Abstract
Attenuated salmonella have been reported to be capable of both selectively growing in tumors and expressing exogenous genes for tumor‐targeted therapy. As 6‐methoxypurine 2′‐deoxyriboside (MoPdR) is similar to 6‐methylpurine 2′‐deoxyriboside in structure, we aimed to evaluate the antitumoral effect of the Escherichia coli purine nucleoside phosphorylase (ePNP) gene, using an attenuated salmonella‐mediated delivery system, in combination with MoPdR. A novel mutant serovar Typhimurium (SC36) was used to carry the pEGFP‐C1‐ePNP vector that contains an enhanced green fluorescent protein and an ePNP gene under the control of the cytomegalovirus promoter. The function of the ePNP expression vector was confirmed in vitro using the enzymic conversion of MoPdR into methoxypurine. We also observed a high bystander effect induced by the ePNP/MoPdR system with a very low proportion (1%) of ePNP‐positive cells and 5 µg/mL MoPdR, although the growth of parental cells was affected appreciably by MoPdR. The killing effect and increased apoptosis induced by SC36 carrying the ePNP expression vector (SC/ePNP) were detected by cytotoxicity assay and propidium iodide staining flow cytometry analysis, in combination with MoPdR. SC/ePNP was given orally to mice bearing mammary carcinomas, and its antitumor effect was evaluated. SC/ePNP plus MoPdR significantly inhibited tumor growth by approximately 86.6–88.7% and prolonged the survival of tumor‐hosting mice. Our data support the view that MoPdR combined with the ePNP gene could be used in gene‐directed enzyme prodrug therapy. Attenuated salmonella could be a promising strategy to improve ePNP/MoPdR bystander killing due to its preferential accumulation and anticancer activity in tumors. (Cancer Sci 2008; 99: 1172–1179)
A hypoxic microenvironment is characteristic of many solid tumors. Current limitations of gene therapies for malignant tumors include lack of cancer‐specific targeting strategy and insufficient tumor delivery.( 1 ) To resolve these problems, Salmonella enterica serovar typhimurium (S. typhimurium) strains, which are facultative anaerobes, have been considered intriguing candidates because of their selective growth in tumors and essential ability to deliver exogenous genes encoding therapeutic proteins.( 2 , 3 ) To increase their applicability for treatments, the S. typhimurium strains were first attenuated by purine and other auxotrophic mutations to improve tumor‐specific targeting as well as to reduce toxicity.( 2 , 3 , 4 , 5 , 6 , 7 ) Then genetic modification of the salmonella lipid A was carried out to reduce septic shock and the lipid A‐attenuated S. typhimurium has been evaluated in a phase I clinical trial.( 4 , 8 ) Recently, Zhao et al.( 9 , 10 , 11 ) developed S. typhimurium strains A1 and A1‐R and detected real‐time imaging of S. typhimurium A1‐R‐induced exogenous gene expression in vitro using fluorescence microscopy. Experience from clinical trials of cancer gene therapy indicates that no single therapeutic strategy can effectively eradicate a fairly large tumor, whereas combined gene therapy represents a more reliable approach to combat tumor growth.( 12 , 13 ) The idea of combining salmonella‐mediated gene therapy with stimulation of the host antitumor immune response drove studies of cancer gene therapy. Therefore these tumor‐targeting bacteria have been used to deliver genes encoding angiogenic inhibitors,( 14 , 15 , 16 ) prodrug‐converting enzymes,( 17 ) or cytokines( 18 , 19 ) aiming to enhance their oncolytic effects.
Of several gene‐directed enzyme prodrug therapy systems, we were interested in the system described by Sorscher et al.( 20 ) It has been reported that the Escherichia coli purine nucleoside phosphorylase (ePNP) gene could convert 6‐methylpurine 2¢‐deoxyriboside (MePdR) into a toxic substance named 6‐methylpurine. As prokaryotic PNP enzymes differ fundamentally in sequence, structure, and function from their eukaryotic counterparts, MePdR should be a poor substrate for mammalian PNP.( 21 , 22 , 23 , 24 , 25 ) Therefore, expression of ePNP is able to kill a number of cancer cells in vitro when a small fraction of cells (e.g. 0.1–3%) express this suicide gene, in combination with MePdR treatment.( 20 , 26 , 27 , 28 , 29 , 30 ) Expression of suicide genes will not be achieved in all the tumor cells, so a bystander effect is necessary to produce toxic metabolites to kill not only the positive cells but also the bystander cells.( 31 , 32 , 33 ) The ePNP/MePdR system differs from other gene‐directed enzyme prodrug therapy systems because the toxic metabolites of this system will readily cross the cell membrane and not require direct cell‐to‐cell contact or the presence of a gap junction.( 34 ) In previous papers, the ePNP/MePdR system has been reported to be an efficient suicide gene/prodrug system with significant antitumor activities on ovarian cancers,( 29 ) gliomas,( 35 ) prostate cancers,( 36 ) melanomas,( 37 ) pancreatic cancers,( 38 , 39 ) hepatomas,( 40 , 41 ) and bladder tumors.( 42 )
Love and Remy( 43 ) reported that metabolism of various methylated purines, such as 6‐methoxypurine, used identical metabolic pathways to 6‐methylpurine. When some cells, such as mammalian cells or purine‐requiring mutants of bacteria, could not use methylated purines, they come to a low growth rate, reduced cell yield, and derepression of purine synthesis. As 6‐methoxypurine 2¢‐deoxyriboside (MoPdR) is similar to MePdR in structure, we wondered whether MoPdR could substitute MePdR and use the identical principle of the ePNP/MePdR strategy to inhibit tumor growth. To fully explore the potentially antitumoral effect of the salmonella‐mediated ePNP gene combined with MoPdR, we constructed a recombinant plasmid expressing the ePNP gene (pEGFP‐C1‐ePNP), which contained an enhanced green fluorescent protein (EGFP) under the control of the cytomegalovirus promoter, and transformed it into a live attenuated purine–auxotrophic strain of S. typhimurium (SC36). Then the function of the ePNP expression vector was confirmed in vitro using the enzymic conversion of MoPdR into methoxypurine. Furthermore, the bystander effect and the apoptosis efficiency of the ePNP/MoPdR system were assessed in vitro by 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide (MTT) assay and flow cytometry analysis. The in vivo antitumor activities were also addressed in terms of tumor growth and survival rate in mice with mammary carcinoma.
Materials and Methods
Plasmids, bacterial strains, and media. Full‐length cDNA of purine nucleoside phosphorylase (EC2.4.2.1, PNPase) gene (DeoD) was amplified using polymerase chain reaction (PCR) from E. coli DH5α genomic DNA. Genomic DNA preparation was carried out as described.( 44 ) Specific primers for PCR were designed according to the published sequences: sense primer 5′‐GGGAATTC GATGGCTACCCCACACATTAA‐3′; and antisense primer 5′‐AT GTCGAC TTACTCTTTATCGCCCAGCAG‐3′. The EcoRI and SalI restriction sites (underlined and italicized) were added to facilitate the PCR product (approximately 720 bp) and inserted into the eukaryotic expression vector pEGFP‐C1 (Clontech) by standard homologous recombination techniques. pEGFP‐C1 encodes a red‐shifted variant of wild‐type GFP that has been optimized for brighter fluorescence and higher expression in mammalian cells. The plasmid constructions were confirmed by DNA sequencing. The auxotrophic S. typhimurium LB5000 (LT2Trp Met Erpsl flaA R–M+) and SL3261 (WARY hisG46 aroA del 407 Fusaricres, R+M+) were provided by Professor Bruce A.D. Stocker (Stanford University School of Medicine, Stanford, CA). A novel purine–auxotrophic mutant SC36 (his G aro A cys pur I) with a low residual virulence was gained by diethyl sulfate mutagenesis of SL3261, as previously described.( 45 ) Bacterial strains were routinely grown at 37°C in Luria broth (LB) or agar supplemented with kanamycin (50 µg/mL). pEGFP‐C1 and pEGFP‐C1‐ePNP were first transformed into LB5000 using electrotransformation in cuvettes (0.2 cm electrode gap; Eppendorf) with a single pulse of 12.5 kV/cm (2.5 kV, 200 Ω, 25 µF). After amplification of the colony, the plasmids were extracted from the transferred LB5000 (3S Spin Plasmid Miniprep Kit V3.1; Shanghai Shenergy Biocolors Bioscience & Technology Co. Ltd.) then introduced into SC36 (as carried out for LB5000) to generate SC/pEGFP and SC/ePNP.
Cells, cell culture, and bacterial infection. Murine mammary carcinoma 4T1 cell lines were obtained from Shanghai No.1 People's Branch Hospital (China). The cells (105 cells/well) were cultured in 12‐well plates overnight in antibiotic‐free RPMI‐1640 media (Gibco BRL) in a 5% CO2 atmosphere at 37°C. Bacteria (SC/pEGFP or SC/ePNP) were shaken in LB broth with kanamycin (50 µg/mL) overnight at 37°C to reach the late logarithmic phase of growth (OD600 of approximately 4) and suspended in antibiotic‐free media. Then 2.5 × 107 c.f.u. of bacteria (at a multiplicity of infection of 1:250) were directly added into 12‐well plates and co‐incubated with tumor cells for 3 h. Subsequently, the cells were washed three times with phosphate‐buffered saline (PBS) and replenished with complete media (containing 100 U/mL penicillin and 100 µg/mL streptomycin) and further cultured for 1 or 2 days. The infected cells were collected and some of them were used to monitor the gene expression by reverse transcription (RT)‐PCR (AccessQuick; Promega). Some of the infected cells (104 cells/well) in 96‐well plates, cultured with complete media (containing 100 U/mL penicillin and 100 µg/mL streptomycin) and MoPdR (previously synthesized using the same principle)( 46 ) were used for cytotoxicity assay( 47 ) and propidium iodide staining flow cytometry analysis.( 48 )
Western blot analysis for caspase‐3. Total proteins of the infected cells were extracted (TRIzol reagent; Invitrogen) and their concentration was determined by Bio‐Rad protein assay buffer. Total proteins were separated on 8–12% polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Amersham, Piscataway, NJ). The membranes were blocked for 1 h in 5% non‐fat dry milk (Shanghai Bright Dairy and Food) in PBS then incubated with primary antibodies, detected by the appropriate secondary antibodies, and revealed with an enhanced chemiluminescence system (Amersham Life Sciences). The primary antibodies were anti‐caspase‐3 (Santa Cruz Biotechnology) and anti‐actin (Sigma).
Stable positive cell lines and functional tests. The cell lines were transfected with pEGFP‐C1 or pEGFP‐C1‐ePNP (Lipofectamine 2000; Invitrogen) and selected with G418 at a concentration of 400 µg/mL. Fresh media with G418 were replaced every 3 days. Fifteen days after the cell culture, the cell clones with G418 resistance (named 4T1/ePNP and 4T1/pEGFP) were obtained. Resistant clones were selected and their ePNP expression was monitored by RT‐PCR. The positive clones highly expressing ePNP were selected for observing the bystander effect by MTT assay( 49 ) and analyzing enzymic conversion by a high‐performance liquid chromatography (HPLC)‐based assay.( 20 )
Bacterial distribution in vivo after oral inoculation. Four‐week‐old female BALB/c mice were obtained from BK Company (Shanghai, China). To eliminate enteric flora, all mice were fed with sterilizing water containing neomycin (5 g/L), streptomycin (5 g/L), and penicillin (5 U/µL). After the first 2 weeks of antibiotic therapy, tumor xenografts were established by subcutaneous inoculation of tumor cells (5 × 106 cells) into the right flanks of mice. When the tumors had reached a mean volume of approximately 80 mm3, mice were fed with 1 × 109 c.f.u. of SC/ePNP. At different time points, three mice in each group were killed and their tumors, livers, spleens, kidneys, and gastrointestinal tracts were excised, weighed, and homogenized in 2 mL of ice‐cold, sterile PBS. The bacteria were quantified by plating serial dilutions of the homogenates onto LB agar plates containing kanamycin (50 µg/mL). The plates were incubated overnight at 37°C and the bacterial colonies were counted.
Assessment of ePNP expression in vivo. The ePNP mRNAs in the tissues of SC/ePNP‐treated mice were determined by RT‐PCR. At different time points, total cellular RNA was isolated from the tissues (TRIzol reagent; Invitrogen) and reverse‐transcribed into cDNA (AccessQuick; Promega). The cDNA was amplified by PCR to determine β‐actin and ePNP mRNA expression. The primer design was based on the published sequences: sense primer 5′‐ACCCACACTGTGGCCCATCTA‐3′ and antisense primer 5′‐CGGAACCGCTCATTGCC‐3′ for β‐actin, and sense primer 5′‐GGGAATTCGATGGCTACCCCACACATTAA‐3′ and antisense primer 5′‐ATGTCGACTTACTCTTTATCGCCCAGCAG‐3′ for ePNP for PCR amplification of 289‐bp and 720‐bp fragments, respectively. To assess the functionality of ePNP/MoPdR in the tissues, an HPLC‐based assay was developed measuring the catabolism of the prodrug MoPdR to methoxypurine. Seven days after oral inoculation, homogenates of tissues (Liquid N2) were generated and lysis was completed by three cycles of freezing and thawing. Cell debris was removed by centrifugation (15 000g × 10 min) followed by determination of the protein content of the supernatants (Bradford protein assay kit; Sangon). Then 100 µL (0.1 mg/sample) of the supernatant was incubated with 900 µL of 5 µg/mL MoPdR at 37°C for 24 h and the enzymic conversion analyzed by HPLC.( 20 )
Analysis of anti‐tumor effect in vivo. To eliminate enteric flora, all mice were fed with sterilizing water containing neomycin (5 g/L), streptomycin (5 g/L), and penicillin (5 U/µL). After the first 2 weeks of antibiotic therapy, the animals were inoculated subcutaneously with 5 × 106 murine tumor cells. When the tumors reached a mean volume of approximately 80 mm3, mice were randomly grouped (n = 5 animals per group) and fed with sterilizing water without antibiotics. All SC/ePNP‐treated mice received 1 × 109 c.f.u. of SC/ePNP and control mice received SC/pEGFP or buffer only. For bacteria colonization, all salmonella‐treated mice were fed with sterilizing water containing kanamycin (800 mg/L) the day before or during the study. Subsequently, intraperitoneal injections of MoPdR (10 mg/kg body weight) were started 7 days after bacterial inoculation, when the mean volume of tumors was over 200 mm3. The MoPdR treatments were used daily, four times. All blank mice received 0.9% normal saline solution. Tumor diameters were measured at regular intervals with calipers, and the tumor volume in mm3 was calculated by the formula: volume = 1/2 × length × width2. Survival rates were monitored after bacterial infection. Mice bearing mammary carcinoma 4T1 were killed when tumors reached 4000 mm3 or beforehand if they showed signs of distress. These time points were defined as survival time.
Statistical analysis. Statistical analysis of the data was carried out using the Student–Newman–Keuls’ test and Primer statistical software (spss Base 10.0 for Windows; SPSS, Chicago, IL). Results were considered statistically significant at P < 0.05.
Results
Vector functional tests in vitro. The function of the ePNP expression vector (pEGFP‐C1‐ePNP) was confirmed by HPLC after SC/ePNP infection in vitro, and the ratio of MoPdR conversion in the supernatants of SC/ePNP‐infected cells was 95% after 96 h (Fig. 1). Moreover, SC/ePNP‐infected cells showed a strong sensitivity to MoPdR (Fig. 2), indicating that plasmid pEGFP‐C1‐ePNP construction was functional.
Figure 1.
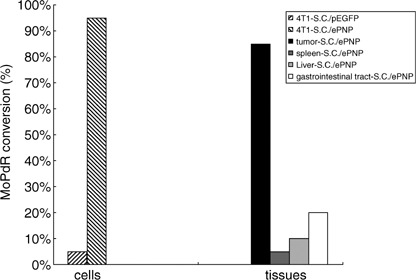
Enzymic conversion. Murine mammary carcinoma 4T1 cells (104 cells/well) were cultured 96‐well plates in media with 5 µg/mL 6‐methoxypurine 2′‐deoxyriboside (MoPdR) for 96 h. The supernatants (0.1 mg/100 µL) of the tissues were incubated with 900 µL of 5 µg/mL MoPdR at 37°C for 24 h. Subsequently, lysates from the cells and tissues were analyzed for the concentrations of MoPdR in the supernatants by high‐performance liquid chromatography at a wavelength of 254 nm. 4T1‐SC/ePNP, right diagonal; 4T1‐SC/pEGFP, left diagonal; gastrointestinal tract‐SC/ePNP, white; liver‐SC/ePNP, hoar; spleen‐SC/ePNP, dark grey; tumor‐SC/ePNP, black.
Figure 2.
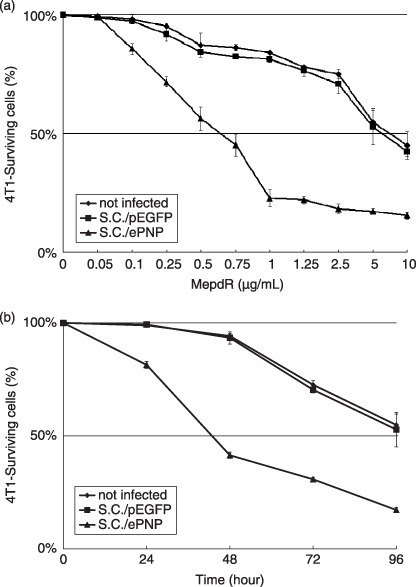
Sensitivity of tumor cells to treatment with the Escherichia coli purine nucleoside phosphorylase gene and 6‐methoxypurine 2′‐deoxyriboside (ePNP/MoPdR). (a) The infected murine mammary carcinoma 4T1 cells were treated with MoPdR concentrations ranging from 0.05 to 10 µg/mL. The absorbance of each well at a wavelength of 570 nm was measured using an enzyme‐linked immunosorbent assay reader (Bio‐Rad) 96 h later. (b) At the concentration of 5 µg/mL MoPdR, cell survival was quantified at indicated time points by 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide assay. Data are representative of at least three separate experiments; each point represents the mean ± SE and is expressed as a percentage relative to the same cells non‐treated. SC/ePNP, triangle; SC/pEGFP, square.
Effects of SC/ePNP increased spontaneous apoptosis of cells. Incubated with different concentrations of MoPdR (ranging from 0 to 10 µg/mL), parental or SC/pEGFP‐infected cells were resistant to MePdR, with an IC50 (kill 50%) higher than 5 µg/mL. In contrast, SC/ePNP‐infected cells were susceptible to MoPdR, with an IC50 lower than 0.75 µg/mL (Fig. 2a). Moreover, the sensitivity of the infected cells to MoPdR was time‐dependent at the concentration of 5 µg/mL MoPdR. The prodrug treatments led to approximately 19% of cell death after 24 h and 83% of cell death after 96 h (Fig. 2b). The apoptosis results showed that SC/ePNP treatment increased the spontaneous apoptosis of the infected cells compared to the control cells at different concentrations of MoPdR (ranging from 0 to 0.75 µg/mL). The greatest increase in the number of apoptosis cells was at the final concentration of 0.75 µg/mL MoPdR, and the ratio was 19.45% (Fig. 3a). As indicated in Fig. 3b, treatment with SC/ePNP and 5 µg/mL MoPdR led significant expression of caspase‐3 to be time‐dependent in 4T1 tumor cell lines, beginning at 24 h and peaking at 48 h after infection, compared with that of SC/pEGFP‐infected cells.
Figure 3.
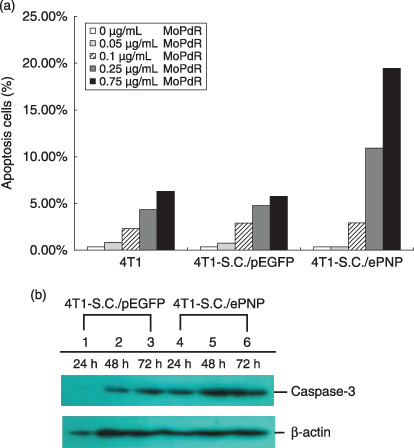
Assessment of apoptosis in 4T1 murine mammary tumor cells. (a) Enhancement of apoptosis. After incubation with 6‐methoxypurine 2′‐deoxyriboside (MoPdR) at different concentrations (0–0.75 µg/mL) for 96 h, harvested cells were fixed overnight with precooled ethanol and treated with 50 µg/mL propidium iodide (Sigma Chemical) and 10 µg/mL RNaseA for 30 min at 37°C. A minimum of 1.5 × 104 events were analyzed on a fluorescence‐activated cell sorting flow cytometer with an argon laser tuned at 488 nm and use of CellQuest software (Becton Dickinson, Rutherford, NJ). Results are expressed as the percentage of apoptotic cells after S. typhimurium carrying pEGFP‐c1 (SC/pEGFP) and S. typhimurium carrying pEGFP‐c1‐ePNP (SC/ePNP) treatment with respect to the same cells non‐treated, in combination with MoPdR. (b) Western blot analysis. The infected cells were treated with 5 µg/mL MoPdR and total cellular proteins were isolated at indicated time points and subjected to Western blot analysis using anti‐caspase and anti‐β‐actin antibody.
Bystander effect. In the absence of ePNP‐positive cells, the growth of parental cells was affected slightly even when the MoPdR concentration reached 5 µg/mL. At the concentration of 5 µg/mL MoPdR, the significant bystander effect could be detected even with a very low proportion (1%) of ePNP‐positive cells. Furthermore, in the conditions of 1 µg/mL MoPdR and 5% ePNP‐positive cells, more than 50% of cells were killed. These results indicated that a high bystander effect was achieved (Fig. 4).
Figure 4.
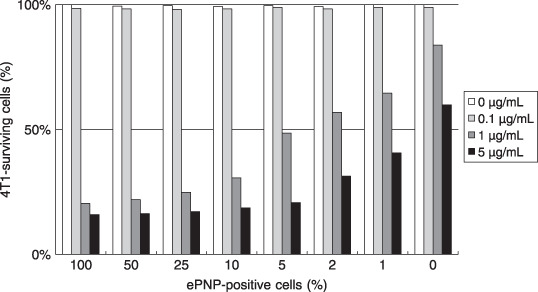
Bystander effect. To investigate the bystander effect in vitro, Escherichia coli purine nucleoside phosphorylase (ePNP)‐positive cells and parental cells were mixed in different proportions (0:100; 1:99; 2:98; 5:95; 10:90; 25:75; 50:50; and 100:0). Twenty‐four hours later, all cells were cultured in RPMI‐1640 media containing 6‐methoxypurine 2′‐deoxyriboside at different concentrations (0–5 µg/mL) for 3 days. The percentage of surviving cells was calculated to evaluate the survival ratios of 4T1 mammary carcinoma cells by 3‐[4,5‐dimethylthiazol‐2‐yl]‐2,5‐diphenyltetrazolium bromide assay. Data are representative of at least three separate experiments. Survival ratios are expressed as percentages relative to the corresponding mixture of untreated control cells.
Bacterial distribution and gene expression in tissues. The kinetics of bacterial distribution after oral inoculation was detected to be in a time‐dependent pattern. Significant accumulation of SC/ePNP in the tumors could be retained for at least 12 days, with a peak level observed at day 7, and the tumor‐to‐normal tissue ratios were as high as 10 000:1. SC/ePNP entirely disappeared from liver and spleen 16 days after oral treatment (Fig. 5a). As shown in Fig. 5b, expression of the ePNP gene was detected in the tumors at day 7 and day 12. In contrast, low expression of the ePNP gene in other tissues could be detected at day 7, but not at day 12. Futhermore, an HPLC‐based assay evaluated the functionality of ePNP/MoPdR in the tissues and showed that higher conversion of MoPdR was detected in tumors than in other tissues (Fig. 1). Taken together, these results showed that SC/ePNP, when given to mice bearing established tumors, was preferentially accumulated and retained in large amounts in tumors for at least 12 days.
Figure 5.
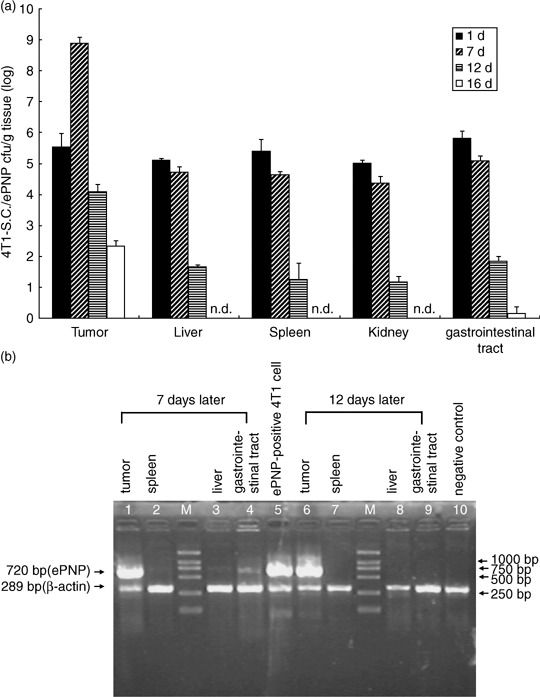
Bacterial distribution in murine tissues. (a) Preferential accumulation of salmonella in the tumors of mice given S. typhimurium carrying pEGFP‐c1‐ePNP (SC/ePNP). Mice bearing mammary carcinoma 4T1 cells were given 1 × 109 c.f.u. of SC/ePNP, orally. The amounts of SC/ePNP accumulated in tumors, livers, spleens, kidneys, and gastrointestinal tracts were determined at 1, 7, 12, and 16 days post‐infection. Each value represents the mean ± SD from three mice. n.d., not detected. (b) Reverse transcription–polymerase chain reaction (RT‐PCR) analysis for gene expression. At different time points after bacterial inoculation (7 and 12 days), the tissues of mice bearing mammary carcinoma 4T1 tumors were tested for ePNP expression by RT‐PCR. M, molecular marker profile.
Antitumoral effect of the ePNP gene combined with MoPdR. We found the intraperitoneal injections of MoPdR were no more than 10 mg/kg body weight/day, otherwise an excessive dose of MoPdR led to significant weight loss of mice. Therefore, this dose was used for all MoPdR treatment experiments in vivo. As shown in Fig. 6a, tumor growth of mice bearing 4T1 mammary carcinoma was significantly retarded by the salmonella‐mediated ePNP/MoPdR system. There was no statistical difference between the four groups (PBS‐treated, MoPdR‐treated, SC/pEGFP‐treated, and SC/ePNP‐treated groups) at any time point during the experiments. The mean tumor volume of mice treated with SC/ePNP plus MoPdR was lowered by 86.6%, 87.2%, 87.6%, and 88.7%, compared with that of mice treated with SC/ePNP (P < 0.001), SC/pEGFP (P < 0.001), MoPdR (P < 0.001), and PBS (P < 0.001), respectively. As shown in Fig. 6b. SC/ePNP plus MoPdR also prolonged the survival of mice with mammary carcinoma as compared with SC/ePNP‐treated (P = 0.0034), SC/pEGFP‐treated (P = 0.0026), MoPdR‐treated (P = 0.0017), and PBS‐treated (P = 0.0015) counterparts. In conclusion, the treatment of the ePNP gene and appropriate dose of MoPdR could significantly suppress tumor growth with the initial mean tumor volume >200 mm3 when the intraperitoneal injections of MoPdR were no more than 10 mg/kg body weight/day.
Figure 6.
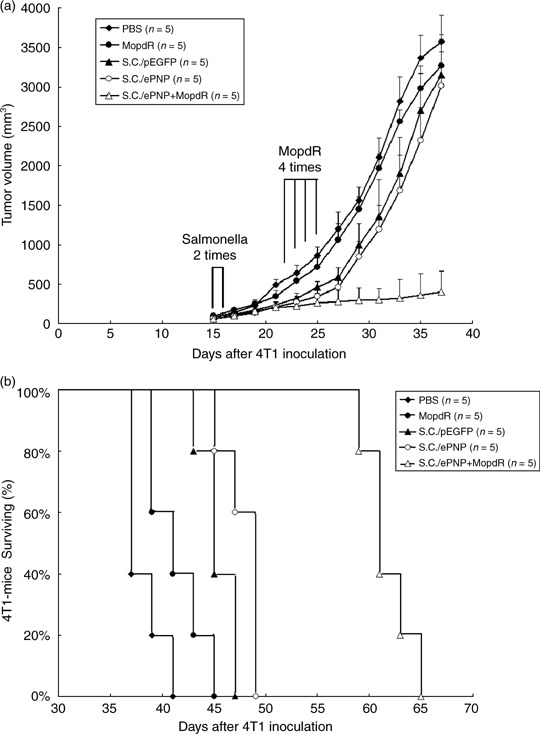
Antitumor effects of the Escherichia coli purine nucleoside phosphorylase (ePNP) gene combined with 6‐methoxypurine 2′‐deoxyriboside MoPdR in mice bearing murine mammary carcinoma 4T1 tumors. (a) When tumors had reached the mean volume of approximately 80 mm3, 4T1‐bearing mice were divided into five groups. There was no statistical difference between the five groups at the start of treatment (phosphate‐buffered saline [PBS], 78.63 ± 8.91; MoPdR, 80.11 ± 10.75; S. typhimurium carrying pEGFP‐c1 [SC/pEGFP], 77.93 ± 10.59; S. typhimurium carrying pEGFP‐c1‐ePNP [SC/ePNP], 81.25 ± 8.47; the combination of SC/ePNP and MoPdR [SC/ePNP+MoPdR], 80.19 ± 12.14). Tumor volumes were compared by two‐tailed Student's t‐test. Data are presented as the mean ± SEM. (b) Survival curves of mice bearing 4T1 tumors among different groups are shown. Data were analyzed by the log–rank test, P < 0.05.
Discussion
Herein, for the first time, we showed a eukaryotic expression vector encoding the ePNP gene through S. typhimurium‐mediated gene delivery in cancer therapy, in combination with MoPdR. Previous studies reported that various methylated purines, such as 6‐methoxypurine and 6‐methylpurine, used identical metabolic pathways and the limited use of them by mammalian cells led to a low growth rate and reduced cell yield.( 43 ) As MoPdR is similar to MePdR in structure, we wondered whether an exploitation of MoPdR could work as functionally as MePdR to retard or stop tumor growth in mice.
The infection of bacteria in human tumor was recognized as early as 1868. Subsequently, tumor‐targeted attenuated bacterial strains, such as S. typhimurium, Clostridium and Bifidobacterium, have been developed as antitumor agents capable of preferentially amplifying within tumors and inhibiting tumors growth.( 2 , 16 , 50 ) Salmonella strains have been reported to have innate antitumor activity towards both primary and metastatic tumors and the ability to deliver proteins capable of metabolizing chemotherapeutic drugs directly within tumors.( 2 , 3 , 4 , 5 , 6 , 7 ) In the work described here, we used a new mutant S. typhimurium named SC36 (his G aro A cys pur I), with low residual virulence gained by diethyl sulfate mutagenesis of SL3261. We observed the SC36‐mediated heterogeneous gene expression in vitro and in vivo and preferential accumulation of SC36 within implanted tumors (1, 5). Our data, combined with that reported previously,( 51 ) suggest that attenuated S. typhimurium appears to only survive in tissues that become hypoxic and provide nutrients for it to grow. This evidence prompted us to explore whether SC36 carrying a eukaryotic expression vector encoding the ePNP gene was capable of both targeting tumor cells and suppressing tumor growth, in combination with MoPdR.
Although the mechanisms contributing to bacterial infection to mammalian cells are not completely understood, Schoen et al.( 52 ) summarized that invasion of bacterial carrier strains into host cells has been shown to be important in cellular mechanisms of bacteria‐mediated delivery. Previous studies done by Critchley et al.( 53 ) reported that bacterial invasion could sensitize mammalian cells to the action of MePdR. In the MTT assay described here, SC/ePNP‐infected cells showed a strong sensitivity to MoPdR (Fig. 2). We also observed that parental or infected cells were susceptible to MoPdR at the final concentration of 10 µg/mL. This is probably responsible for the toxicity of MoPdR to cells, and an excessive dose of MoPdR led to significant cell death. Furthermore, propidium iodide staining showed that the ePNP/MoPdR system increased spontaneous apoptosis (Fig. 3), consistent with previous reports.( 30 ) It is possible that the concomitant presence of apoptotic cells, bacterial products such as lipopolysaccharides,( 54 ) and bacterial DNA( 55 ) could act as ‘danger signals’( 56 , 57 ) for these infiltrating cells. The local abundance of these danger signals associated with the phagocytosis of dead cancer cells by activated macrophages is likely to initiate an immune response against specific cancer antigens and increase spontaneous apoptosis.( 53 ) We also observed a high bystander killing effect induced by the ePNP/MoPdR system with 1% ePNP‐positive cells and 5 µg/mL MoPdR, although the growth of parental cells was affected appreciably by MoPdR (Fig. 4). These data suggest that S. typhimurium could act as an antitumor agent to deliver genes, and apoptosis induced by the ePNP/MoPdR system was observed to have a high bystander effect.
In this study, with the help of MoPdR, we showed that attenuated S. typhimurium carrying the ePNP gene expression vector, given orally, significantly slowed tumor growth and prolonged survival periods in mice with established tumors (Fig. 6). Furthermore, in vivo studies show that the combination of MoPdR and the ePNP gene has positive synergistic antitumoral properties against experimental murine tumors, compared with treatments using MoPdR or the ePNP gene alone. To achieve complete tumor regression in further experiments, multiple inoculations of the bacteria might be important to prolong gene production and to increase antitumor activity.( 58 ) Zhao et al.( 9 , 10 , 11 ) showed that weekly treatment of bacteria was more effective than a two‐dose treatment. Moreover, this combined approach could be improved by using cytokines (e.g. interleukin [IL]‐4, IL‐12, or IL‐18) that have the capacity to induce the production of γ‐interferon to achieve the inhibition of cancer growth and reduce the dose of prodrugs.( 19 ) Alternatively, combining this approach with other therapeutic strategies, such as radiotherapy, might be more effective for tumor regression and prodrug abasement.( 59 ) Although attenuated S. typhimurium is effective to deliver plasmids carrying exogenous genes, the continuous loss of recombinant plasmids during cell division was observed and the mice required antibiotic treatment to reduce competing microflora. To make SC36 safer for clinical trials, it should be important to develop tumor‐specific expression vectors. Mengesha et al.( 60 ) developed a hypoxia inducible promoter (HIP‐1) system and found that HIP‐1 could confine gene expression strictly to the tumor. By this token, the use of tumor‐specific gene promoters is an attractive option for salmonella‐mediated gene delivery of the ePNP/MoPdR system.
In conclusion, MoPdR could be used in antitumor therapy associated with the ePNP gene. Because of some characteristics of attenuated salmonella, such as preferential accumulation, selective expression, and positive synergistic antitumor activity, our data support the idea that tumor‐targeting S. typhimurium could improve antitumor efficacy of the ePNP/MoPdR system in murine models. As substantial weight loss, limiting lethality, or other toxicities were not observed in these experiments with appropriate MoPdR doses, we expect that the simple method detailed in this study will be applicable for more tumor models in further research.
Acknowledgments
We thank the State Key Laboratory of Genetic Engineering of Fudan University for technical assistance. Thanks also to Mr Jiachi Liu for reviewing this paper.
References
- 1. Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer‐specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci USA 2005; 102: 14034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pawelek JM, Low KB, Bermudes D. Tumor‐targeted Salmonella as a novel anticancer vector. Cancer Res 1997; 57: 4537–44. [PubMed] [Google Scholar]
- 3. Low KB, Ittensohn M, Le T et al . Lipid A mutant Salmonella with suppressed virulence and TNFα induction retain tumor‐targeting in vivo . Nat Biotechnol 1999; 17: 37–41. [DOI] [PubMed] [Google Scholar]
- 4. Pawelek JM, Low KB, Bermudes D. Bacteria as tumour‐targeting vectors. Lancet Oncol 2003; 4: 548–56. [DOI] [PubMed] [Google Scholar]
- 5. Clairmont C, Lee KC, Pike J et al . Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium . J Infect Dis 2000; 181: 1996–2002. [DOI] [PubMed] [Google Scholar]
- 6. Steven A, Rosenberg SA, Spiess PJ, Kleiner DE. Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium . J Immunother 2002; 25: 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saltzman DA, Katsanis E, Heise CP et al . Antitumor mechanisms of attenuated Salmonella typhimurium containing the gene for human interleukin‐2: a novel antitumor agent? J Ped Surg 1997; 32: 301–6. [DOI] [PubMed] [Google Scholar]
- 8. Toso JF, Gill VJ, Hwu P et al . Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 2002; 20: 142–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao M, Yang M, Li X‐M et al . Tumor‐targeting bacterial therapy with amino acid auxotrophs of GFP‐expressing Salmonella typhimurium . Proc Natl Acad Sci USA 2005; 102: 755–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao M, Yang M, Ma H et al . Targeted therapy with a Salmonella typhimurium leucine–arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res 2006; 66: 7647–52. [DOI] [PubMed] [Google Scholar]
- 11. Zhao M, Jack G, Ma HY, Yang M, Sheldon P, Robert MH. Monotherapy with a tumor‐targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA 2007; 104: 10170–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dachs GU, Tupper J, Tozer GM. From bench to bedside for gene‐directed enzyme prodrug therapy of cancer. Anticancer Drugs 2005; 16: 349–59. [DOI] [PubMed] [Google Scholar]
- 13. Barzon L, Boscaro M, Palù G. Endocrine aspects of cancer gene therapy. Endocr Rev 2004; 25: 1–44. [DOI] [PubMed] [Google Scholar]
- 14. Lee CH, Wu CL, Shiau AL. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J Gene Med 2005; 6: 1382–93. [DOI] [PubMed] [Google Scholar]
- 15. Lee CH, Wu CL, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin‐1 gene leads to tumor‐specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Ther 2005; 12: 175–84. [DOI] [PubMed] [Google Scholar]
- 16. Li X, Fu GF, Fan YR et al . Bifidobacterium adolescentis as a delivery system of endostatin for cancer gene therapy: selective inhibitor of angiogenesis and hypoxic tumor growth. Cancer Gene Ther 2003; 10: 105–11. [DOI] [PubMed] [Google Scholar]
- 17. King I, Bermudes D, Lin S et al . Tumor‐targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther 2002; 13: 1225–33. [DOI] [PubMed] [Google Scholar]
- 18. Yamada H, Matsumoto S, Matsumoto T, Yamada T, Yamashita U. Murine IL‐2 secreting recombinant Bacillus Calmette–Guerin augments macrophagemediated cytotoxicity against murine bladder cancer MBT‐2. J Urol 2000; 164: 526–31. [PubMed] [Google Scholar]
- 19. Agorio C, Schreiber F, Sheppard M et al . Live attenuated Salmonella as a vector for oral cytokine gene therapy in melanoma. J Gene Med 2007; 9: 416–23. [DOI] [PubMed] [Google Scholar]
- 20. Sorscher EJ, Peng S, Bebok Z, Allan PW, Bennett LL Jr, Parker WB. Tumor cell bystander killing in colonic carcinoma utilizing the E. coli Deo D gene and generation of toxic purines. Gene Ther 1994; 1: 233–8. [PubMed] [Google Scholar]
- 21. Jensen KF, Nygaard P. Purine nucleoside phosphorylase from Escherichia coli and Salmonella typhimurium. Purification and some properties. Eur J Biochem 1975; 51: 253–65. [DOI] [PubMed] [Google Scholar]
- 22. Ealick SE, Greenhough TJ, Babu YS et al . Three‐dimensional structure of human erythrocytic purine nucleoside phosphorylase at 3.2 Å resolution. J Biol Chem 1990; 265: 1812–20. [DOI] [PubMed] [Google Scholar]
- 23. Mao C, Cook WJ, Zhou M, Koszalka GW, Krenitsky TA, Ealick SE. The crystal structure of Escherichia coli purine nucleoside phosphorylase: a comparison with the human enzyme reveals a conserved topology. Structure 1997; 5: 1373–83. [DOI] [PubMed] [Google Scholar]
- 24. Secrist JA III, Parker WB, Allan PW et al . Gene therapy of cancer: activation of nucleoside prodrugs with E. coli purine nucleoside phosphorylase. Nucleosides Nucleotides 1999; 18: 745–57. [DOI] [PubMed] [Google Scholar]
- 25. Bzowska A, Kulikowska E, Shugar D. Purine nucleoside phosphorylases: properties, functions and clinical aspects. Pharmacol Therapeutics 2000; 88: 349–425. [DOI] [PubMed] [Google Scholar]
- 26. Hughes B, Parker WB, Gadi VK, Garver RI, Sorscher EJ. Tumor specific killing with high bystander toxicity using the human tyrosinase promoter to express the E.coli PNP gene. Cancer Res 1995; 55: 3339–45. [PubMed] [Google Scholar]
- 27. Lockett LJ, Molloy PL, Russell PJ, Both GW. Relative efficiency of tumor cell killing in vitro by two enzyme–prodrug systems delivered by identical adenovirus vectors. Clin Cancer Res 1997; 3: 2075–80. [PubMed] [Google Scholar]
- 28. Park BJ, Brown CK, Hu Y et al . Augmentation of melanoma‐specific gene expression using a tandem melanocyte‐specific enhancer results in increased cytotoxicity of the purine nucleoside phosphorylase gene in melanoma. Hum Gene Ther 1999; 10: 889–98. [DOI] [PubMed] [Google Scholar]
- 29. Gadi VK, Alexander SD, Kudlow JE, Allan P, Parker WB, Sorscher EJ. In vivo sensitization of ovarian tumors to chemotherapy by expression of E. coli purine nucleoside phosphorylase in a small fraction of tumor cells. Gene Ther 2000; 7: 1738–43. [DOI] [PubMed] [Google Scholar]
- 30. Krohne TU, Shankara S, Geissler M et al . Mechanisms of cell death induced by suicide genes encoding purine nucleoside phosphorylase and thymidine kinase in human hepatocellular carcinoma cells in vitro . Hepatology 2001; 34: 511–18. [DOI] [PubMed] [Google Scholar]
- 31. Da Costa L, Jen J, He TC, Kinzler KW, Vogelstein B. Converting cancer genes into killer genes. Proc Natl Acad Sci USA 1996; 93: 4192–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nestler UM, Heinkelein M, Lucke J et al . Foamy virus vectors for suicide gene therapy. Gene Ther 1997; 4: 1270–7. [DOI] [PubMed] [Google Scholar]
- 33. Parker WB, King SA, Allan PW et al . In vivo gene therapy of cancer with E. coli purine nucleoside phosphorylase. Hum Gene Ther 1997; 8: 1637–44. [DOI] [PubMed] [Google Scholar]
- 34. Hughes BW, King SA, Allan PW, Parker WB, Sorscher EJ. Cell to cell contact is not required for bystander cell killing by E.coli purine nucleoside phosphorylase. J Biol Chem 1998; 273: 2322–8. [DOI] [PubMed] [Google Scholar]
- 35. Bharara S, Sorscher EJ, Gillespie GY et al . Antibiotic‐mediated chemoprotection enhances adaptation of E. coli PNP for Herpes simplex virus based glioma therapy. Hum Gene Ther 2005; 16: 1–9. [DOI] [PubMed] [Google Scholar]
- 36. Martiniello‐Wilks R, Dane A, Jeyakumar G et al . Gene‐directed enzyme prodrug therapy for prostate cancer in a mouse model that imitates the development of human disease. J Gene Med 2004; 6: 43–54. [DOI] [PubMed] [Google Scholar]
- 37. Hughes BW, Wells AH, Bebok Z et al . Bystander killing of melanoma cells using the human tyrosinase promoter to express the Escherichia coli purine nucleoside phosphorylase gene. Cancer Res 1995; 55: 3339–45. [PubMed] [Google Scholar]
- 38. Deharvengt S, Wack S, Uhring M, Aprahamian M, Hajri A. Suicide gene/prodrug therapy for pancreatic adenocarcinoma by E. coli purine nucleoside phosphorylase and 6‐methylpurine 2′‐deoxyriboside. Pancreas 2004; 28: e54–64. [DOI] [PubMed] [Google Scholar]
- 39. Deharvengt S, Wack S, Aprahamian M, Hajri A. Transcriptional tumor‐selective promoter targeting of E. coli purine nucleoside phosphorylase for pancreatic cancer suicide gene therapy. J Gene Med 2005; 7: 672–80. [DOI] [PubMed] [Google Scholar]
- 40. Cai X, Zhou J, Lin J, Sun X, Xue X, Li C. Experimental studies on PNP suicide gene therapy of hepatoma. J Huazhong Univ Sci Technolog Med Sci 2005; 25: 178–81. [DOI] [PubMed] [Google Scholar]
- 41. Cai XK, Zhou JL, Zhou HJ, Zhang L, Wu JH, Lin JS. [Killing effect of PNP/MeP‐dR suicide gene system driven by an AFP promoter AF0.3 on AFP‐positive hepatoma cells.] Ai Zheng 2006; 25: 1334–9. (In Chinese.) [PubMed] [Google Scholar]
- 42. Kikuchi E, Menendez S, Ozu C et al . Delivery of replication‐competent retrovirus expressing Escherichia coli purine nucleoside phosphorylase increases the metabolism of the prodrug, fludarabine phosphate and suppresses the growth of bladder tumor xenografts. Cancer Gene Ther 2007; 14: 279–86. [DOI] [PubMed] [Google Scholar]
- 43. Love SH, Remy CN. Metabolism of methylated purines in Escherichia coli: derepression of purine biosynthesis. J Bacteriol 1966; 91: 1037–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Persing DH, Smith TF, Tenover FC, White TJ, eds. Diagnostic Molecular Microbiology: Principles and Applications. Washington DC: American; Society for Microbiology, 1993. [Google Scholar]
- 45. Roth JR. Genetic techniques in studies of bacterial metabolism. Meth Enzymol 1970; 17: 3–35. [Google Scholar]
- 46. Gao T, Li WZ, Liang SH et al . Enzymatic synthesis of 6‐methylpurine‐2′‐deoxyriboside by recombinant purine nucleoside phosphorylase. Industrial Microbiol 2007; 37: 8–13. [Google Scholar]
- 47. Mosmann T. Rapid colorimetric assay for celluar growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth 1983; 65: 55. [DOI] [PubMed] [Google Scholar]
- 48. Sasaki DT, Dumas SE, Engleman EG. Discrimination of viable and non‐viable cells using propidium iodide in two color immunofluorescence. Cytometry 1987; 8: 413–20. [DOI] [PubMed] [Google Scholar]
- 49. Tanaka T, Kanai F, Okabe S et al . Adenovirus‐mediated prodrug gene therapy for carcinoembryonic antigen‐producing human gastric carcinoma cells in vitro. Cancer Res 1996; 56: 1341–5. [PubMed] [Google Scholar]
- 50. Theys J, Landuyt W, Nuyts S et al . Specific targeting of cytosine deaminase to solid tumors by engineered Clostridium acetobutylicum. Cancer Gene Ther 2001; 8: 294–7. [DOI] [PubMed] [Google Scholar]
- 51. Rosenberg SA, Spiess PJ, Kleiner DE. Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium . J Immunother 2002; 25: 218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schoen C, Stritzker J, Goebel W, Pilgrim S. Bacteria as DNA vaccine carriers for genetic immunization. Int J Med Microbiol 2004; 294: 319–35. [DOI] [PubMed] [Google Scholar]
- 53. Critchley RJ, Jezzard S, Radford KJ et al. Potential therapeutic applications of recombinant, invasive E. coli . Gene Ther 2004; 11: 1224–33. [DOI] [PubMed] [Google Scholar]
- 54. Gallucci S, Lolkema ML, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med 1999; 5: 1249–55. [DOI] [PubMed] [Google Scholar]
- 55. Hemmi H, Takeuchi O, Kawai T, et al . A Toll‐like receptor recognizes bacterial DNA. Nature 2000; 408: 740–5. [DOI] [PubMed] [Google Scholar]
- 56. Matzinger P. An innate sense of danger. Semin Immunol 1998; 10: 399–415. [DOI] [PubMed] [Google Scholar]
- 57. Melero I, Vile RG, Colombo MP. Feeding dendritic cells with tumor antigens: self‐service buffet or a la carte? Gene Ther 2000; 7: 1167–70. [DOI] [PubMed] [Google Scholar]
- 58. Lee CH, Wu CL, Shiau AL. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. J Gene Med 2004; 6: 1382–93. [DOI] [PubMed] [Google Scholar]
- 59. Platt J, Sodi S, Kelley M et al . Antitumour effects of genetically engineered Salmonella in combination with radiation. Eur J Cancer 2000; 36: 2397–402. [DOI] [PubMed] [Google Scholar]
- 60. Mengesh AL, Dubois P, Lambin W et al . Development of a flexible and potent hypoxia‐inducible promoter for tumor‐targeted gene expression in attenuated salmonella. Cancer Biol Ther 2006; 5: 1120–28. [DOI] [PubMed] [Google Scholar]


