Abstract
Mesothelioma is an aggressive cancer often caused by chronic asbestos exposure, and its prognosis is very poor despite the therapies currently used. Due to the long latency period between asbestos exposure and tumor development, the worldwide incidence will increase substantially in the next decades. Thus, novel effective therapies are warranted to improve the prognosis. The ERC/mesothelin gene (MSLN) is expressed in wide variety of human cancers, including mesotheliomas, and encodes a precursor protein cleaved by proteases to generate C‐ERC/mesothelin and N‐ERC/mesothelin. In this study, we investigated the antitumor activity of C‐ERC/mesothelin‐specific mouse monoclonal antibody, 22A31, against tumors derived from a human mesothelioma cell line, ACC‐MESO‐4, in a xenograft experimental model using female BALB/c athymic nude mice. Treatment with 22A31 did not inhibit cell proliferation of ACC‐MESO‐4 in vitro; however, therapeutic treatment with 22A31 drastically inhibited tumor growth in vivo. 22A31 induced antibody‐dependent cell‐mediated cytotoxicity by natural killer (NK) cells, but not macrophages, in vitro. Consistently, the F(ab′)2 fragment of 22A31 did not inhibit tumor growth in vivo, nor did it induce antibody‐dependent cell mediated cytotoxicity (ADCC) in vitro. Moreover, NK cell depletion diminished the antitumor effect of 22A31. Thus, 22A31 induced NK cell‐mediated ADCC and exerted antitumor activity in vivo. 22A31 could have potential as a therapeutic tool to treat C‐ERC/mesothelin‐expressing cancers including mesothelioma.
(Cancer Sci 2010; 101: 969–974)
Mesothelioma is an aggressive cancer stemming from transformation of mesothleial cells, which is usually associated with chronic asbestos exposure.( 1 ) Due to the long latency period between asbestos exposure and tumor development, the worldwide incidence will increase substantially in the next decades.( 2 , 3 ) The prognosis is very poor with a median survival of 4 to12 months despite the therapies currently used, including surgery, radiotherapy, and chemotherapy.( 4 , 5 ) Because of the inefficacy of the conventional treatments, novel effective therapies are necessary for improving the prognosis of this devastating disease.
Erc was identified in renal cell cancers of the Eker rat, and is the homolog of human MSLN.( 6 , 7 ) The ERC/mesothelin gene encodes a 71 kDa precursor protein, which is cleaved by proteases to yield 31‐kDa N‐terminal (N‐ERC/mesothelin) and 40‐kDa C‐terminal (C‐ERC/mesothelin) proteins. ( 8 , 9 ) N‐ERC/mesothelin, originally identified as megakaryocyte‐potentiating factor (MPF), is a soluble protein released into the extracellular space and is now used as a serum marker of mesothelioma.( 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 ) C‐ERC/mesothelin is a glycoprotein tethered to the cell surface by a glycosyl phosphatidyl inositol anchor, and it was reported that this protein promotes anchorage‐independent growth and prevents anoikis.( 8 , 9 , 22 ) C‐ERC/mesothelin is expressed not only in normal mesothelial cells of the pleura, pericardium, and peritoneum, but also in malignant cells of mesotheliomas, pancreatic ductal carcinomas, ovarian cancers, and some other cancers.( 8 , 23 , 24 , 25 , 26 ) Moreover, C‐ERC/mesothelin is a possible target for immunotherapy because of its frequent expression.( 9 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 )
In this study, we investigated the antitumor activity of an anti‐C‐ERC/mesothelin monoclonal antibody (mAb) (22A31) that we have devised against tumors derived from a mesothelioma cell line.( 35 ) Treatment with 22A31 itself did not affect tumor cell growth in vitro, but induce antibody‐depend cell mediated cytotoxicity (ADCC) with natural killer (NK) cells, but not macrophages. 22A31 consistently exerted an antitumor effect in vivo, and which was not observed when the F(ab′)2 fragment of 22A31 was used or NK cells were depleted in mice. These results suggest that 22A31 is a possible therapeutic tool for C‐ERC/mesothelin‐expressing tumors including mesothelioma in clinical therapy.
Materials and Methods
Mice. Female BALB/c athymic nude (BALB/c nu/nu) mice at 7 weeks of age were purchased from Charles River Japan (Yokohama, Japan). Rag‐2‐deficient (RAG‐2−/−) C57BL/6 mice were derived as described previously.( 36 ) All mice were maintained under specific pathogen‐free conditions and all in vivo studies were approved by the Institute Animal Care and Use Committee of Juntendo University.
Cells and antibodies. ACC‐MESO‐4 cells, derived from human mesothelioma, were provided by RIKEN cell bank (Ibaraki, Japan), and cultured in RPMI‐1640 medium supplemented with 10% fetal calf serum.( 37 ) Huh7 cells, derived from human hepatocellular carcinoma, were purchased from RIKEN Cell Bank, and cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal calf serum. NCI‐H226 cells, derived from human mesothelioma, were provided by the American Type Culture Collection (Manassas, VA, USA), and cultured in RPMI‐1640 medium supplemented with 10% fetal calf serum. C‐ERC/mesothelin‐specific mouse monoclonal antibody, 22A31, was prepared in our laboratory as previously described.( 35 ) Normal mouse IgG1κ was purchased from Sigma (St. Louis, MO, USA). F(ab′)2 fragment was prepared in our laboratory as previously described.( 38 ) Generation of F(ab′)2‐22A31 was confirmed by SDS‐polyacrylamide gel electrophoresis (SDS‐PAGE) using 10% Laemmli gel and Coomassie Brilliant Blue (CBB) staining.
Flow cytometric analysis. C‐ERC/mesothlin expression on cell surface was analyzed by flow cytometry as previously described.( 35 ) Briefly, 1 × 106 cells were incubated with 1 μg/mL of 22A31 or 1 μg/mL of normal mouse IgG1κ diluted in serum free medium (100 μL) at 4°C for 30 min. After washing with PBS, cells were resuspended in 100 μL of serum free medium containing 2 μg/mL of Alexa Flour 488‐conjugated goat antimouse IgG (Molecular Probes, Eugene, OR, USA) to detect the primary antibodies, and incubated at 4°C for 30 min. After washing with PBS, the stained cells were analyzed by a FACScan (BD Bioscience, San Jose, CA, USA).
Antitumor activity of 22A31 mAb in BALB/c nu/nu mice. To assess the antitumor activity of 22A31, 2 × 106 of tumor cells were inoculated subcutaneously into the right flank of BALB/c nu/nu mice. We commenced antibody treatments with 22A31 or isotype matched control IgG by intratumoral injection or intraperitoneal injection twice per week, when tumors become visible (4 mm × 4 mm, 16 mm2). In some experiments, mice were treated with 200 μg of anti‐asialo‐GM1 Ab (Wako Pure Chemicals, Osaka, Japan) or control rabbit Ig every 4 days starting 2 days before 22A31 treatment. NK cell depletion was confirmed by flow cytometric analysis in anti‐asialo GM1 Ab‐treated mice. Tumor bearing‐mice were monitored for tumor development and progression. Tumor size was determined by caliper measurement of the largest (a) and smallest perpendicular diameters (b) and height (c), and was calculated using following the formula: Volume = 4 πa × b × c/3. After the completion of the experiment, mice were euthanized.
Immunohistochemistry. Three‐cm thick tissue sections were prepared from archival formalin‐fixed, paraffin‐embedded specimens. After deparaffinization, the tissue sections were heated in 10 mm citrate buffer (pH6) for antigen retrieval and then treated with 3% hydrogen peroxide. Then, the sections were incubated with antibody solutions diluted in Tris‐buffered saline with 0.1% Tween 20 overnight at 4°C. We used biotin‐conjugated 22A31 (1:300 dilution) as the primary antibody. Diaminobenzidine was used as the substrate for peroxidase. DX50F‐3, DP‐25, and DP2‐BSW (Olympus. Tokyo, Japan) were used for the acquisition of images.
Cell proliferation assay. Tumor cells grown under regular culture condition were harvested and resuspended in RPMI‐1640 medium at a concentration 8 × 104 cells/mL. Twenty‐five μL of cell suspensions and 25 μL of antibody solutions were added to 96‐well plates. After 72 h of incubation at 37°C with 5% CO2 atmosphere, XTT assay was performed using 50 μL of XTT mixture containing 200 μL/mL of XTT and 25 μm of menatione (Roche Diagnostics, Mannheim, Germany) for 4 h. Absorbance at 470–650 nm was measured in an ELISA reader (E‐MAX; Molecular Devices, Sunnyvale, CA, USA).
Antibody‐dependent cell mediated cytotoxicity (ADCC) assay. Cytotoxic activity was tested by a 4‐h 51Cr release assay as previously described.( 36 ) Freshly isolated splenic and peritoneal mononuclear cells derived from B6 RAG‐2−/− mice were stained with phycoerythrin (PE)‐conjugated anti‐DX5 mAb (eBioscience, San Diego, CA, USA) or PE‐conjugated anti‐CD14 mAb (eBioscience), and DX5 + NK cells or CD14 + macrophages were enriched by auto MACS using PE microbeads (Miltenyi Biotec, Bergisch Glabach, Germany), respectively, according to the manufacturer’s instructions. These purified cells were subsequently used as the effector cells in the ADCC assay.
Complement‐dependent cytotoxicity (CDC) assay. Complement‐mediated lysis with rabbit serum (Low‐Tox M; Cedarlane Laboratories, Hornby, ON, Canada) was performed as previously described.( 39 ) After incubation, cellular viability was determined by Trypan blue exclusion. Cytotoxicity index (C.I.) was calculated using the following formula: % C.I. = (sample % cell death − control % cell death)/(100 − control % cell death) × 100%.
Statistical analysis. We analyzed the data of xenografts treated with antibodies, using JMP and SAS version 8.1.3 (SAS Institute, Cary, CA, USA). To compare the volume of tumors between groups, the Mann–Whitney U‐test was useed. The two‐sample t‐test was used for cell proliferation, and the ADCC and CDC assays. P < 0.05 was considered statistically significant.
Results
C‐ERC/mesothelin expression in target cells. Cell surface expression of C‐ERC/mesothelin on ACC‐MESO‐4 tumor cells and NCI‐H226 tumor cells, but not Huh7 tumor cells, was demonstrated by both flow cytometric and immunohistochemical analysis (Fig. 1). Thus, ACC‐MESO‐4 and NCI‐H226 tumor cells constitutively expressed C‐ERC/mesothelin even after xenograft transplantation into nude mice.
Figure 1.
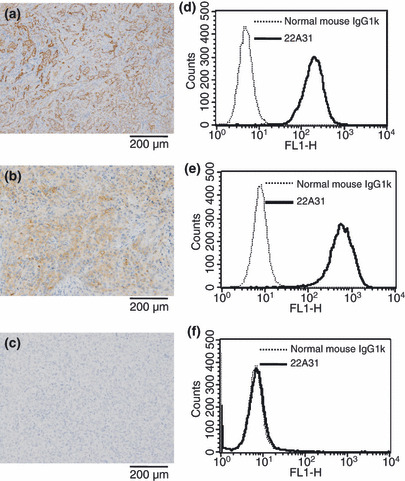
Expression of C‐ERC/mesothelin in ACC‐MESO‐4, NCI‐H226, and Huh7 cells. Immunohistochemical localization of C‐ERC/mesothelin in ACC‐MESO‐4 cell‐ (a), NCI‐H226 cell‐ (b), and Huh7 cell‐ (c) derived xenografts. Indicated panel is representative of xenograft specimens. Original magnification, ×100. Surface expression of C‐ERC/mesothelin on ACC‐MESO‐4 cells (d), NCI‐H226 cells (e), and Huh7 cells (f) analyzed by flow cytometry. Solid line denotes staining of 22A31 and detected with Alexa Flour‐488. Dashed line denotes staining normal mouse IgG1κ.
Antitumor activity of 22A31 in mice xenograft model. When 22A31 was intraperitonealy injected into ACC‐MESO‐4 tumor‐bearing mice, 22A31 did not significantly inhibit tumor growth compared with normal mouse IgG1κ (Fig. 2a). However, intratumoral injection of 22A31 significantly inhibited the growth of ACC‐MESO‐4 tumors, but not Huh7 tumors, compared with normal mouse IgG1κ (Fig. 2b,c). Moreover, this antitumor effect of 22A31 was demonstrated to occur in a dose‐dependent manner (Fig. 2b). These results suggest that 22A31 exerts an antitumor effect against C‐ERC/mesothelin‐expressing tumor cells in vivo when administrated intratumorally.
Figure 2.
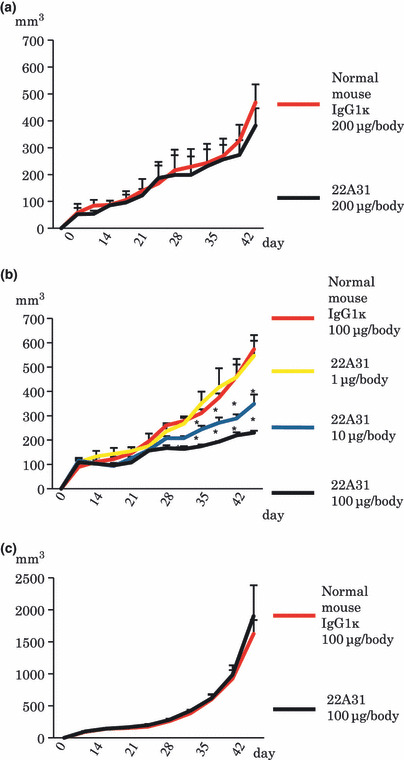
In vivo antitumor activity of 22A31. (a) Antitumor effect of 22A31 intraperitoneal injection against ACC‐MESO‐4 tumor cell. (b) Antitumor effect of 22A31 intratumoral injection against ACC‐MESO‐4 tumor cells. (c) Antitumor effect of 22A31 intratumoral injection against C‐ERC/mesothelin‐negative Huh7 tumor cells. 22A31 or normal mouse IgG1κ (control Ab) was given twice per week. Mice were treated 10 times with the respective antibody. Median tumor volume and SD of xenografts are indicated (n = 3–5). Asterisk mark indicates statistical significance.
22A31 triggered ADCC in vitro. Then, we examined the possible mechanisms involved in the 22A31‐induced antitumor effect in vitro. The cell proliferation assay using XTT did not show a direct inhibitory effect of 22A31 on the growth of ACC‐MESO‐4 tumor cells in vitro (Fig. 3a). Furthermore, 22A31 did not induce CDC against ACC‐MESO‐4 cells (Fig. 3b). However, 22A31 induced ADCC when ACC‐MESO‐4 cells were incubated with purified NK cells, and that was in an effector/target ratio‐dependent manner (Fig. 3c). 22A31 also induced ADCC against C‐ERC/mesothlelin‐expressing NCI‐H226 cells, but not Huh7 cells, which did not express C‐ERC/mesothelin when incubated with purified NK cells (Fig. 3d,e), although both cells might be sensitive to direct NK‐mediated cytotoxicity. Besides, 22A31 did not induce ADCC against C‐ERC/mesothlelin‐expressing ACC‐MESO‐4 when incubated with purified macrophages (Fig. 3f).
Figure 3.
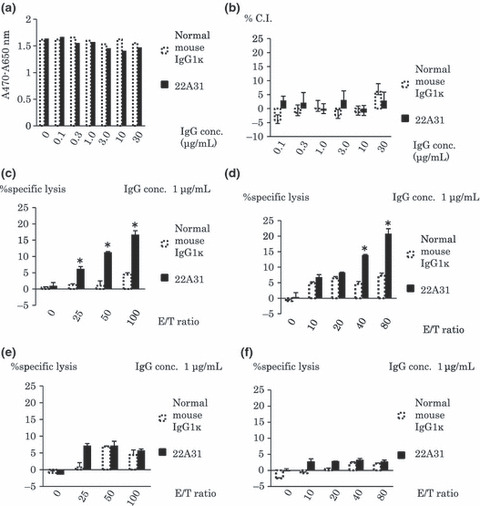
In vitro antitumor activity of 22A31. (a) Direct effect of 22A31 on ACC‐MESO‐4 tumor cell growth. After 72 h of incubation with indicated concentration of 22A31 (black bar) or normal mouse IgG1k (white bar), XTT assay was performed and absorbance was presented. (b) 22A31‐induced complement‐dependent cytotoxicity (CDC). %Cyto‐toxicity index (C.I.) of the indicated concentrations of 22A31 or normal mouse IgG1k against ACC‐MES‐4 tumor cells are indicated. (c,d) 22A31‐inducd antibody‐dependent cell mediated cytotoxicity (ADCC) against ACC‐MESO‐4 tumor cells (c) or NCI‐H226 tumor cells (d) with purified natural killer (NK) cells. ADCC induced by 1 μg/mL of 33A31 or normal mouse IgG1κ was examined at indicated effector/target ratios when purified NK cells were used as effector cells. (e) 22A31‐inducd ADCC against C‐ERC/mesothelin‐negative Huh7 tumor cells with purified NK cells. ADCC induced by 1 μg/mL of 33A31 or normal mouse IgG1κ was examined at indicated effector/target ratios when purified NK cells were used as effector cells. (f) 22A31‐inducd ADCC against ACC‐MESO‐4 tumor cells with purified macrophages. ADCC induced by 1 μg/mL of 33A31 or normal mouse IgG1κ was examined at indicated effector/target ratios when purified macrophages were used as effector cells. Asterisk indicates statistical significance.
Critical contribution of Fc portion and NK cells to antitumor effect of 22A31 in vivo. The Fc portion of antibodies, which binds to the Fc receptor on NK cells and complement, is critical for ADCC induction.( 40 ) Thus, we examined the antitumor effect of 22A31 F(ab′)2 fragment (Fig. 4a). The F(ab′)2 fragment of 22A31 did not induce ADCC in vitro (Fig. 4b), and administration of 22A31 F(ab′)2 fragment consistently did not inhibit the growth of ACC‐MESO‐4 tumor compared with intact 22A31 in vivo (Fig. 4c). Moreover, the antitumor effect of 22A31 was inhibited when NK cells were depleted in mice by anti‐asialo‐GM1 Ab treatment (Fig. 4d). Taken together, these results suggested that 22A31 exerts antitumor effect against C‐ERC/mesothelin‐expressing tumor cells via NK cell‐mediated ADCC in vivo.
Figure 4.
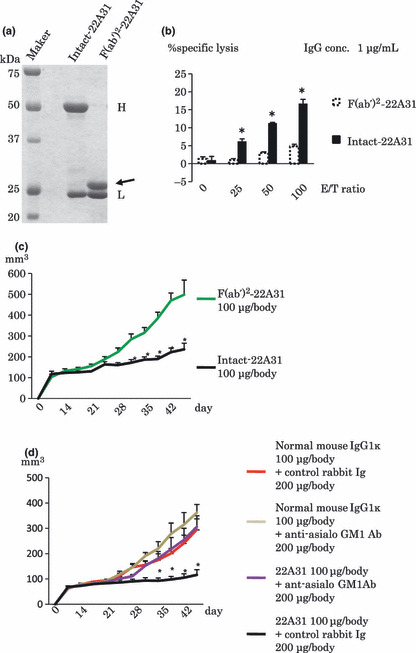
Contribution of the Fc portion and natural killer (NK) cells to the antitumor effect of 22A31 in vivo. (a) Preparation of 22A31 F(ab′)2 fragment. Ten μg of intact‐22A31 or F(ab′)2‐22A31 were electrophoresed using SDS‐PAGE and visualized Coomassie Brilliant Blue (CBB)‐staining. Arrow indicates heavy chain of F(ab′)22‐22A31. H and L indicate heavy chain of intact‐22A31 and light chain of both antibodies. (b) Intact‐ or F(ab′)2‐22A31‐inducd ADCC against ACC‐MESO‐4 tumor cells with purified NK cells. ADCC induced by 1 μg/mL of Intact‐ or F(ab′)2‐22A31 was examined at indicated effector/target ratios when purified NK cells were used as effector cells. (c) Antitumor effect of intact‐ or F(ab′)2‐22A31 against ACC‐MESO‐4. One hundred μg/body of Intact‐22A31 or F(ab′)2‐22A31 was intratumorally injected twice per week. Mice were treated 10 times with the respective antibody. Median tumor volume and SD of xenografts are indicated (n = 5). (d) Antitumor effect of 22A31 in NK cell‐depleted mice. ACC‐MESO‐4 tumor‐bearing mice were treated with 200 μg/body of anti‐asialo GM1 Ab to deplete NK cells or control rabbit Ig, then intratumorally injected with 100 μg/body of intact‐22A31 or control Ig twice per week as described in the Materials and Methods. Median tumor volume and SD of xenografts are indicated (n = 5). Asterisk indicates statistical significance.
Discussion
C‐ERC/mesothelin is expressed in various human cancer cells, particularly in malignant mesothelioma, which has poor prognosis.( 4 , 5 ) C‐ERC/mesothelin is believed to be a possible target for immunotherapy against these cancers; thus we here examined the therapeutic antitumor effect of anti‐C‐ERC/mesothelin monoclonal antibody, 22A31, which we had previously developed.( 35 )
In a xenograft model using BALB/c nu/nu mice, intratumoral administration of 22A31 inhibited the growth of C‐ERC/mesothelin‐expressing ACC‐MESO‐4 tumor cells in a dose‐dependent manner, but not the growth of C‐ERC/mesothelin‐negative Huh‐7 tumor cells. 22A31 induced ADCC against C‐ERC/mesothelin‐expressing ACC‐MESO‐4 cells and NCI‐H226 cells, but not Huh7 tumor cells, and this 22A31‐mediaed ADCC was observed with incubation with purified NK cells, but not purified macrophages. Moreover, the antitumor effect of 22A31 was inhibited when the mice were treated with the F(ab′)2 fragment of 22A31 or NK cells were depleted during the treatment with intact 22A31. Thus, 22A31 effected NK cell‐mediated ADCC which resulted in an antitumor effect against C‐ERC/mesothelin‐expressing human cancers in vivo, indicating the possible utility of 22A31 in clinical therapy.
It was reported that C‐ERC/mesothelin promotes anchorage‐independent growth and prevents anoikis.( 22 ) However, there are no anatomical and historical abnormalities in C‐ERC/mesothelin knockout mice, and physiological and pathogenic functions of C‐ERC/mesothelin have not been fully revealed.( 41 ) We did not observe any inhibitory effect of 22A31 on the proliferation of some C‐ERC/mesothelin‐expressing tumor cells including ACC‐MESO‐4 tumor cells in vitro. Moreover, we did not detect any 22A31‐induced apoptosis or autophagic cell death by caspase‐3/7 activity assay or LC‐3 immunoblotting analysis. These results indicate that 22A31 does not inhibit tumor growth directly. Further studies are needed to clarify the roles of C‐ERC/mesothelin in tumor cells, which will provide critical information for improving the antitumor effect of anti‐C‐ERC/mesothelin mAb.
It is now commonly accepted that tumor‐targeting mAbs, such as anti‐HER2/neu/ErbB‐2 mAb and anti‐epidermal growth factor mAb, demonstrate significant therapeutic effects in cancer patients.( 42 ) Direct antitumor effect by tumor‐targeting mAbs is possibly involved; however, multiple mechanisms, including ADCC and CDC, have been reported to generally contribute to the antitumor effect of tumor‐targeting mAbs.( 43 ) Moreover, it was reported that induction of tumor antigen‐specific cytotoxic T cells (CTL) is critical for successful antibody‐based tumor‐targeting therapy.( 44 , 45 ) Thus, 22A31 treatment possibly induced ACC‐MESO‐4 tumor‐specific CTL during the therapy; however, we could not examine this possibility because we used a xenograft experimental model featuring athymic nude mice. Further basic studies using humanized mice, such as NOD/Shi‐scid/IL‐2 receptor γ null (NOG) mice with humanized 22A31, are needed to reveal the complete mechanisms of the antitumor effect of 22A31 in vivo. ( 46 )
C‐ERC/mesothelin is expressed not only in mesotheliomas, but also in pancreatic ductal carcinomas, ovarian cancers, and some other cancers.( 8 , 23 , 24 , 25 , 26 ) Pancreatic ductal carcinoma is also a particularly devastating disease because of its poor prognosis. The overall 5‐year survival rate of these patients is under 10%.( 47 , 48 ) Early diagnosis of ovarian cancers is so difficult that the 5‐year survival is only 35–40%.( 49 ) Novel therapic strategies against C‐ERC/mesothelin‐expressing tumors, including mesothelioma, pancreatic cancer, and ovarian cancer, are warranted. The results presented here suggest the possible utility of 22A31 to treat cancers; however, intraperitoneal administration of 22A31 did not significantly exert antitumor effect, possibly due to insufficient intratumor concentration of 22A31. Thus, further studies are needed to advance drug delivery within the tumor mass to augment the therapeutic effect of 22A31. We are now going to humanize 22A31 with the hope that this may improve the prognosis of patients with C‐ERC/mesothelin‐expressing tumors.
Acknowledgments
We would like to thank Masumi Maruo, Hidehiro Okura, Sun Guo Dong, Wang Lu, Piao Xiang Hua, Kazu Shiomi, Danqing Zhang, Shuji Matsuoka, Toshiyuki Kobayashi, and members of the Department of Gastroenterology, Juntendo Hospital, for their help in the preparation of this study. This work was supported by a Grant‐in‐Aid for Cancer Research and Grants‐in‐Aid for Scientific Research from the Ministry of Education, Culture, Sports and Science and Technology of Japan and the Ministry of Health, Labor and Welfare of Japan. This study was partially supported by a consignment expense for the molecular imaging program on ‘Research Base for PET Diagnosis’ from the Ministry of Education, Culture, Sport, and Science and Technology, Government of Japan.
References
- 1. Britton M. The epidemiology of mesothelioma. Semin Surg Oncol 2002; 29: 18–25. [DOI] [PubMed] [Google Scholar]
- 2. Connelly RR, Spirtas R, Myers MH, Percy CL, Fraumeni JF Jr. Demographic patterns for mesothelioma in the United States. J Natl Cancer Inst 1987; 78: 1053–60. [PubMed] [Google Scholar]
- 3. Ismaril‐Khan R, Robinson LA, Williams CC Jr, Garrett CR, Bepler G, Simon GR. Malignant pleural mesothelioma, a comprehensive review. Cancer Control 2006; 13: 255–63. [DOI] [PubMed] [Google Scholar]
- 4. Pass H. Malignant pleural mesothelioma, surgical roles and novel therapies. Clin Lung Cancer 2001; 3: 102–17. [DOI] [PubMed] [Google Scholar]
- 5. Kanazawa N, Ioka A, Tsukuma H, Ajiki W, Oshima A. Incidence and survival of mesothelioma in Osaka, Japan. Jpn J Clin Oncol 2006; 36: 254–7. [DOI] [PubMed] [Google Scholar]
- 6. Hino O, Kobayashi E, Nishizawa M et al. Renal carcinogenesis in the Eker rat. J Cancer Res Clin Oncol 1995; 121: 602–5. [DOI] [PubMed] [Google Scholar]
- 7. Yamashita Y, Yokoyama M, Kobayashi E, Takai S, Hino O. Mapping and determination of the cDNA sequence of the Erc gene preferentially expressed in renal cell carcinoma in the Tsc2 gene mutant (Eker) rat model. Biochem Biophys Res Commun 2000; 275: 134–40. [DOI] [PubMed] [Google Scholar]
- 8. Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA 1996; 93: 136–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raffit H, Tapan B, Ira P. Mesothelin: a new target for immunotherapy. Clin Cancer Res 2004; 10: 3937–42. [DOI] [PubMed] [Google Scholar]
- 10. Yamaguchi N, Hattori K, Oh‐eda M, Kojima T, Imai N, Ochi N. A novel cytokine exhibiting megakaryocyte potentiating activity from a human pancreatic tumor cell line HPC‐Y5. J Biol Chem 1994; 269: 805–8. [PubMed] [Google Scholar]
- 11. Kojima T, Oh‐eda M, Hattori K et al. Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J Biol Chem 1995; 270: 21984–90. [DOI] [PubMed] [Google Scholar]
- 12. Maeda M, Hino O. Molecular tumor marker for asbestos‐related mesothelioma: serum diagnostic markers. Pathol Int 2006; 56: 649–54. [DOI] [PubMed] [Google Scholar]
- 13. Maeda M, Hino O. Blood test for asbestos‐related mesothelioma. Oncology 2006; 71: 26–31. [DOI] [PubMed] [Google Scholar]
- 14. Shiomi K, Miyamoto H, Segawa T et al. Novel ELISA system for detection of N‐ERC/mesothelin in the sera of mesothelioma patients. Cancer Sci 2006; 97: 928–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shiomi K, Hagiwara Y, Sonoue K et al. Sensitive and specific new enzyme‐linked immunosorbent assay for N‐ERC/meosthelin increases its potential as a useful serum tumor marker for mesothelioma. Clin Cancer Res 2008; 14: 1431–7. [DOI] [PubMed] [Google Scholar]
- 16. Hino O, Shiomi K. Diagnostic biomarker of asbestos‐related mesothelioma: example of translational research. Cancer Sci 2007; 98: 1147–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scholler N, Fu N, Yang Y et al. Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma. Proc Natl Acad Sci USA 1999; 96: 11531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Onda M, Nagata S, Ho M et al. Megakaryocyte potentiation factor cleaved from mesothelin precursor is a useful tumor marker in the serum of patients with mesothelioma. Clin Cancer Res 2006; 12: 4225–31. [DOI] [PubMed] [Google Scholar]
- 19. Hassan R, Remaley AT, Sampson ML et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 2006; 12: 447–53. [DOI] [PubMed] [Google Scholar]
- 20. Robinson BW, Creaney J, Lake R et al. Mesothelin‐family proteins and diagnosis of mesothelioma. Lancet 2003; 15: 1612–6. [DOI] [PubMed] [Google Scholar]
- 21. Robinson BW, Creaney J, Lake R et al. Soluble mesothelin‐related protein – a blood test for mesothelioma. Lung Cancer 2005; 49: S109–11. [DOI] [PubMed] [Google Scholar]
- 22. Uehara N, Matshoka Y, Tsubura A. Mesothelin promotes anchorage‐independent growth and prevents anoikis via extracellular signal‐regulated kinase signaling pathway in human breast cancer cells. Mol Cancer Res 2008; 6: 186–93. [DOI] [PubMed] [Google Scholar]
- 23. Argani P, Iacobuzio‐Donahue C, Ryu B et al. Mesothelin is overexpressed in the vast majority of ductal adenocarinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res 2001; 7: 3862–8. [PubMed] [Google Scholar]
- 24. Baruch AC, Wang H, Staerkel GA, Evans DB, Hwang RF, Krishnamurthy S. Immunocytochemical study of the expression of mesothelin in fine‐needle aspiration biopsy specimens of pancreatic adenocarcinoma. Diagn Cytopathol 2007; 35: 143–7. [DOI] [PubMed] [Google Scholar]
- 25. Yaziji H, Battifora H, Barry TS et al. Evaluation of 12 antibodies for distinguishing epithelioid mesothelioma from adenocarcinoma: identification of a three‐antibody immunohistochemical panel with maximal sensitivity and specificity. Mod Pathol 2006; 19: 514–23. [DOI] [PubMed] [Google Scholar]
- 26. Inami K, Kajino K, Abe M et al. Secretion of N‐ERC/mesothelin and expression of C‐ERC/mesothelin in human pancreatic ductal carcinoma. Oncol Rep 2008; 20: 1375–80. [PubMed] [Google Scholar]
- 27. Li M, Bharadwaj U, Zhang R et al. Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Mol Cancer Ther 2008; 7: 286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hung CF, Tsai YC, He L, Wu TC. Control of mesothelin‐expressing ovarian cancer using adoptive transfer of mesothelin peptide‐specific CD8 + T cells. Gene Ther 2007; 14: 921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hassan R, Btoaddus VC, Wilson S, Liewehr DJ, Zhang J. Anti‐mesothelin immunotoxin SS1P in combination with gemcitabine results in increased activity against mesothelin‐expressing tumor xenografts. Clin Cancer Res 2007; 13: 7166–71. [DOI] [PubMed] [Google Scholar]
- 30. Hassan R, Ebel W, Routhier EL et al. Preclinical evaluation of MORAb‐009, a chimeric antibody targeting tumor‐associated mesothelin. Cancer Immun 2007; 7: 20. [PMC free article] [PubMed] [Google Scholar]
- 31. Hassan R, Bullock S, Premkumar A et al. Phase I study of SS1P, a recombinant anti‐mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin‐expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res 2007; 13: 5144–9. [DOI] [PubMed] [Google Scholar]
- 32. Hassan R, Williams‐Gould J, Steinberg SM et al. Tumor‐directed radiation and the immunotoxin SS1P in the treatment of mesothelin‐expressing tumor xenografts. Clin Cancer Res 2006; 12: 4983–8. [DOI] [PubMed] [Google Scholar]
- 33. Sato N, Hassan R, Axworthy DB et al. Pretargeted radioimmunotherapy of mesothelin‐expressing cancer using a tetravalent single‐chain Fv‐streptavidin fusion protein. J Nucl Med 2005; 46: 1201–9. [PubMed] [Google Scholar]
- 34. Thomas AM, Santarsiero LM, Lutz ER et al. Mesothelin‐specific CD8 (+) T cell responses provide evidence of in vivo cross‐priming by antigen‐presenting cells in vaccinated pancreatic cancer patients. J Exp Med 2004; 200: 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishikawa K, Segawa T, Hagiwara Y, Maeda M, Abe M, Hino O. Establishment of novel monoclonal antibody to human ERC/mesothelin useful for study and diagnosis of ERC/mesothelin‐expressing cancers. Pathol Int 2009; 59: 161–6. [DOI] [PubMed] [Google Scholar]
- 36. Takeda K, Hayakawa Y, Smyth MJ et al. Involvement of tumor necrosis factor‐related apoptosis‐inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med 2001; 7: 94–100. [DOI] [PubMed] [Google Scholar]
- 37. Usami N, Fukui T, Kondo M et al. Establishment and characterization of four malignant pleural mesothelioma cell lines from Japanese patients. Cancer Sci 2006; 97: 387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson DS, Wu J, Peluso P, Nock S. Improved method for pepsinolysis of mouse IgG(1) molecules to F(ab′)2(2) fragments. J Immunol Methods 2002; 260: 29–36. [DOI] [PubMed] [Google Scholar]
- 39. Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lpr mice correlates with the disappearance of natural killer type 1‐positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med 1993; 177: 155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rouard H, Tamasdan S, Moncuit J et al. Fc receptors as targets for immunotherapy. Int Rev Immunol 1997; 16: 147–85. [DOI] [PubMed] [Google Scholar]
- 41. Bera TK, Pastan I. Mesothelin is not required for normal mouse development or reproduction. Mol Cell Biol 2000; 20: 2902–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pegram MD, Lipton A, Hayes DF et al. Phase II study of receptor‐enhanced chemosensitivity using recombinant humanized anti‐p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu‐overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 1998; 16: 2659–71. [DOI] [PubMed] [Google Scholar]
- 43. Takeda K, Okumura K, Smyth MJ. Combination antibody‐based cancer immunotherapy. Cancer Sci 2007; 98: 1297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. zum Büschenfelde CM, Hermann C, Schmidt B, Peschel C, Bernhard H. Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I‐restricted HER2‐specific T lymphocytes against HER2‐overexpressing tumor cells. Cancer Res 2002; 62: 2244–7. [PubMed] [Google Scholar]
- 45. Dhodapkar KM, Krasovsky J, Williamson B, Dhodapkar MV. Antitumor monoclonal antibodies enhance cross‐presentation of cellular antigens and the generation of myeloma‐specific killer T cells by dendritic cells. J Exp Med 2002; 195: 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ito M, Kobayashi K, Nakahata T. NOD/Shi‐scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr Top Microbiol Immunol 2008; 324: 53–76. [DOI] [PubMed] [Google Scholar]
- 47. Wray CJ, Ahmad SA, Matthews JB, Lowy AM. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology 2005; 128: 1626–41. [DOI] [PubMed] [Google Scholar]
- 48. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004; 363: 1049–57. [DOI] [PubMed] [Google Scholar]
- 49. Singh AP, Senapati S, Ponnusamy MP et al. Clinical potential of mucins in diagnosis, prognosis, and therapy of ovarian cancer. Lancet Oncol 2008; 9: 1076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]


