Abstract
Fundamental studies have suggested that matrix metalloproteinases‐7 (MMP‐7) expression is associated with chemoresistance and constitutes a prognostic factor in several solid tumors. The present study assessed the prognostic and predictive value of MMP‐7 in tumors of patients with advanced non‐small cell lung cancer (NSCLC) treated with platinum‐based chemotherapy. In total, 159 patients with stage III and IV NSCLC were retrospectively enrolled. Immunohistochemistry was performed to evaluate the expression of MMP‐7, apoptosis‐related proteins Bcl‐2, Bax, Fas and FasL and the Ki‐67 proliferation marker. The TUNEL (terminal deoxynucleotidyl transferase‐mediated deoxyuridine triphosphate nick‐end labeling) method was performed to investigate tumor apoptosis. Ninety carcinomas (56.6%) were identified as high expression of MMP‐7. Overexpression of MMP‐7 was more frequent in adenocarcinomas than in squamous cell carcinomas (P = 0.032). The expression of MMP‐7 was positively related with Ki‐67 index and Bcl‐2, but not apoptosis index. MMP‐7 status was correlated inversely with response to chemotherapy in overall patients (response rates, 20.0% and 35.8%, for patients with high‐MMP‐7 and low‐MMP‐7 tumors, respectively, P = 0.036), especially in adenocarcinoma (P = 0.021), but not in patients with squamous cell carcinomas (P = 0.373). The overall survival was significantly lower in NSCLC patients with high MMP‐7 than in those with low MMP‐7 (P < 0.001). A Cox regression analyses also demonstrated MMP‐7 status to be a significant prognostic factor (hazard ratio, 5.49; P = 0.001). These findings suggest that the expression level of MMP‐7 in tumor cells is predictive of response to chemotherapy and outcome in patients with advanced NSCLC receiving platinum‐based chemotherapy. (Cancer Sci 2008; 99: 2185–2192)
Lung cancer is the leading cause of cancer‐related death in the world and non‐small cell lung cancers (NSCLC) comprise more than 75% of all lung cancers. About 70% of NSCLC cases are advanced at diagnosis and treated with chemotherapy and radiotherapy. Platinum‐based combinations with newer agents have been widely accepted as first‐line options in the treatment of advanced NSCLC, but the frequent development of platinum‐resistance is a major obstacle at present.( 1 ) A large effort has been devoted and the precise mechanisms remain unclear. Therefore, identification and implementation of markers predictive of response appear to be one way to select sensitive regimen based on the biological characteristics of the tumors.
Increasing evidence indicates that local microenvironment of the tumor plays an important role in the development of chemoresistance.( 2 ) Matrix metalloproteinases (MMP) maintain the proper homeostasis of extracellular matrix (ECM) and stabilize cell–matrix interaction.( 3 , 4 ) As the smallest (28 kDa) member of the MMP family, MMP‐7 (matrilysin) has been related to tumor invasion and metastasis.( 5 ) More recently, fundamental data implicated the role of MMP‐7 in the development of chemoresistance. Vargo‐Gogola et al.( 6 ) reported that transfection of MMP‐7 expression vector resulted in a population of cells that showed less sensitivity to the apoptosis induced by staurosporine and mitomycin. Similarly, Mitsiades et al.( 7 ) found that transfection cloning MMP‐7 sequence can confer apoptosis resistance to doxorubicin in SW480 colon carcinoma cells and SK‐N‐MC Ewing's sarcoma cells. Moreover, Almendro et al.( 8 ) demonstrated that reversal of oxaliplatin chemoresistance can be obtained by MMP‐7 inhibitor in colorectal cancer cell lines. Indeed, our pilot study has observed a protective activity of MMP‐7 for cisplatin‐induced apoptosis in A549 lung adenocarcinoma cells in vitro (unpublished data).
Taking these findings into consideration, we hypothesized that MMP‐7 status in NSCLC may predict the response to chemotherapy. Although MMP‐7 has been revealed as a significant prognostic factor in several carcinomas,( 9 , 10 ) the predictive value of MMP‐7 in human lung tumors has been largely neglected. By far, only a limited number of studies concerning its role in lung carcinomas and the results have remained inconclusive.( 11 , 12 , 13 ) Most importantly, none has investigated its clinic significance in the context of chemoresponsiveness. Therefore, we conducted this retrospective clinical study on MMP‐7 expression associated with prognosis in advanced NSCLC patients. We focused on the possible relationship between MMP‐7 status and chemotherapeutical sensitivity. An additional evaluation of apoptosis‐related proteins expression in relation to MMP‐7 was performed to further clarify its profound functions in the progression of NSCLC.
Materials and Methods
Study population. The patients were retrospectively collected at three university‐affiliated hospitals: the Third Affiliated Hospital of Sun Yat‐sen University, Cancer Center of Sun Yat‐sen University and First Affiliated Hospital of Sun Yat‐sen University between January 2000 and December 2002. Inclusion criteria for this study were patients who had primary NSCLC staging III to IV, had received at least two cycles of platinum‐based chemotherapy as initial treatment modality and had complete clinicopathologic data. Exclusion criteria for this study were (a) patients who have previous treatment with either chemotherapy or radiotherapy and (b) patients who have only cytological specimens such as sputum, bronchial washings and needle aspirations and (c) patients who could not be assessed for response. In total, the paraffin‐embedded tissue specimens from one hundred and fifty‐nine patients who met the above criteria were retrospectively investigated. This study was performed under an institutional review board approved protocol to investigate molecular markers relevant to lung cancer pathogenesis.
Accordingly, the clinical records of enrolled patients were fully documented. Clinicopathologic data collected included age, sex, smoking history, performance status (using the Eastern Cooperative Oncology Group scale), date of initial diagnosis, histopathologic diagnosis, grade of tumor differentiation, pathologic tumor stage and date of death or last follow‐up. Histologic diagnosis and grade of differentiation were assigned in accordance with the WHO criteria for lung tumors,( 14 ) and pathologic stage was based on the revised international system.( 15 ) This report included follow‐up data as of December 31, 2007.
All of the patients who received at least two courses of chemotherapy were evaluated for response. Second‐line chemotherapy or radiotherapy was administered when clinically indicated. We used the standard response criteria to evaluate response to chemotherapy.( 16 ) Complete response (CR) was defined as the disappearance of all signs of disease both at clinical examination and on the computed tomography scan. Partial response (PR) was defined by a reduction of >50% in the sum of products of the largest perpendicular diameters of all tumor localizations, with no new tumor lesions. Stable disease (SD) was defined by a <50% decrease or a <25% increase in tumor size. Progressive disease (PD was defined as an increase in the size of tumor lesions by >25% or the appearance of a new lesion. The response rate was defined as the number of CR patients plus the number of PR patients divided by the total number of patients. Patients were followed every 3–6 months by history, physical examination, chest X‐ray and additional imaging tests when there was suspicion for metastases. Overall survival was calculated as the time between the beginning of chemotherapy and death or last follow‐up. All data from patients were reviewed by the authors without knowledge of histopathological status.
Immunohistochemistry (IHC). The pretreatment biopsy specimens for all enrolled patients were retrospectively collected. Formaldehyde‐fixed paraffin‐embedded tissue specimens were cut into 4‐µm sections and mounted on poly l‐lysine‐coated slides. For each patient, a representative tissue block containing adequate tumor cells and non‐neoplastic lung tissue was selected. All section was stained with hematoxylin and eosin and reviewed to confirm the histopathologic diagnosis and adequacy of specimens for immunohistochemistry (IHC) analysis. Table 1 summarizes the features of these markers. IHC was performed as previously described. In brief, sections were deparaffinized in xylene and rehydrated in graded alcohols and water. The slides were then heated in a microwave for 10 min in a 10‐mM citrate buffer solution at pH 6.0, and cooled to room temperature for 20 min. After quenching the endogenous peroxidase activity with 0.3% hydrogen peroxide (in absolute methanol) for 30 min, the sections were blocked for 2 h at room temperature with 5% bovine serum albumin (Sigma Chemical). Subsequently, duplicate sections were incubated overnight at 4°C with the primary specific antibodies at an appropriate dilution (Table 1). After several rinses in phosphate‐buffered saline (PBS), the sections were incubated in the biotinylated secondary antibody. Then, sections were incubated with streptavidin linked with peroxidase and visualized with 3,3′‐diaminobenzidine tetrahydrochloride as the chromogen (Invitrogen, Carlsbad, CA, USA). Slides were rinsed in PBS, exposed to diaminobenzidine, and counterstained with Mayer's hematoxylin. The negative controls for these proteins were made by the omission of the primary antibody during the process of immunohistochemical staining.
Table 1.
Characteristics of antibodies used in this study
| Antibody | Manufacturer | Clone | Dilution | Localization |
|---|---|---|---|---|
| MMP‐7 | Santa Cruz Biotechnology Inc. | JL07 | 1:100 | Cytoplasmic |
| Bcl‐2 | DAKO, Glostrup, Denmark | 124 | 1:50 | Cytoplasmic |
| Bax | Santa Cruz Biotechnology Inc. | P‐19 | 1:150 | Cytoplasmic |
| Fas | Santa Cruz Biotechnology Inc. | C‐20 | 1:200 | Membrane and cytoplasmic |
| FasL | Santa Cruz Biotechnology Inc. | N‐20 | 1:200 | Membrane and cytoplasmic |
| Ki‐67 | DAKO, Glostrup, Denmark | MIB‐1 | 1:50 | Nuclear |
Evaluation of immunohistochemical results. All of the immunostained sections were analyzed by two pathologists unaware of clinicopathological data. Staining intensity in tumor cells was graded into three groups: 1 = weak, 2 = moderate and 3 = strong. Strong intensity corresponded with that in control samples used as standards. Weak intensity was similar to that noted in benign bronchial epithelium. Moderate intensity was classified as a staining intensity between weak and strong. In each case, at least 1000 cells were counted in 10 different areas using the 40× objective lens. Percentage of the positively stained cancer cells was evaluated using a continuous scale (0–100%) and graded on a scale of 0–4: 0, none; 1, 1–25%; 2, 26–50%; 3, 51–75%; 4, >75%. For further analysis, the extent of staining was defined as the product of grades of the extent and intensity of staining to determine the cutoff value for high expression of the proteins. Because the cutoff line of 2 demonstrated the most significance in relation to the Ki‐67 proliferation index,( 11 ) we presented the extent of staining, which was classified into high (grades 2–4) and low (grades 0 and 1) expression for clarity of data presentation. In addition, the percentage of carcinoma cells with positive staining of Ki‐67 (moderate or strong) among 1000 malignant cells in a given specimen was scored as the Ki‐67 proliferation index.
Terminal deoxynucleotide transferase‐mediated dUTP nick end labeling assay. For histological evaluation of apoptosis, the sections were processed by terminal deoxynucleotidyl tranferase‐mediated deoxyuridine triphosphate nick end‐labeling (TUNEL) method, using an In Situ Cell Death Detection Kit (Roche Diagnostics) following manufacturer's instructions. Briefly, sections were deparaffinized and rehydrated with xylene and a graded series of alcohols (100%, 90%, 80% and 70%) for 2 min each. This was followed by a 20‐min incubation of sections with proteinase K (Roche; pH 7.5) at room temperature. After two washes with PBS, sections were incubated with 0.3% hydrogen peroxide (H2O2) in methanol for 30 min to quench endogenous peroxidase (POD) activity. Sections were then rinsed twice in PBS and reacted with 50 µL of the TUNEL reaction mixture (Roche) for 60 min in a humidified chamber at 37°C. The sections were then rinsed three times in PBS and incubated for an additional 30 min with 50 µL of the Converter‐POD (Roche) followed by 10 min with diaminobenzidine (DAB; Roche). This procedure ensures the detection of TUNEL‐labeled cells. For positive controls, sections were treated with DNase I to induce DNA strand breaks, or peroxidase blocking solution was excluded. Negative controls were achieved by omitting terminal deoxynucleotidyl transferase (TdT; Roche). Apoptotic cells were determined based on observations of TUNEL‐staining sections and serial HE‐staining sections. TUNEL‐staining cells, if they represented the histologic features of necrosis in HE‐staining sections, were not considered to be apoptotic cells. For apoptotic detection, 10 000 tumor cells (1000 tumor cells each in 10 different fields) were evaluated at high magnification (40×). The apoptotic index was defined as the number of apoptotic cells per 1000 tumor cells.
Statistical analysis. Response‐rate calculations were based on all eligible patients. Follow‐up time was measured from the onset of chemotherapy. Overall patient survival was calculated from date of diagnosis to date of last follow‐up examination (censored) or date of death (event). Comparisons between two groups of patients according to MMP‐7 status were done using the the χ2 test or Fisher's exact test as appropriate for categorical data. The correlations between MMP‐7 expression and apoptosis‐related proteins were examined using Pearson correlation analysis. The associations between expression of MMP‐7 and Ki‐67 proliferation index or the apoptotic index were analyzed by Mann–Whitney U‐test. Bivariate correlations between immunohistochemical expression, patient or tumor characteristics, and response to chemotherapy were examined using the χ2 test or Fisher's exact test as appropriate. To simultaneously examine the effect of more than one factor on response to chemotherapy, multivariate logistic regression analysis was used. Survival curves were estimated using the Kaplan–Meier method, and differences in overall survival between groups were determined using the log‐rank test. Cox proportional hazards regression analysis was used to measure the association of clinicopathologic variables to overall survival. Statistical significance for model parameters was based on the likelihood ratio test. In all tests, a P‐value <0.05 was considered to indicate statistical significance. All analyses were done using SPSS for Windows 12.0 software package (SPSS, Chicago, IL, USA).
Results
Clinicopathologic data. The clinicopathologic features of the patients studied are summarized in Table 2. The median patient age was 54.1 years (range, 39–74 years). One hundred and eighteen patients (74.2%) were male. One hundred and twenty‐three patients (77.4%) had a history of smoking. There were 77 patients with a performance score (PS) of 0, whereas 82 patients had a PS of 1 or 2. Adenocarcinoma (n = 81) was the primary tumor type in this patient sample, whereas squamous cell carcinoma was the second most common type (42.5% of the patients). There were 62 patients (39.0%) who had a well or moderately differentiated histologic grade, and 97 patients (61.0%) whose grade was poor or undifferentiated. There were 96 patients (60.4%) with tumors at stages IIIA to IIIB, and 63 patients (39.6%) who had tumors at stage IV.
Table 2.
Clinical characteristics of patients
| Characteristic | Number | % | |
|---|---|---|---|
| Age, years | |||
| Median | 54.1 | ||
| Range | 39–74 | ||
| Sex | |||
| Male | 118 | 74.2 | |
| Female | 41 | 25.8 | |
| PS | |||
| 0 | 77 | 48.4 | |
| 1 | 65 | 40.9 | |
| 2 | 17 | 10.7 | |
| Histology | |||
| Adenocarcinoma | 81 | 50.9 | |
| Squamous cell carcinoma | 67 | 42.5 | |
| Large‐cell carcinoma | 7 | 4.4 | |
| Others | 4 | 2.5 | |
| Pathologic stage | |||
| III | 96 | 60.4 | |
| IV | 63 | 39.6 | |
| Chemotherapy regimen | |||
| Platinum + Paclitaxel | 56 | 35.2 | |
| Platinum + Taxotere | 42 | 26.4 | |
| Platinum + Gemcitabine | 33 | 20.8 | |
| Platinum + Vinorelbine | 23 | 14.5 | |
| Platinum + others | 5 | 3.1 | |
| Treatment response | |||
| CR | 8 | 5.0 | |
| PR | 34 | 21.4 | |
| SD | 78 | 49.1 | |
| PD | 39 | 24.5 | |
Abbreviation: PS, performance status; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Systemic chemotherapy using carboplatin or cis‐diamminedichloroplatinum (CDDP) was performed in all entrolled patients, with surgical resection in 35 patients (22.0%) and radiotherapy in 49 patients (30.8%). Regarding chemotherapeutic regimens, paclitaxel (35.2%) and taxotere (26.4%) were the most common options in combination with platium, followed by gemcitabine (20.8%). Two to eight courses (mean: four courses) of chemotherapy were administered.
Association of MMP‐7 with clinicopathologic characteristics. As previous reported,( 11 ) MMP‐7 staining appeared in tumor cells in the form of a heterogeneous cytoplasmic staining pattern, and typical staining patterns for other apoptosis‐relative protein are shown in Figure 1. Among the 159 carcinomas studied, 90 carcinomas (56.6%) were identified as high MMP‐7 expression, and 69 carcinomas (43.4%) were low MMP‐7 expression. Clinicopathologic variables stratified by MMP‐7 status are listed in Table 3. Regarding tumor histology, the occurrence of high MMP‐7 expression was 49.3% (n = 33) in squamous cell carcinomas and 66.7% (n = 54) in adenocarcinomas. The high expression of MMP‐7 was more frequent in adenocarcinomas than in squamous cell carcinomas (χ2 = 4.589, P = 0.032). However, no correlation was observed between MMP‐7 expression and clinical stage or tumor differentiation. Furthermore, the incidence of high MMP‐7 expression was similar in patients received surgery or not (45.7%versus 59.7%, P = 0.085). Also, the expression of MMP‐7 was not related with the use of radiation therapy (χ2 = 1.189, P = 0.602).
Figure 1.
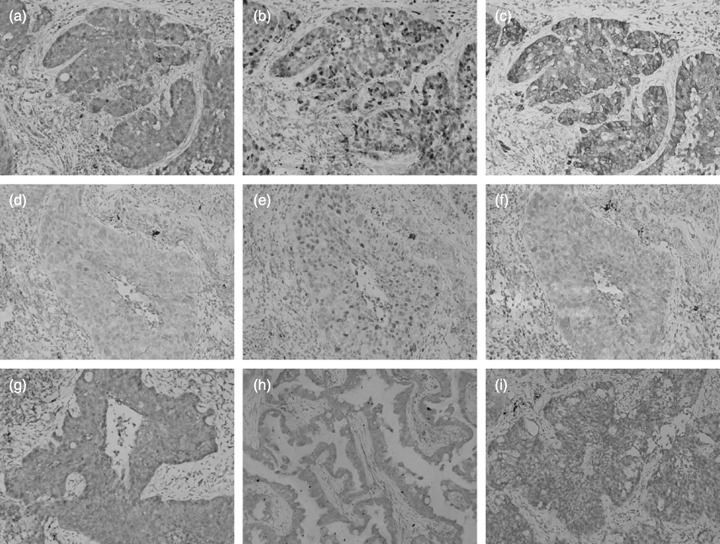
Representative immunohistochemical staining of non‐small cell lung cancer (×200). A squamous cell carcinoma with (a) high expression of matrix metalloproteinases‐7 (MMP‐7; Grade 4), (b) high Ki‐67 proliferation index and (c) high expression of Bcl‐2 (grade 4). An adenocarcinoma with (d) low expression of MMP‐7 (Grade 1), (e) low Ki‐67 proliferation index and (f) low expression of Bcl‐2 (Grade 1). (g) High expression of Fas in an adenocarcinoma (grade 4). (h) High expression of FasL in an adenocarcinoma (grade 4). (i) High expression of Bax in a squamous cell carcinoma (Grade 3).
Table 3.
MMP‐7 expression and clinicopathologic characteristics
| Characteristics | No. of patients | MMP‐7 | P‐value | |
|---|---|---|---|---|
| High | Low | |||
| Total | 159 | 90 | 69 | |
| Age (years) | 0.560 | |||
| <60 | 88 | 49 | 40 | |
| ≥60 | 71 | 42 | 29 | |
| Histology | 0.032 | |||
| Adenocarcinoma | 81 | 54 | 27 | |
| Squamous | 67 | 33 | 34 | |
| Differentiation | 0.492 | |||
| Well or moderate | 62 | 33 | 29 | |
| Poor or undifferentiated | 97 | 57 | 40 | |
| Tumor status | 0.673 | |||
| T1–T2 | 57 | 31 | 26 | |
| T3–T4 | 102 | 59 | 43 | |
| Nodal status | 0.417 | |||
| N1–N2 | 68 | 41 | 27 | |
| N3–N4 | 91 | 49 | 42 | |
| Stage | 0.165 | |||
| III | 96 | 35 | 61 | |
| IV | 63 | 14 | 41 | |
| Fas expression | 0.055 | |||
| High | 83 | 41 | 42 | |
| Low | 76 | 49 | 27 | |
| FasL expression | 0.157 | |||
| High | 130 | 77 | 53 | |
| Low | 29 | 13 | 16 | |
| Bcl‐2 expression | 0.042 | |||
| High | 106 | 66 | 40 | |
| Low | 53 | 24 | 29 | |
| Bax expression | 0.885 | |||
| High | 123 | 70 | 53 | |
| Low | 36 | 20 | 16 | |
Association of MMP‐7 with apoptosis‐related proteins. Among the 159 carcinomas studied, high expression of Bax, Bcl‐2, Fas and FasL was observed in 123 (77.4%), 106 (66.7%), 83 (52.2%), and 130 (81.8%) patients, respectively. We next studied the MMP‐7 expression in relation to other apoptosis‐related proteins in NSCLC. The percentage of MMP‐7 overexpression was 62.3% in high‐Bcl‐2 tumors and 45.3% in low‐Bcl‐2 tumors. The MMP‐7 status was positively correlated with the expression of Bcl‐2 (r = 0.162, P = 0.042). Although the statistic levels remained insignificant (r = –0.152, P = 0.056), there was a trend seen between Fas expression and MMP‐7 status, in which those with a low expression of Fas tended to have a high expression of MMP‐7.
Association of MMP‐7 expression with tumor proliferation and apoptosis. Given that MMP‐7 has been implicated in tumorigenesis and growth, we also compared the expression of MMP‐7 with that of Ki‐67 proliferative marker and apoptosis index. Regarding the Ki‐67 proliferation index, it varied greatly among the 159 NSCLC we studied (mean, 36.2 ± 19.7%). The Ki‐67 proliferation index was 38.1 ± 10.9% in high‐MMP‐7 tumors and 33.7 ± 9.7% in low‐MMP‐7 tumors. The Ki‐67 proliferation index was significantly higher in high‐MMP‐7 tumors than in low‐MMP‐7 tumors (Z = –3.541, P = 0 .031). The mean of the apoptotic index in 159 tumors was 13.9 ± 9.1. However, there was no statistically significant correlation between MMP‐7 expression and the apoptotic index determined by TUNEL methods. The apoptotic index was 14.2 ± 6.7 in low‐MMP7 tumors and 13.4 ± 6.3 in high‐MMP‐7 tumors (Z = –1.421, P = 0.155).
Predictive value of MMP‐7 expression for response to platinum‐based chemotherapy. The overall response rate was 42/159 (26.4%); 78 patients (49.1%) had stable disease, whereas 39 patients (24.5%) progressed after two or three cycles of chemotherapy. We next analyzed the relationship between MMP‐7 status and response to chemotherapy in all patients who received platinum‐based chemotherapy. Among the 90 patients with high MMP‐7 expression, 31 (34.4%) patients had SD and 41 (45.6%) patients had PD. In 69 patients with low MMP‐7 expression, 24 (34.8%) patients achieved CR or PR, 27 (39.1%) patients had SD, and 18 (26.1%) patients had PD. As shown in univariate analysis (Table 4), MMP‐7 expression was significantly correlated with response to chemotherapy (χ2 = 4.391, P = 0.036), as well as stage (χ2 = 10.100, P = 0.001) and PS status (χ2 = 4.151, P = 0.042). Expression of Bcl‐2 or Bax was not correlated with response to chemotherapy. Especially, MMP‐7 status was correlated with response to chemotherapy in adenocarcinoma (response rates, 18.2% and 42.3%, for patients with high‐MMP‐7 and low‐MMP‐7 tumors, respectively, χ2 = 5.351, P = 0.021), but not in patients with squamous cell carcinomas (χ2 = 0.792, P = 0.373). Combination of high expression of MMP‐7 with the expression of Bcl‐2 did not reveal any prognostic significance. Multivariate logistic regression analysis was performed for the correlation between chemotherapy response and characteristics, including stage, MMP‐7 and PS, which were significant in univariate analysis. However, only stage (OR 5.18, 95% CI: 3.53–14.67, P = 0.004), but not MMP‐7 (OR 2.54, 95% CI: 0.63–5.97, P = 0.054) or PS (OR 1.01, 95% CI: 0.53–2.40, P = 0.703), was identified as an independent predictor for the response to chemotherapy in overall cohort.
Table 4.
Response to platinum‐based chemotherapy according to various characteristics
| Immuostaining | Overall (n = 159) | Adeno (n = 81) | Squamous (n = 67) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CR + PR | % | P * | CR + PR | % | P | CR + PR | % | P | |
| Age (years) | 0.058 | 0.089 | 0.913 | ||||||
| <60 | 18 | 20.5 | 6 | 16.7 | 12 | 27.9 | |||
| ≥60 | 24 | 33.8 | 15 | 33.3 | 7 | 29.2 | |||
| PS | 0.042 | 0.114 | 0.056 | ||||||
| 0 | 26 | 33.8 | 14 | 33.3 | 11 | 40.7 | |||
| 1–2 | 16 | 19.5 | 7 | 17.9 | 8 | 19.5 | |||
| Stage | 0.001 | 0.019 | 0.018 | ||||||
| III | 34 | 35.4 | 17 | 35.4 | 16 | 38.5 | |||
| IV | 8 | 12.7 | 4 | 12.1 | 3 | 11.5 | |||
| Differentiation | 0.213 | 0.985 | 0.691 | ||||||
| Well or Moderate | 13 | 21.0 | 8 | 25.8 | 5 | 25.0 | |||
| Poor or undifferentiated | 29 | 29.9 | 13 | 26.0 | 14 | 29.8 | |||
| MMP‐7 | 0.036 | 0.021 | 0.373 | ||||||
| High | 18 | 20.0 | 10 | 18.2 | 8 | 23.5 | |||
| Low | 24 | 35.8 | 11 | 42.3 | 11 | 33.3 | |||
| Bcl‐2 | 0.056 | 0.433 | 0.309 | ||||||
| High | 23 | 21.7 | 12 | 23.1 | 11 | 24.4 | |||
| Low | 19 | 35.8 | 9 | 31.0 | 8 | 36.4 | |||
| Bcl‐2/MMP‐7 | 0.836 | 0.543 | 0.974 | ||||||
| Both high | 18 | 27.3 | 10 | 29.4 | 8 | 28.6 | |||
| Others | 24 | 36.6 | 11 | 23.4 | 11 | 28.2 | |||
| Bax | 0.833 | 0.444 | 0.869 | ||||||
| High | 32 | 26.0 | 15 | 24.6 | 15 | 28.8 | |||
| Low | 10 | 27.8 | 6 | 30.0 | 4 | 26.7 | |||
Abbreviation: Adeno, adenocarcinoma; CR, complete response; PD, progressive disease; PR, partial response; PS, performance status; SD, stable disease.
χ2 test or Fisher's exact test as appropriate.
Association of MMP‐7 expression with overall survival. During the follow‐up, 140 (88.1%) patients died because of lung cancer, whereas 19 (11.9%) patients were alive at the end of the follow‐up. The median follow‐up duration of patients was 20.8 months (range, 2–44 months). The 5‐year survival was 18.8% (n = 13) in patients with low‐MMP‐7 tumors and 6.7% (n = 6) in patients with high‐MMP‐7 tumors (χ2 = 5.501, P = 0.019). The estimated survival distributions were calculated by the Kaplan–Meier method. Overall, the median survival time was 16 months for 69 patients with low‐MMP‐7 expression (95% CI: 11–20 months) and was 10 months for 90 patients with high‐MMP‐7 expression (95% CI: 9–11 months). Figure 2 illustrates patient survival over time according to MMP‐7 expression.
Figure 2.
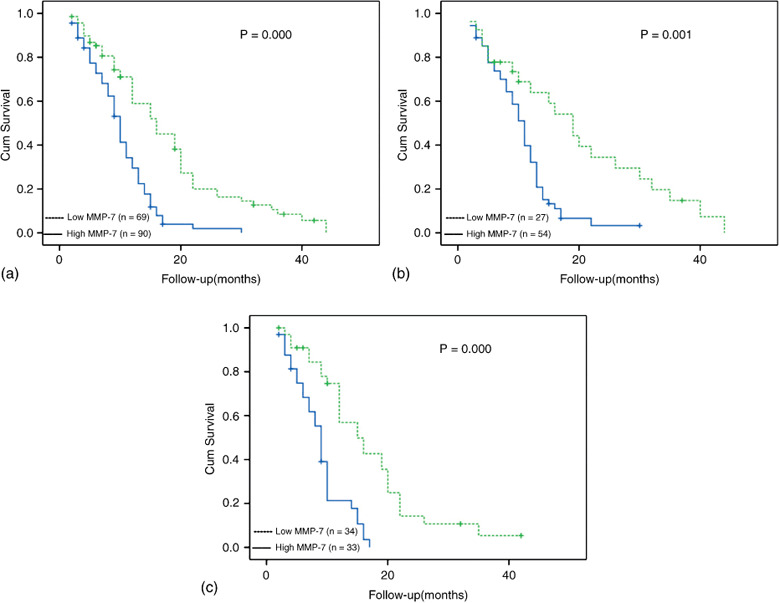
Overall survival of 159 patients with advanced non‐small cell lung cancer (NSCLC) according to of matrix metalloproteinases‐7 (MMP‐7) expression status: (a) in 159 NSCLC patients; (b) in 81 patients with adenocarcinomas; and (c) in 67 patients with squamous cell carcinomas.
In univariate analysis, there were highly significant associations between survival and tumor stage, PS, MMP‐7 expression level and patient age in overall patients (Table 5). The predictive value of MMP‐7 remained regardless of the tumor histology (Fig. 2b,c). Consequently, a multivariate regression analysis using the Cox proportional hazards regression model was performed. The model revealed MMP‐7 status (hazard ratio, 5.49; P = 0.001) and the tumor stage (hazard ratio, 5.96; P < 0.001) to be significant prognostic factors for NSCLC patients (Table 5).
Table 5.
Analysis of prognostic factors for overall survival
| Prognostic factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P * | HR | 95% CI | P ** | |
| Age (<60 versus≥60 years) | 2.97 | 1.88–7.21 | 0.042 | 1.58 | 0.64–3.79 | 0.210 |
| PS (0 versus 1–2) | 3.98 | 1.13–9.91 | 0.019 | 2.65 | 2.44–6.63 | 0.058 |
| Differentiation (Well or moderate versus poor or undifferentiated) | 1.72 | 0.70–4.23 | 0.198 | |||
| Stage (III versus IV) | 18.61 | 1.10–17.28 | 0.000 | 5.96 | 2.40–17.30 | 0.000 |
| Response (Responder versus Nonresponder † ) | 2.31 | 0.85–5.96 | 0.063 | |||
| Bcl‐2 expression (high versus low) | 2.09 | 0.78–5.62 | 0.075 | |||
| MMP‐7 expression (high versus low) | 11.31 | 7.24–29.33 | 0.000 | 5.49 | 1.01–8.30 | 0.001 |
Abbreviation: PS, performance status; HR, hazard ratio; 95% CI, 95% confidence interval.
Log‐rank test.
Cox proportional‐hazards regression model.
Stable disease, progressive disease, not evaluable.
Discussion
The present study explored the predictive value of MMP‐7 expression in tumor samples of patients with advanced NSCLC receiving platinum‐based regimen. MMP‐7 overexpression was found to be associated with poor response to platinum‐chemotherapy in overall NSCLC and especially in adenocarcinoma patients. Furthermore, MMP‐7 status was an independent predictor for prognosis of NSCLC patients. This study rendered further evidence for the multifunction of MMP‐7 in progression of NSCLC.
Impact of MMP‐7 on proliferation and apoptosis. In line with Liu et al.,( 11 ) MMP‐7 expression has a significant correlation with Ki‐67 proliferation index in our NSCLC cohort, but not with the apoptotic index. The impact of MMP‐7 on apoptosis and proliferation has been extensively debated in literature.( 6 , 7 , 17 , 18 ) Some researchers found that MMP‐7 may influence the early stage of carcinogenesis by accelerating proliferation and inhibiting apoptosis of malignant cells.( 19 , 20 , 21 ) Modification of non‐ECM proteins, such as heparin‐binding EGF‐like growth factor (HB‐EGF) precursor (proHB‐EGF)( 22 ) and insulin‐like growth factor binding proteins (IGFBP),( 23 ) is one of the mechanisms by which MMP‐7 plays a role in early tumorigenesis.( 5 ) However, a conflicting report by Liu et al.( 24 ) has shown an inverse relationship between MMP‐7 expression and Ki‐67 proliferation index in advanced gastric carcinoma. And we recently reported an inhibitive ability of MMP‐7 on the proliferation of A549 lung adenocarcinoma cells in vitro.( 25 ) The discrepancy may lie in the disparate microenvironment between in vivo and in vitro,( 7 ) the diverse secretion patterns of MMP‐7( 26 ) and the nature of targets.( 27 )
Both anti‐ and proapoptotic activity of MMP‐7 have been reported in several normal and tumor epithelial cell lines.( 6 , 7 , 17 , 18 ) Despite the presence of proapoptotic property, Vargo‐Gogola and his colleague( 7 ) emphasized that the contribution of this enzyme to tumor formation may be partly by exposing the epithelia to an apoptotic selective pressure, resulting in selective expansion of resistance to apoptotic stimuli. Increasing evidence indicates that MMP‐7 may be multifunctional, with apparent paradoxical effects on tumor progression.( 28 ) In support of this conjecture, we have observed its dual‐effect in modulating the sensitivity of A549 cells to FasL‐mediated apoptosis,( 25 ) which suggests its complex role in the apoptotic–antiapoptotic process. The imbalanced regulation of MMP‐7 on apoptosis and proliferation may indicate the different behavior of this metalloproteinase in lung cancer development, from normal epithelium to dysplastic epithelium and finally to invasive carcinoma.
MMP‐7 and chemotherapy sensitivity. One of the major findings in the present study is that elevated MMP‐7 expression has a close association with decreased response to platinum‐based chemotherapy in patients with NSCLC. To the best of our knowledge, the present study is the first report to show that intratumoral MMP‐7 expression in NSCLC, especially in adenocarcinoma, is associated with response to combination chemotherapy including platinum. These results provide new insight for the biological properties and clinical relevance of the MMP‐7 in NSCLC.
Many chemotherapeutic drugs cause the cytotoxicity of cancer cells by apoptosis. Both extrinsic (or receptor‐mediated) pathway and intrinsic (or mitochondria‐mediated) pathway have been involved in platinum‐induced cell death.( 29 , 30 ) Owing to the proteolytic down‐regulation of membrane‐bound Fas (mFas) or membrane‐bound Fas ligand (mFasL) protein by MMP‐7, the Fas/FasL receptor pathway has become the focus of previous research.( 7 , 17 , 18 ) Furthermore, an inverse correlation between MMP‐7 and Fas expression has been revealed in patients with colorectal carcinoma( 10 ) and gastric carcinoma,( 31 ) respectively. Nevertheless, the expression of Fas or FasL protein in relation to MMP‐7 status was never explored in lung cancers. In our study, although MMP‐7 had a close correlation with Fas, the statistical level was insignificant.
Interestingly, the present study revealed that high MMP‐7 expression was associated with Bcl‐2 overexpression. Crawford et al.( 32 ) observed that Bcl‐2 was expressed specifically in the metaplastic ducts of MMP‐7‐expressing pancreata in wild‐type mice, whereas ductal structures in pancreata of MMP‐7−/– mice did not stain for Bcl‐2. They speculated that MMP‐7 might directly stimulate the expression of Bcl‐2. Indeed, we have observed that chronic exposure to MMP‐7 can elevate the level of Bcl‐2 protein in A549 cells (unpublished data). Therefore, current clinical study has confirmed our experimental data in vitro. More importantly, recent evidence from the laboratory has associated the persistence of Bcl‐2 overexpression with cisplatin‐resistance in several cancer cells line.( 33 , 34 ) Huang et al.( 35 ) demonstrated that small interfering RNA (siRNA) targeting of the Bcl‐2 gene restored the sensitivity to CDDP in A549/CDDP cells. Based on these findings, the poor response to platinum‐based chemotherapy in NSCLC patients may be, at least partly, ascribed to sustained induction of Bcl‐2 by MMP‐7 overexpression.
Besides the depression of apoptosis, MMP‐7 mRNA level was found to be positively correlated with transcription factor Ets‐1 in patients with lung cancers( 12 ) and the role of Ets‐1 in cisplatin‐resistance has been established.( 36 ) Recent study proved that MMP‐7 treatment indirectly activated the epidermal growth factor receptor (EGFR)‐mediated MEK–ERK signaling pathway,( 37 ) which can successively up‐regulate DNA repair genes.( 38 ) Therefore, the effect of MMP‐7 on chemoresistance may not only depend on the forms present in the local concentration of MMP‐7, cellular and pericellular distribution, bioavailability of local concentration of MMP‐7 in the microenvironment and the time when the MMP‐7 is presented to the tumor cells, but also on its interaction with other factors. Collectively, the complicated mechanisms for MMP‐7‐induced chemoresistance warrant more thorough and explicit investigations.
MMP‐7 and survival. The impact of MMP‐7 expression on the prognosis in NSCLC has been evaluated by a few studies.( 11 , 12 , 13 , 39 , 40 ) Only one study in the literature has found MMP‐7 status to be a significant predictor for the overall survival in NSCLC.( 11 ) In our cohort, the overall survival was significantly lower in patients with high MMP‐7 expression than in those with low expression. Regardless of disrelation with other clinicpathological characteristics, MMP‐7 status was significantly associated with survival by the multivariate analysis. Thus, our results confirmed MMP‐7 expression to be a critical factor in predicting the survival of NSCLC patients. Furthermore, Liu et al.( 11 ) reported that the MMP‐7 status was a significant prognostic factor for patients with squamous cell carcinomas, but not with adenocarcinoma. Consistent with most of previous studies,( 13 , 40 ) adenocarcinomas exhibited a higher proportion of MMP‐7 overexpression as compared with other histological subtypes in our study. Nevertheless, we did not find that the prognostic value of MMP‐7 differs among histological types.
The present study has several potential limitations. First, the present study was a retrospective one at three institutions and the sample size was not large. Second, patient backgrounds, including chemotherapeutic combinations used, were not uniform. The heterogeneity in the use of surgery and radiotherapy may cause bias in the conclusion. Finally, MMP‐7 and relevant markers are unevenly expressed in lung cancer and evaluation of IHC has its own limitation because of its semiquantitative nature. Also, the evaluation criteria for expression (such as cutoff threshold) were arbitrary.
Although further larger prospective clinical studies employing standard chemotherapy regimens are warranted to validate the role of MMP‐7 expression in NSCLC, the present results have several clinical implications. The current results suggest that the prognosis of patients with high expression of MMP‐7 would be poor after chemoradiotherapy with platinum, which plays a central role in the management of advanced NSCLC. In addition, concurrent chemoradiotherapy with platinum is usually associated with significant toxicity. Therefore, it is reasonable to consider an alternative treatment strategy for patients with high expression of MMP‐7. MMP‐7 may become a new immunohistochemical marker or add to the current predictors of chemotherapeutic responsiveness and prognosis in NSCLC patients.
Conclusion
In brief, current results validated our laboratory observation, and provided further support for using MMP‐7 as a therapeutic target. Thus, our results might have significant clinical relevance with regard to prognosis and therapy. Conversely, it suggests that MMP‐7 protein in tumor tissue could potentially serve as a tumor marker to predict platinum resistance of NSCLC. Such a prognostic classification and stratification based on this aspect of tumor biology might be crucial for clinical decision making on adjuvant or neoadjuvant treatments of NSCLC. To date, many phase III studies using broad‐spectrum MMP inhibitors have yielded disappointing results; our results raise a concern regarding adjuvant treatment that specifically modulates the expression or function of MMP‐7 to improve the effectiveness for some NSCLC patients.
References
- 1. Cosaert J, Quoix E. Platinum drugs in the treatment of non‐small‐cell lung cancer. Br J Cancer 2002; 87: 825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet 2008; 371: 771–83. [DOI] [PubMed] [Google Scholar]
- 3. Rintoul RC, Sethi T. Extracellular matrix regulation of drug resistance in small‐cell lung cancer. Clin Sci (Lond) 2002; 102: 417–24. [PubMed] [Google Scholar]
- 4. Sethi T, Rintoul RC, Moore SM et al . Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo . Nat Med 1999; 5: 662–8. [DOI] [PubMed] [Google Scholar]
- 5. Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase‐7 (Matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med 2006; 231: 20–7. [DOI] [PubMed] [Google Scholar]
- 6. Vargo‐Gogola T, Fingleton B, Crawford HC, Matrisian LM. Matrilysin (matrix metalloproteinase‐7) selects for apoptosis‐resistant mammary cells in vivo. Cancer Res 2002; 62: 5559–63. [PubMed] [Google Scholar]
- 7. Mitsiades N, Yu WH, Poulaki V, Tsokos M, Stamenkovic I. Matrix metalloproteinase‐7‐mediated cleavage of Fas ligand protects tumor cells from chemotherapeutic drug cytotoxicity. Cancer Res 2001; 61: 577–81. [PubMed] [Google Scholar]
- 8. Almendro V, Maurel J, Augé J et al . Role of metalloproteinase‐7 in oxaliplatin acquired resistance in colorectal cancer cell lines. ASCO Meeting Abstracts 2006; 24: 20042. [Google Scholar]
- 9. Miyata Y, Iwata T, Ohba K, Kanda S, Nishikido M, Kanetake H. Expression of matrix metalloproteinase‐7 on cancer cells and tissue endothelial cells in renal cell carcinoma: prognostic implications and clinical significance for invasion and metastasis. Clin Cancer Res 2006; 12: 6998–7003. [DOI] [PubMed] [Google Scholar]
- 10. Wang WS, Chen PM, Wang HS, Liang WY, Su Y. Matrix metalloproteinase‐7 increases resistance to Fas‐mediated apoptosis and is a poor prognostic factor of patients with colorectal carcinoma. Carcinogenesis 2006; 27: 1113–20. [DOI] [PubMed] [Google Scholar]
- 11. Liu D, Nakano J, Ishikawa S et al . Overexpression of matrix metalloproteinase‐7 (MMP‐7) correlates with tumor proliferation, and a poor prognosis in non‐small cell lung cancer. Lung Cancer 2007; 58: 384–91. [DOI] [PubMed] [Google Scholar]
- 12. Yukiue H, Moiriyama S, Kobayashi Y et al . Clinical significance of matrix metalloproteinase‐7 and Ets‐1 gene expressio n in patients with lung cancer. J Surg Res 2001; 101: 242–7. [DOI] [PubMed] [Google Scholar]
- 13. Leinonen T, Pirinen R, Bohm J, Johansson R, Ropponen K, Kosma VM. Expression of matrix metalloproteinases 7 and 9 in non‐small cell lung cancer. Relation to clinicopathological factors, beta‐catenin and prognosis. Lung Cancer 2006; 51: 313–21. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization . Histological Typing of Lung Tumours, 2nd edn. Geneva: World Health Organization, 1999; 21–66. [Google Scholar]
- 15. Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997; 111: 1710–17. [DOI] [PubMed] [Google Scholar]
- 16. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981; 47: 207–14. [DOI] [PubMed] [Google Scholar]
- 17. Strand S, Vollmer P, van den Abeelen L et al . Cleavage of CD95 by matrix metalloproteinase‐7 induces apoptosis resistance in tumour cells. Oncogene 2004; 23: 3732–6. [DOI] [PubMed] [Google Scholar]
- 18. Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol 1999; 9: 1441–7. [DOI] [PubMed] [Google Scholar]
- 19. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 2000; 18: 1135–49. [DOI] [PubMed] [Google Scholar]
- 20. Wilson CL, Heppner KJ, Labosky PA et al . Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci 1997; 94: 1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hulboy DL, Gautam S, Fingleton B, Matrisian LM. The influence of matrix metalloproteinase‐7 on early mammary tumorigenesis in the multiple intestinal neoplasia mouse. Oncol Rep 2004; 12: 13–17. [PubMed] [Google Scholar]
- 22. Yu WH, Woessner JFJ, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP‐7 with heparin‐binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev 2002; 16: 307–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyamoto S, Yano K, Sugimoto S et al . Matrix metalloproteinase‐7 facilitates insulin‐like growth factor bioavailability through its proteinase activity on insulin‐like growth factor binding protein 3. Cancer Res 2004; 64: 665–71. [DOI] [PubMed] [Google Scholar]
- 24. Liu XP, Oga A, Suehiro Y, Furuya T, Kawauchi S, Sasaki K. Inverse relationship between matrilysin expression and proliferative activity of cells in advanced gastric carcinoma. Hum Pathol 2002; 33: 741–7. [DOI] [PubMed] [Google Scholar]
- 25. Liu H, Huang J, Wu B, Zhou Y, Zhu J, Zhang T. Matrilysin inhibits proliferation and modulates sensitivity of lung cancer cells to FasL‐mediated apoptosis. Med Oncol 2008. (Mar 14) [Epub ahead of print]. [DOI] [PubMed]
- 26. Harrell PC, McCawley LJ, Fingleton B et al . Proliferative effects of apical, but not basal, matrix metalloproteinase‐7 activity in polarized MDCK cells. Exp Cell Res 2005; 303: 308–20. [DOI] [PubMed] [Google Scholar]
- 27. Nailin H, Yasushi I, Masako K et al . MMP‐7 (matrilysin) accelerated growth of human umbilical vein endothelial cells. Cancer Lett 2002; 177: 95–100. [DOI] [PubMed] [Google Scholar]
- 28. Kendra JG, Zena W, Farrah K. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev 2007; 87: 69–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghobrial IM, Witzig TE, Alex A. Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin 2005; 55: 178–94. [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez VM, Fuertes MA, Alonso C, Perez JM. Is cisplatin‐induced cell death always produced by apoptosis? Mol Pharmacol 2001; 59: 657. [DOI] [PubMed] [Google Scholar]
- 31. Hu D‐M, Wang S‐F, Feng Y‐Z. Expression of matrix metalloproteinase‐7 and Fas and their significances in gastric carcinoma. World J Gastroenterol 2006; 14: 3237–40. [Google Scholar]
- 32. Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase‐7 is expressed by pancreatic cancer precursors and regulates acinar‐to‐ductal metaplasia in exocrine pancreas. J Clin Invest 2002; 109: 1437–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Losert D, Pratscher B, Soutschek J et al . Bcl‐2 downregulation sensitizes nonsmall cell lung cancer cells to cisplatin, but not to docetaxel. Anticancer Drugs 2007; 18: 755–61. [DOI] [PubMed] [Google Scholar]
- 34. Kumar Biswas S, Huang J, Persaud S, Basu A. Down‐regulation of Bcl‐2 is associated with cisplatin resistance in human small cell lung cancer H69 cells. Mol Cancer Ther 2004; 3: 327–34. [PubMed] [Google Scholar]
- 35. Huang Z, Lei X, Zhong M, Zhu B, Tang S, Liao D. Bcl‐2 small interfering RNA sensitizes cisplatin‐resistant human lung adenocarcinoma A549/DDP cell to cisplatin and diallyl disulfide. Acta Biochim Biophys Sin (Shanghai) 2007; 39: 835–43. [DOI] [PubMed] [Google Scholar]
- 36. Wilson LA, Yamamoto H, Singh G. Role of the transcription factor Ets‐1 in cisplatin resistance. Mol Cancer Ther 2004; 3: 823–32. [PubMed] [Google Scholar]
- 37. Tan X, Egami H, Abe M, Nozawa F, Hirota M, Ogawa M. Involvement of MMP‐7 in invasion of pancreatic cancer cells through activation of the EGFR mediated MEK‐ERK signal transduction pathway. J Clin Pathol 2005; 58: 1242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andrieux LO, Fautrel A, Bessard A, Guillouzo A, Baffet G, Langouët S. GATA‐1 is essential in EGF‐mediated induction of nucleotide excision repair activity and ERCC1 expression through ERK2 in human hepatoma cells. Cancer Res 2007; 67: 2114–23. [DOI] [PubMed] [Google Scholar]
- 39. Lin TS, Chiou SH, Wang LS et al . Expression spectra of matrix metalloproteinases in metastatic non‐small cell lung cancer. Oncol Rep 2004; 12: 717–23. [PubMed] [Google Scholar]
- 40. Kawano N, Osawa H, Ito T et al . Expression of gelatinase A, tissue inhibitor of metalloproteinases‐2, matrilysin, and trypsin (ogen) in lung neoplasms: an immunohistochemical study. Hum Pathol 1997; 28: 613–22. [DOI] [PubMed] [Google Scholar]


