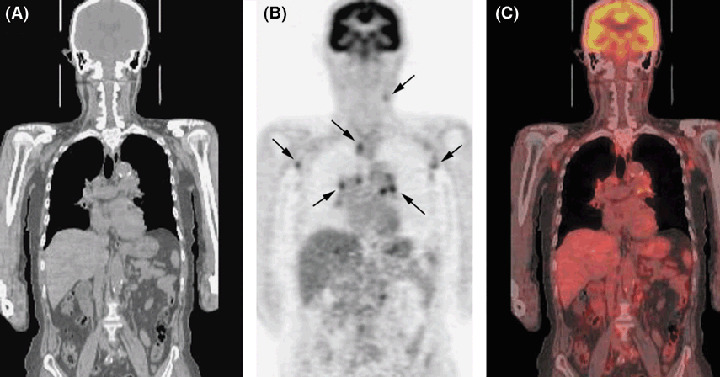
Detection of recurrent lymphoma by positron emission tomography‐computed tomography (PET‐CT) with 18F‐FDG. A 73‐year‐old man with peripheral T‐cell lymphoma in the left cervix underwent six courses of chemotherapy with THP‐COP. After 2 years, cervical CT demonstrated recurrence at a left cervical lymph node. PET‐CT was performed for restaging. (A) CT, (B) PET and (C) coronal view on PET‐CT show increased uptake of 18F‐FDG in multiple lymph nodes <1 cm (arrows).
Figure 4.
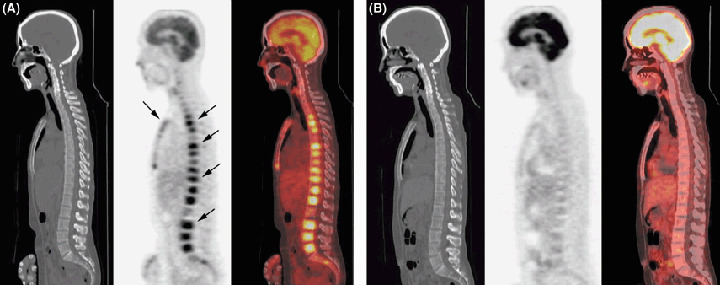
Positron emission tomography‐computed tomography (PET‐CT) with 18F‐FDG before and after therapy. PET‐CT in a 43‐year‐old woman with intravascular lymphoma. (A) PET‐CT before therapy clearly shows increased uptake of 18F‐FDG diffusely in the bone marrow (arrows). CT on the left does not show any abnormality. (B) After six cycles of therapy with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone, PET‐CT shows no evidence of increased uptake in the bone marrow, indicating that there was no residual lymphoma.
The diagnostic performance of PET in restaging is excellent for non‐small‐cell lung,( 33 ) breast,( 34 ) esophageal,( 35 , 36 , 37 ) colorectal,( 38 , 39 ) and head and neck( 40 , 41 ) cancer, as well as lymphoma,( 42 , 43 ) melanoma,( 44 ) and sarcoma.( 45 ) Sensitivity, specificity, and accuracy of PET in restaging these tumor types are 80–95%, 75–90%, and 80–90%, respectively.
The typical use of PET in tumor restaging is in the detection of recurrence in patients who have elevated levels of tumor markers without symptoms. PET is suitable due to the non‐invasive nature of the examination and the high‐contrast visualization of recurrent tumors in many organs, even if tumors are deep inside the abdominal cavity.
Monitoring tumor response
Owing to its characteristics of functional or metabolic imaging, PET may have an advantage for the monitoring of tumor response. Conventional imaging assesses tumor response based on the morphological changes in the tumor. These changes usually occur after a certain time interval following effective chemotherapy. Therefore, multiple doses of cytotoxic drugs are sometimes administered even if they are ineffective. It would be beneficial if patients without response could be identified early enough to avoid ineffective treatment and to reduce undesirable side‐effects and cost, and then to determine the alternative therapy. Patients who demonstrate a response at an early stage can continue treatment, thereby tailoring the therapy for the individual patient.
Previous studies have demonstrated the ability of PET to detect early changes in metabolism shortly after chemotherapy and radiotherapy.( 46 , 47 ) Evaluation of tumor response to therapy has previously been made according to changes in tumor size measured by CT. A growing body of evidence proves that metabolic change in a tumor is more accurate for the assessment of tumor response. Guidelines for the evaluation of tumor response and response classification criteria may be revised in future to take this into account.
Decreases in cellular glucose transport and glycolysis occur before shrinkage of the tumor mass. Clinical studies clearly demonstrated that PET predicts the pathological response of breast cancer to neoadjuvant chemotherapy.( 48 , 49 ) In these studies, PET could accurately predict the pathological response after just a single course of chemotherapy. Thus the value of PET in monitoring the response to treatment is clarified in breast cancer. Other neoplasms, such as lymphoma,( 50 ) and non‐small‐cell lung,( 51 ) head and neck,( 52 ) esophageal,( 53 ) and gastric( 54 ) cancer as well as liver metastasis( 55 ) show decreases in glucose metabolism after early stages of chemotherapy.
Molecular‐targeted therapy also decreases 18F‐FDG uptake in the early stage. Preliminary observation of gefitinib therapy for patients with epidermal growth factor receptor (EGFR) mutation‐positive adenocarcinoma of the lung has demonstrated a rapid and significant decrease in the uptake of 18F‐FDG in responding tumors after two doses of gefitinib therapy (Fig. 5).
Figure 5.
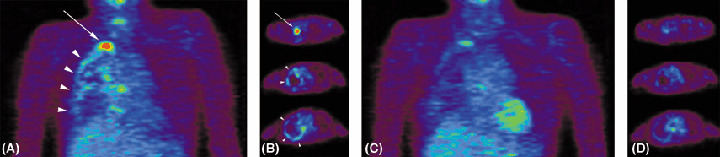
Positron emission tomography (PET) with 18F‐FDG before and after two doses of chemotherapy. A 57‐year‐old woman with non‐small‐cell lung cancer developed pleural dissemination of the tumor 9 months after upper and middle lobectomy of the right lung. (A) Coronal view and (B) axial view on PET performed at that time showed a markedly increased uptake of 18F‐FDG in the right apical portion (arrow) and also along the pleura (arrowheads). (C) Coronal view and (D) axial view on PET performed after two doses of gefitinib (250 mg/day) clearly shows decreased uptake of 18F‐FDG, indicating that the therapy was effective.
For patients whose tumors do not show a decrease in the 18F‐FDG uptake, the present therapy may be ineffective. The clinical significance of PET has been strengthened by the finding that a decrease in 18F‐FDG uptake as early as 1–2 weeks after the first course of therapy may be correlated with the outcome of patients with a variety of neoplasms.( 48 , 49 , 51 , 52 ) Lack of decrease in the uptake implies that the present therapeutic regimen may be ineffective; however, the optimal time course to evaluate the responsiveness has to be determined according to the type of cancer and therapy. Evaluation of the efficacy of the present therapy and determination of the best therapeutic options could be accelerated by use of PET.
References
- 1. Bos R, Van Der Hoeven JJ, Van Der Wall E et al. Biologic correlates of (18) fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol 2002; 20: 379–87. [DOI] [PubMed] [Google Scholar]
- 2. Kubota K. From tumor biology to clinical Pet: a review of positron emission tomography (PET) in oncology. Ann Nucl Med 2001; 15: 471–86. [DOI] [PubMed] [Google Scholar]
- 3. Kubota K, Matsuzawa T, Fujiwara T et al. Differential diagnosis of lung tumor with positron emission tomography: a prospective study. J Nucl Med 1990; 31: 1927–33. [PubMed] [Google Scholar]
- 4. Kole AC, Nieweg KO, Prium J et al. Standardized uptake value and quantification of metabolism for breast cancer imaging with FDG and L‐[1‐11C] tyrosine PET. J Nucl Med 1997; 38: 692–6. [PubMed] [Google Scholar]
- 5. Hustinx R, Lemaire C, Jerusalem G et al. Whole‐body tumor imaging using PET and 2‐18F‐fluoro‐L‐tyrosine: Preliminary evaluation and comparison with 18F‐FDG. J Nucl Med 2003; 44: 533–9. [PubMed] [Google Scholar]
- 6. Pauleit D, Floeth F, Tellmann L et al. Comparison of O‐(2‐18F‐fluoroethyl)‐L‐tyrosine PET and 3‐123I‐iodo‐alpha‐methyl‐L‐tyrosine SPECT in brain tumors. J Nucl Med 2004; 45: 374–81. [PubMed] [Google Scholar]
- 7. Amano S, Inoue T, Tomiyoshi K et al. In vivo comparison of PET and SPECT radiopharmaceuticals in detecting breast cancer. J Nucl Med 1998; 39: 1424–7. [PubMed] [Google Scholar]
- 8. Buck AK, Schirrmeister H, Hetzel M et al. 3‐deoxy‐3‐[18F] fluorothymidine‐positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res 2002; 62: 3331–4. [PubMed] [Google Scholar]
- 9. Hara T, Kosaka N, Kishi H. PET imaging of prostate cancer using carbon‐11‐choline. J Nucl Med 1998; 39: 990–5. [PubMed] [Google Scholar]
- 10. Schmid DT, John H, Zweifel R et al. Fluorocholine PET/CT in patients with prostate cancer: initial experience. Radiology 2005; 235: 623–8. [DOI] [PubMed] [Google Scholar]
- 11. Koh WJ, Bergman KS, Rasey JS et al. Evaluation of oxygenation status during fractionated radiotherapy in human non‐small cell lung cancers using [F‐18]fluoromisonidazole positron emission tomography. Int J Radiat Oncol Biol Phys 1995; 33: 391–8. [DOI] [PubMed] [Google Scholar]
- 12. Dehdashti F, Flanagan FL, Mortimer JE et al. Positron emission tomographic assessment of ‘metabolic flare’ to predict response of metabolic breast cancer to antiestrogen therapy. Eur J Nucl Med 1999; 26: 51–6. [DOI] [PubMed] [Google Scholar]
- 13. Dimitrakopoulou‐Strauss A, Strauss LG, Schlag P et al. Fluorine‐18‐fluorouracil to predict therapy response in liver metastases from colorectal carcinoma. J Nuclr Med 1998; 39: 1197–202. [PubMed] [Google Scholar]
- 14. Gangloff A, Hsueh WA, Kesner AL et al. Estimation of paclitaxel biodistribution and uptake in human‐derived xenografts in vivo with 18F‐fluoropaclitaxel. J Nucl Med 2005; 46: 1866–71. [PubMed] [Google Scholar]
- 15. Toretsky J, Levenson A, Weinberg IN, Tait JF, Uren A, Mease RC. Preparation of F‐18 labeled annexin V: a potential PET radiopharmaceutical for imaging cell death. Nucl Med Biol 2004; 31: 747–52. [DOI] [PubMed] [Google Scholar]
- 16. Khan N, Oriuchi N, Ninomiya H, Higuchi T, Kamada H, Endo K. Positron emission tomographic imaging with 11C‐choline in differential diagnosis of head and neck tumors: comparison with 18F‐FDG PET. Ann Nucl Med 2004; 18: 409–17. [DOI] [PubMed] [Google Scholar]
- 17. Tian M, Zhang H, Oriuchi N, Higuchi T, Endo K. Comparison of 11C‐choline PET and FDG PET for the differential diagnosis of malignant tumors. Eur J Nucl Med Mol Imaging 2004; 31: 1064–72. [DOI] [PubMed] [Google Scholar]
- 18. Ogawa T, Shishido F, Kanno I et al. Cerebral glioma: evaluation with methionine PET. Radiology 1993; 186: 45–53. [DOI] [PubMed] [Google Scholar]
- 19. Yasukawa T, Yoshikawa K, Aoyagi H et al. Usefulness of PET with 11C‐methionine for the detection of hilar and mediastinal lymph node metastasis in lung cancer. J Nucl Med 2000; 41: 283–90. [PubMed] [Google Scholar]
- 20. Inoue T, Koyama K, Oriuchi N et al. Detection of malignant tumors: whole‐body PET with fluorine 18 alpha‐methyl tyrosine versus FDG – preliminary study. Radiology 2001; 220: 54–62. [DOI] [PubMed] [Google Scholar]
- 21. Tomiyoshi K, Inoue T, Higuchi T et al. Metabolic studies of [18F‐alpha‐methyl]tyrosine in mice bearing colorectal carcinoma LS‐180. Anticancer Drugs 1999; 10: 329–36. [DOI] [PubMed] [Google Scholar]
- 22. Shields AF, Grierson JR, Dohmen BM et al. Imaging proliferation in vivo with [F‐18]FLT and positron emission tomography. Nat Med 1998; 4: 1334–6. [DOI] [PubMed] [Google Scholar]
- 23. Vesselle H, Grierson J, Muzi M et al. In vivo validation of 3′deoxy‐3′‐[18F] fluorothymidine ([18F] FLT) as a proliferation imaging tracer in humans: correlation of [18F] FLT uptake by positron emission tomography with Ki‐67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 2002; 8: 3315–23. [PubMed] [Google Scholar]
- 24. Leyton J, Latigo JR, Perumal M, Dhaliwal H, He Q, Aboagye EO. Early detection of tumor response to chemotherapy by 3′‐deoxy‐3′‐[18F] fluorothymidine positron emission tomography: the effect of cisplatin on a fibrosarcoma tumor model in vivo . Cancer Res 2005; 65: 4202–10. [DOI] [PubMed] [Google Scholar]
- 25. Bading JR, Shahinian AH, Vail A et al. Pharmacokinetics of the thymidine analog 2′‐fluoro‐5‐methyl‐1‐beta‐D‐arabinofuranosyluracil (FMAU) in tumor‐bearing rats. Nucl Med Biol 2004; 31: 407–18. [DOI] [PubMed] [Google Scholar]
- 26. Mangner TJ, Klecker RW, Anderson L, Shields AF. Synthesis of 2′‐deoxy‐2′‐[18F] fluoro‐beta‐D‐arabinofuranosyl nucleosides, [18F]FAU, [18F]FMAU, [18F]FBAU and [18F]FIAU, as potential PET agents for imaging cellular proliferation. Synthesis of [18F]‐labelled FAU, FMAU, FBAU, FIAU. Nucl Med Biol 2003; 30: 215–24. [DOI] [PubMed] [Google Scholar]
- 27. Rischin D, Hicks RJ, Fisher R et al. Trans‐Tasman Radiation Oncology Group Study 98.02. Prognostic significance of [18F]‐misonidazole positron emission tomography‐detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans‐Tasman Radiation Oncology Group Study 98.02. J Clin Oncol 2006; 24: 2098–104. [DOI] [PubMed] [Google Scholar]
- 28. O'Donoghue JA, Zanzonico P, Pugachev A et al. Assessment of regional tumor hypoxia using 18F‐fluoromisonidazole and 64Cu(II)‐diacetyl‐bis (N4‐methylthiosemicarbazone) positron emission tomography: Comparative study featuring micro PET imaging, PO2 probe measurement, autoradiography, and fluorescent microscopy in the R3327‐AT and FaDu rat tumor models. Int J Radiat Oncol Biol Phys 2005; 61: 1493–502. [DOI] [PubMed] [Google Scholar]
- 29. Fujibayashi Y, Taniuchi H, Yonekura Y, Ohtani H, Konishi J, Yokoyama A. Copper‐62‐ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J Nucl Med 1997; 38: 1155–60. [PubMed] [Google Scholar]
- 30. Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu‐ATSM: relationship to therapeutic response. A preliminary report. Int J Radiat Oncol Biol Phys 2003; 55: 1233–8. [DOI] [PubMed] [Google Scholar]
- 31. Mortimer JE, Dehdashti F, Siegel BA, Katzenellenbogen JA, Fracasso P, Welch MJ. Positron emission tomography with 2‐[18F] fluoro‐2‐deoxy‐D‐glucose and 16alpha‐[18F] fluoro‐17beta‐estradiol in breast cancer: correlation with estrogen receptor status and response to systemic therapy. Clin Cancer Res 1996; 2: 933–9. [PubMed] [Google Scholar]
- 32. Larson SM, Morris M, Gunther I et al. Tumor localization of 16beta‐18F‐fluoro‐5alpha‐dihydrotestosterone versus 18F‐FDG in patients with progressive, metastatic prostate cancer. J Nucl Med 2004; 45: 366–73. [PubMed] [Google Scholar]
- 33. Keidar Z, Haim N, Guralnik L et al. PET/CT using 18F‐FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. J Nucl Med 2004; 45: 1640–6. [PubMed] [Google Scholar]
- 34. Kim TS, Moon WK, Lee DS et al. Fluorodeoxyglucose positron emission tomography for detection of recurrent or metastatic breast cancer. World J Surg 2001; 25: 829–34. [DOI] [PubMed] [Google Scholar]
- 35. Kato H, Miyazaki T, Nakajima M, Fukuchi M, Manda R, Kuwano H. Value of positron emission tomography in the diagnosis of recurrent oesophageal carcinoma. Br J Surg 2004; 91: 1004–9. [DOI] [PubMed] [Google Scholar]
- 36. Westerterp M, Van Westreenen HL, Reitsma JB et al. Esophageal cancer: CT, endoscopic US, and FDG PET for assessment of response to neoadjuvant therapy – systematic review. Radiology 2005; 236: 841–51. [DOI] [PubMed] [Google Scholar]
- 37. Cerfolio RJ, Bryant AS, Ohja B, Bartolucci AA, Eloubeidi MA. The accuracy of endoscopic ultrasonography with fine‐needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg 2005; 129: 1232–41. [DOI] [PubMed] [Google Scholar]
- 38. Israel O, Mor M, Guralnik L et al. 18F‐FDG PET/CT useful for imaging and management of patients with suspected occult recurrence of cancer? J Nucl Med 2004; 45: 2045–51. [PubMed] [Google Scholar]
- 39. Even‐Sapir E, Parag Y, Lerman H et al. Detection of recurrence in patients with rectal cancer: PET/CT after abdominoperineal or anterior resection. Radiology 2004; 232: 815–22. [DOI] [PubMed] [Google Scholar]
- 40. Porceddu SV, Jarmolowski E, Hicks RJ et al. Utility of positron emission tomography for the detection of disease in residual neck nodes after (chemo) radiotherapy in head and neck cancer. Head Neck 2005; 27: 175–81. [DOI] [PubMed] [Google Scholar]
- 41. Yao M, Graham MM, Smith RB et al. Value of FDG PET in assessment of treatment response and surveillance in head‐and‐neck cancer patients after intensity modulated radiation treatment: a preliminary report. Int J Radiat Oncol Biol Phys 2004; 60: 1410–8. [DOI] [PubMed] [Google Scholar]
- 42. Juweid ME, Wiseman GA, Vose JM et al. Response assessment of aggressive non‐Hodgkin's lymphoma by integrated International Workshop Criteria and fluorine‐18‐fluorodeoxyglucose positron emission tomography. J Clin Oncol 2005; 23: 4652–61. [DOI] [PubMed] [Google Scholar]
- 43. Spaepen K, Stroobants S, Dupont P et al. Prognostic value of positron emission tomography (PET) with fluorine‐18 fluorodeoxyglucose ([18F]FDG) after first‐line chemotherapy in non‐Hodgkin's lymphoma: is [18F]FDG‐PET a valid alternative to conventional diagnostic methods? J Clin Oncol 2001; 19: 414–9. [DOI] [PubMed] [Google Scholar]
- 44. Fuster D, Chiang S, Johnson G, Schuchter LM, Zhuang H, Alavi A. Is 18F‐FDG PET more accurate than standard diagnostic procedures in the detection of suspected recurrent melanoma? J Nucl Med 2004; 45: 1323–7. [PubMed] [Google Scholar]
- 45. Hawkins DS, Schuetze SM, Butrynski JE et al. [18F]Fluorodeoxyglucose positron emission tomography predicts outcome for Ewing sarcoma family of tumors. J Clin Oncol 2005; 23: 8828–34. [DOI] [PubMed] [Google Scholar]
- 46. Price P, Jones T. Can positron emission tomography (PET) be used to detect subclinical response to cancer therapy? The EC PET Oncology Concerted Action and the EORTC PET Study Group. Eur J Cancer 1995; 31A: 1924–7. [DOI] [PubMed] [Google Scholar]
- 47. Chaiken L, Rege S, Hoh C et al. Positron emission tomography with fluorodeoxyglucose to evaluate tumor response and control after radiation therapy. Int J Radiat Oncol Biol Phys 1993; 27: 455–64. [DOI] [PubMed] [Google Scholar]
- 48. Smith IC, Welch AE, Hutcheon AW et al. Positron emission tomography using [18F]‐fluorodeoxy‐D‐glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol 2000; 18: 1676–88. [DOI] [PubMed] [Google Scholar]
- 49. Schelling M, Avril N, Nahrig J et al. Positron emission tomography using [18F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol 2000; 18: 1689–95. [DOI] [PubMed] [Google Scholar]
- 50. Kostakoglu L, Coleman M, Leonard JP, Kuji I, Zoe H, Goldsmith SJ. PET predicts prognosis after 1 cycle of chemotherapy in aggressive lymphoma and Hodgkin's disease. J Nucl Med 2002; 43: 1018–27. [PubMed] [Google Scholar]
- 51. Weber WA, Petersen V, Schmidt B et al. Positron emission tomography in non‐small‐cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol 2003; 21: 2651–7. [DOI] [PubMed] [Google Scholar]
- 52. Brun E, Kjellen E, Tennvall J et al. FDG PET studies during treatment: prediction of therapy outcome in head and neck squamous cell carcinoma. Head Neck 2002; 24: 127–35. [DOI] [PubMed] [Google Scholar]
- 53. Weber WA, Ott K, Becker K et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 2001; 19: 3058–65. [DOI] [PubMed] [Google Scholar]
- 54. Ott K, Fink U, Becker K et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 2003; 21: 4604–10. [DOI] [PubMed] [Google Scholar]
- 55. Findlay M, Young H, Cunningham D et al. Noninvasive monitoring of tumor metabolism using fluorodeoxyglucose and positron emission tomography in colorectal cancer liver metastases: correlation with tumor response to fluorouracil. J Clin Oncol 1996; 14: 700–8. [DOI] [PubMed] [Google Scholar]


