Abstract
Metronomic chemotherapy is the frequent administration of low doses of chemotherapeutic agents targeting tumor‐associated endothelial cells. We examined the efficacy of metronomic irinotecan combined with low‐intensity ultrasound (US) in human uterine sarcoma and evaluated its antiangiogenesis mechanism by measuring the circulating endothelial progenitor cells (CEP), a surrogate marker of angiogenesis. A human uterine sarcoma cell line, FU‐MMT‐3, was used in the present study because this tumor is one of the most malignant neoplasms of human solid tumors and it also has a high angiogenesis property. The combination of low‐dose irinotecan and US irradiation significantly inhibited the tube formation of HUVEC and vascular endothelial growth factor expression of tumor cells in vitro. The FU‐MMT‐3 xenografts in nude mice were treated using US at a low intensity (2.0 w/cm2, 1 MHz) for 4 min three times per week each after the intraperitoneal administration of irinotecan; this treatment was continued for 5 weeks. The tumor vascularity was assessed by contrast‐enhanced color Doppler US in real time. The combination treatment significantly inhibited the mobilization of CEP and intratumoral vascularity compared with the control. This combination therapy showed a significant reduction in tumor volume, resulting in a significant prolongation of survival, in comparison with each treatment alone. These results suggest that the effect of metronomic chemotherapy for human uterine sarcoma was accelerated by US irradiation in vivo and this combination might therefore be potentially effective for new cancer therapy. (Cancer Sci 2011; 102: 452–459)
Uterine sarcomas are among the most aggressive uterine malignancies. Most uterine sarcomas have a poor prognosis, with an estimated 2‐year survival of <50%, even when discovered at an early stage.( 1 ) These tumors show a poor response to radiotherapy or any of the chemotherapeutic agents with substantial toxic effects that are currently in use.( 1 ) Previous studies in uterine sarcomas apparently showed a higher expression of vascular endothelial growth factor (VEGF)‐A and angiopoietin‐2 genes, as well as a higher frequency of lymphovascular invasion and high‐microvascular density in these tumors, compared with other human uterine carcinomas.( 2 )
The antiangiogenic strategy known as metronomic chemotherapy,( 3 , 4 ) with frequent and long‐term administration of low‐dose chemotherapeutic agents has recently been showing promising results. The rationale for this approach is that activated tumor vascular endothelial cells might be more sensitive to lower doses of chemotherapeutic drugs compared with normal or cancer cells, when exposed in a metronomic manner.( 3 , 5 ) Growing evidence suggests that metronomic delivery of drugs, such as cyclophosphamide,( 4 ) paclitaxel,( 6 ) vinblastine,( 7 ) taxanes( 8 ) and irinotecan,( 9 , 10 ) at doses much lower than the conventional dose, may have antiangiogenic properties and thus may be more effective than traditional chemotherapy. Irinotecan is a topoisomerase inhibitor that shows antiangiogenic activity in colorectal cancers( 11 ) and glioma( 12 ) when administered in a metronomic dose, but its antiangiogenic effect has not yet been investigated in uterine sarcoma.
Ultrasound has been shown to enhance the antitumor effect of a chemotherapeutic agent in vitro and in vivo. ( 13 , 14 ) Transiently increased permeability of the cell membrane, which is called sonoporation, is one of the mechanisms of the US‐enhanced chemotherapy.( 15 ) The drug efficiency of TNP‐470, an antiangiogenic agent is enhanced in combination with low‐intensity US irradiation without any major side‐effects.( 16 ) The present study is the first to examine the therapeutic effect of metronomic chemotherapy combined with US irradiation for human solid malignances in vitro and in vivo. The circulating endothelial progenitor cells (CEP), a surrogate marker of angiogenesis,( 17 , 18 ) were measured during the treatment period to evaluate the antiangiogenesis process in vivo.
Materials and Methods
Cell line and nude mice. A human uterine sarcoma cell line, FU‐MMT‐3, was previously established in this laboratory from a patient with uterine sarcoma. FU‐MMT‐3 shows highly progressive properties both in vitro and in vivo, and the immunophenotype, tumorigenicity and cytogenetic characteristics have been reported.( 19 ) Five‐ to 6‐week‐old female BALB/cA Jcl‐nu athymic nude mice were purchased from Clea (Tokyo, Japan). This in vivo experiment was approved by the Institutional Animal Care and Use Committee of Fukuoka University (No. 0803222).
Chemicals and ultrasound. Irinotecan (CPT‐11) and SN38, the active metabolite of irinotecan, were obtained from Daiichi‐Sankyo Co. Ltd (Tokyo, Japan). SN‐38 was dissolved in 100% DMSO to yield a 10 mM stock solution for the in vitro studies. The Sonitron 2000 (Richmar, Inola, OK, USA) was used for low‐intensity ultrasound irradiation both in vitro and in vivo, as described previously.( 16 )
Cell cytotoxicity assay. FU‐MMT‐3 cells were plated at a density of 1 x 104 cells/well in 96 flat‐bottomed plates, and were then treated either with various concentrations of SN38 (0.1–5000 nM) or at various intensities of ultrasound (0.1–3 W/cm2). A cell cytotoxicity assay was performed on day 4 using 3‐(4,5‐dimethylthiazol‐2‐yl)‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sulfophenyl)‐2H‐tetrazolium, inner salt MTS.
Combination of low‐dose SN38 and US in vitro. To evaluate the expression of human VEGF and TSP‐1, 1 × 105 FU‐MMT‐3 cells were seeded in 24‐multiwell dishes, then 24 h later the cells were cultivated with lower and inactive doses of SN38 (1, 5 nM) in Ham’s DMEM‐12 with 10% FBS, and the medium was changed every 3 days. Low‐intensity US (1 MHz, 1 W/cm2, 50% duty cycle, 60 s) was irradiated on the second and eighth days of the culture. Both the cell‐cultured medium and cells were collected to measure the secretion and mRNA expression of VEGF and TSP‐1 at two different time points (days 4 and 9).
Human VEGF and TSP‐1 gene expression on tumor cells. Quantitative real‐time PCR (qRT‐PCR) analysis was performed with 7500 Fast system using TaqMan Gene Expression Assays according to the manufacturer’s instructions (all from Applied Biosystems, Foster City, CA, USA). VEGF‐A and TSP‐1 validated primers were purchased from Applied Biosystems (Assay ID; Hs99999070_m1; Hs00170236_m1). Amplification was normalized to β‐actin and the quantification of gene expression was performed using the ΔΔCt method.
Human VEGF and TSP‐1 detection in conditioned media. Detection of VEGF and TSP‐1 in conditioned media was determined by a sandwich enzyme immunoassay using Quantikine ELISA kits (R & D systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. All experiments were repeated, independently, three times with at least two samples for each concentration.
In vitro tube formation assay. All experiments in angiogenesis were conducted in triplicate in 24‐multiwell dishes using an Angiogenesis kit (Kurabo, Osaka, Japan) according to the manufacturer’s instructions. Briefly, human umbilical vein endothelial cells (HUVEC) co‐cultured with human fibroblasts were cultivated with SN38 (1, 5 nM) in the medium containing 10 ng/mL of VEGF. Low‐intensity US was irradiated on the second and eighth days of the culture. After 10 days, co‐cultured cells were stained with mouse antihuman CD31 (Kurabo) and observed using a microscope (BX51; Olympus, Tokyo, Japan). The visualization of microvessel‐like tube formation of HUVEC and analysis was done according to the manufacturer’s instructions. Briefly, the area and tube length were quantitatively analyzed with the Kurabo Angiogenesis Image Analyzer (Kurabo) in five different fields of each well.
Drug administration and ultrasound irradiation in vivo. The mice were injected subcutaneously with 2 × 106 FU‐MMT‐3 cells in 0.2 mL medium in the right auxiliary region of the flank. Tumor growth was monitored by measuring the weekly volume twice, calculated as V = a × b2/2 (a = length; b = width), and mice weight was measured weekly.
This study investigated two types of treatment strategies in vivo; namely, early and late regimen. Early regimen: mice bearing the resultant tumors measuring 50–100 mm3 in diameter were randomly separated into three treatment groups (five mice in each) and continued for 8 weeks. (i) Maximum tolerated dose (MTD) of irinotecan 50 mg/kg weekly; (ii) metronomic dose of irinotecan (1 or 5 mg/kg) daily; and (iii) non‐treatment as the control group.( 12 , 20 ) Late regimen: mice bearing tumors measuring 300–500 mm3 were randomly separated into three treatment groups (ten mice in each) and continued for 5 weeks. (i) Metronomic irinotecan (1 or 5 mg/kg) daily; (ii) US alone; and (iii) combination of metronomic irinotecan and US (1 and 5 mg/kg + US) treatments were performed. Ultrasound was performed as described in a previous study.( 16 ) Briefly, US was administered at a low‐intensity (1 MHz, 2.0 W/cm2, 50% duty cycle) for 4 min three times per week after each intraperitoneal administration of metronomic irinotecan. An autopsy was performed on five mice of each group following death and the other five mice were used to measure the survival time.
Evaluation of CEP with flow cytometry. For evaluation of CEP, blood was obtained from anesthetized mice via retro‐orbital sinus bleeding and prepared for CEP labeling as described previously.( 18 ) The following directly conjugated antibodies were used to detect CEP in murine peripheral blood: rat anti‐mouse CD45‐PerCP, Flk1‐PE (mouse VEGFR‐2), CD31‐APC (platelet/endothelial adhesion molecule‐1) and CD117‐FITC (c‐kit receptor; all from BD Biosciences, San Jose, CA, USA). CD45−Flk‐1+CD31+CD117+ cells were counted as CEP. Flow cytometry was done using a FACSCalibur flow cytometer (BD Biosciences) and acquired data was analyzed using FLOWJO analysis software (Treestar, Ashland, OR, USA). The number of CEP detected for each mouse was expressed as a percentage of peripheral blood mononuclear cells detected for that mouse.
Evaluation of tumor vascularity with contrasted color Doppler US. The intratumoral vascularity in the xenografts of the late treatment regimen was examined two times in each group using contrast‐enhanced color Doppler US (SSD‐4000; Aloka Ltd, Tokyo, Japan) with a 7.5‐MHz curved array transducer (UST‐987‐7.5; Aloka Ltd), as shown in a previous study.( 16 ) Briefly, mice were anesthetized with intraperitoneal pentobarbital injection (160 mg/kg), and then the intratumoral blood flow was enhanced by i.v. injection of Sonazoid (0.015 mL/kg; Daiichi Sankyo). The vascularity index (VI) of tumor in the color Doppler US images was quantitatively evaluated using Image J 1.38X software (NIH, Bethesda, MD, USA).
Evaluation of tissue VEGF, mircovascular density and apoptosis. The deparaffinized sections or cryosections from each tumor were incubated with 5% skim milk in PBS for 30 min. The sections were then incubated with monoclonal rabbit anti‐human VEGF‐A (dilution 1:200; Calbiochem, Darmstadt, Germany) or rat anti‐mouse CD31 primary antibody (dilution 1:100; PharMingen, San Diego, CA, USA). Cytoplasmic staining was scored positively for VEGF( 20 ) (×400). The microvessel density (MVD) quantification in the highest vascularized area (×400) was examined in each tumor. TUNEL was performed using the in situ cell death detection kit (Roche Diagnostics, K.K., Basel, Switzerland) according to the manufacturer’s instructions.
Statistical analysis. All data were expressed as the mean ± SD. The Mann–Whitney U‐test on the in vivo analysis results or one‐way anova was performed on the in vitro sets of data. Differences were considered to be significant when the P value was <0.05. These statistical analyses were done using the StatView 5.0 software package (SAS institute, Inc., Cary, NC, USA) for Macintosh. Survival curves were plotted by the method of Kaplan–Meier, and then they were examined for survival differences with log‐rank statistics. ***P < 0.001; **P < 0.01; *P < 0.05.
Results
Antiangiogenic effect of low dose SN38 and US. The 96‐h SN38 exposure inhibited the cell growth of FU‐MMT‐3 in a concentration‐dependent manner (Fig. 1A), and the calculated IC50 value was 200 ± 33.1 nM. Ultrasound also inhibited the cell growth of FU‐MMT‐3 in an intensity‐dependent manner (Fig. 1B), and the calculated IC50 value was 2.27 ± 0.6 W/cm2. Next, low doses of SN38 (1 and 5 nM) and 1 W/cm2 US alone or in combination was used to examine the modification of VEGF and TSP‐1 expression in FU‐MMT‐3. Low‐dose SN38 significantly inhibited VEGF mRNA expression and secretion compared with the controls (Fig. 1C,D). Ultrasound significantly inhibited VEGF expression on day 4 but not on day 9 (Fig. 1C,D). Moreover, the combination treatment significantly decreased the VEGF compared with each treatment alone.
Figure 1.
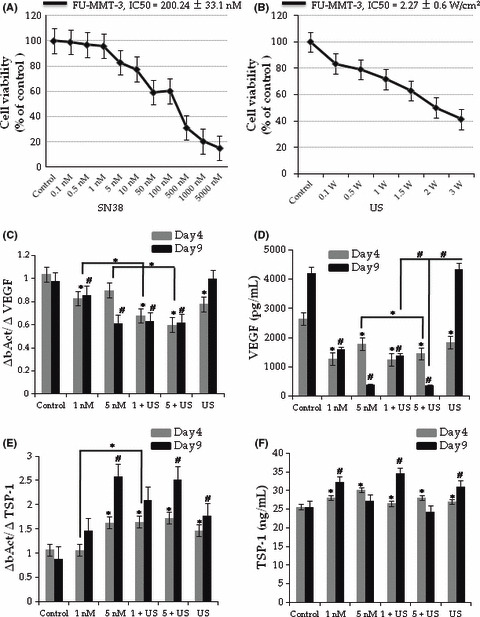
Antiangiogenic and antitumor effect of low dose SN38 and ultrasound (US). The IC50 values of SN38 (A), US (B) in FU‐MMT‐3 cells. Low dose SN38 and US irradiation significantly inhibited the vascular endothelial growth factor (VEGF) mRNA expression (C) and secretion (D). In contrast, mRNA expression and secretion of TSP‐1 significantly increased in all treatment groups compared with the control (E,F) on day 4. (*P < 0.01 vs Day 4, #P < 0.01 vs Day 9).
The TSP‐1 mRNA expression in FU‐MMT‐3 cells exposed to 5 nM SN38 significantly decreased (Fig. 1E). However, 5 nM SN38 showed a significant antiproliferative effect, and the secretion level was therefore lower in comparison with the non‐treated cells (control) on day 9 (Fig. 1F). Therefore, 1 nM and US alone significantly increased mRNA expression and secretion of TSP‐1 (Fig. 1E) compared with the controls. The combination of 1 nM and US significantly increased both TSP‐1 mRNA expression and secretion compared with each treatment alone.
Inhibition of tube formation in vitro. The HUVEC efficiently formed tubes when cultured in the presence of VEGF (Fig. 2A, control). A quantitative analysis of the area (Fig. 2B) and length (Fig. 2C) of tube formation by HUVEC revealed that US alone or low‐dose SN‐38 alone significantly inhibited tube formation. Moreover, the combination of SN38 and US treatment showed significant synergistic effects compared with each treatment alone.
Figure 2.
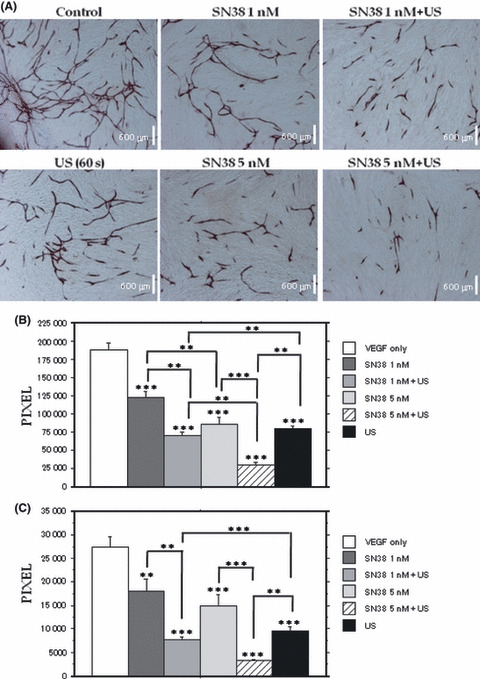
Inhibition of tube formation in vitro. Tube formation of HUVEC (A). Quantitative analysis of tube area (B) and tube length (C) using an image analyzer. Combination of low dose SN38 and ultrasound (US) irradiation significantly inhibit tube formation compared with each treatment alone.
Suppression of tumor growth. Early regimen: either the administration of metronomic irinotecan (1, or 5 mg/kg, daily), or MTD irinotecan (50 mg/kg, weekly) significantly inhibited the growth of the FU‐MMT‐3 xenografts compared with the controls (1 mg/kg, P < 0.05; 5 mg/kg, P < 0.001; MTD, P < 0.01) (Fig. 3A, top). However, significant bodyweight loss was observed after administration of the MTD irinotecan treatment (Fig. 3A, bottom). All xenografts (5/5) treated with metronomic 5 mg/kg irinotecan showed complete responses from the third week of treatments without any side‐effects.
Figure 3.
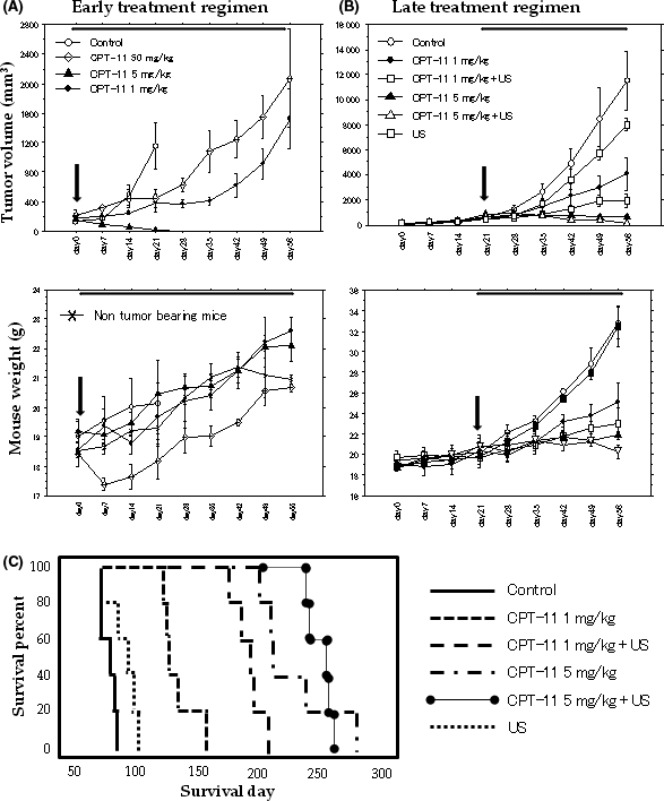
Inhibition of FU‐MMT‐3 tumor growth and prolongation of survival in vivo. Effects of early (A) and late (B) regimens on tumor growth. Top, tumor volume; bottom, mouse weights. Arrows indicate the beginning of each regimen. The combination treatment of irinotecan and ultrasound (US) significantly enhanced the suppression of tumor growth compared with each treatment alone. (C) Survival of mice with FU‐MMT‐3 tumors treated with the late regimen. Combination treatments demonstrate an effective prolongation of survival compared with each treatment alone.
Late regimen: All the metronomic irinotecan and US treatments significantly inhibited the growth of the FU‐MMT‐3 xenografts compared with the controls. The combination treatment of irinotecan and US significantly enhanced the suppression of tumor growth in comparison to each treatment alone (1 mg/kg + US vs 1 mg/kg, P < 0.05; and vs US alone, P < 0.001; or 5 mg/kg + US vs 5 mg/kg, P < 0.001; and vs US alone, P < 0.001) (Fig. 3B, top). In the combination of metronomic 5 mg/kg irinotecan and US, a complete response was seen in 7/10 mice by the end of the fifth week of treatment. No weight loss was observed in these combined treatment groups (Fig. 3B, bottom). Figure 3C shows the survival curves of the treatment groups and control mice in the late regimen. Ultrasound or metronomic irinotecan alone increased survival compared with the control (US, P < 0.05; 1 mg/kg, P < 0.001; 5 mg/kg, P < 0.001). Both combination treatment groups demonstrated an effective prolongation of survival, and superior survival curves were actually obtained compared with each treatment alone (1 mg/kg + US, P < 0.01; 5 mg/kg + US, P < 0.01).
Suppression of CEP. Early regimen: the CEP levels were significantly decreased in the metronomic irinotecan groups compared with the controls and MTD (Fig. 4A).
Figure 4.
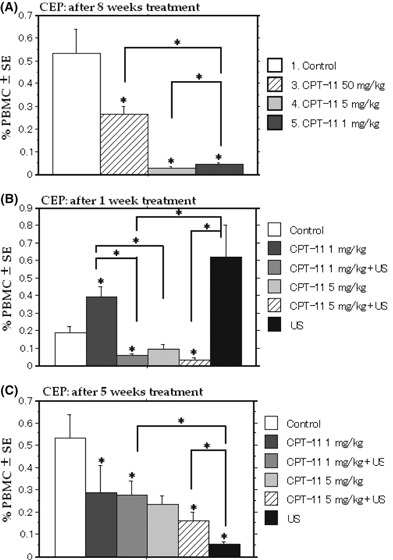
Suppression of circulating endothelial progenitor cells (CEP) in mice treated with early and late regimens. The CEP levels are significantly decreased in the metronomic irinotecan group compared with the controls and the MTD irinotecan group (A). The number of CEP is significantly decreased in the first week (B) or the fifth week (C) of treatment in the combined treatment groups compared with each treatment. US, ultrasound.
Late regimen: the number of CEP was significantly decreased in the first week of treatment only in the combined treatment groups (Fig. 4B) compared with the control. The CEP were decreased in all groups but 5 mg/kg compared with the control at the end of the treatment (Fig. 4C). Furthermore, a significantly enhanced inhibitory effect was obtained using the combined treatment compared with the control.
Reduced vascularity of xenografts in the late regimen. Figure 5A shows the tumor vascularity after the fourth week of treatments and the control in the late regimen. After 1 week of treatment, the VI of the FU‐MMT‐3 xenografts treated with metronomic 5 mg/kg irinotecan was significantly lower than that of the control (Fig. 5B). The VI of the combination of metronomic 5 mg/kg irinotecan and US was also significantly lower compared with US alone. After the fourth week of treatment, the VI in the metronomic irinotecan alone was significantly lower than that of the control, whereas no significant reduction was observed in the US alone treatment (Fig. 5C). The reduction in the VI was significantly enhanced by the combined treatment compared with each treatment alone.
Figure 5.
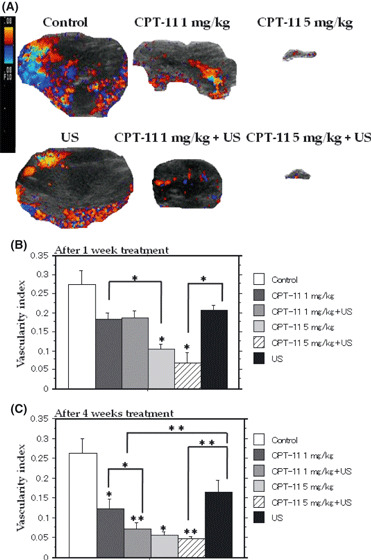
Decrease of vascularity of xenografts in the late regimen. Color Doppler ultrasound (US) pictures of xenografts after 4 weeks of treatment with the late regimen (A). Both the tumor volume and vessel density decreased in each treatment group compared with the control. Metronomic 5 mg/kg irinotecan and its combination with US significantly reduced the vascularity index compared with the control after 1 week of treatment (B). The vascularity indexes of all treated tumors were decreased compared with the controls in the fourth week of treatment (C). Moreover, combined treatment showed a significantly enhanced effect compared with each treatment alone.
Enhanced antiangiogenesis and antitumor activity of the combination treatment. The percentages of VEGF immuno‐stained cells (Fig. 6A–F) were significantly decreased in the metronomic irinotecan alone and US alone group compared with the control. The percentage of VEGF immuno‐stained cells was decreased in the combination of metronomic 1 mg/kg irinotecan and US xenografts compared with each treatment alone. The mean MVD (Fig. 6G–L) of xenografts in the 1 mg/kg irinotecan group was significantly lower than that of the controls. The reduction of MVD was significantly enhanced in the xenografts treated with the combination of metronomic 1 mg/kg irinotecan and US compared with that of the control and the treatment with US alone. An increase in the number of apoptotic cells was observed in all treated groups. The TUNEL scores in the metronomic irinotecan groups were significantly higher than those of the control and US (Fig. 6M–R). The score of the combination of metronomic 1 mg/kg irinotecan and US was significantly higher than each treatment alone or the control. The mRNA expression of VEGF (Fig. 6S) was significantly decreased in xenografts treated by metronomic irinotecan compared with the control. The mRNA level of VEGF was decreased in the combination treatment (1 mg/kg + US) compared with each treatment alone.
Figure 6.
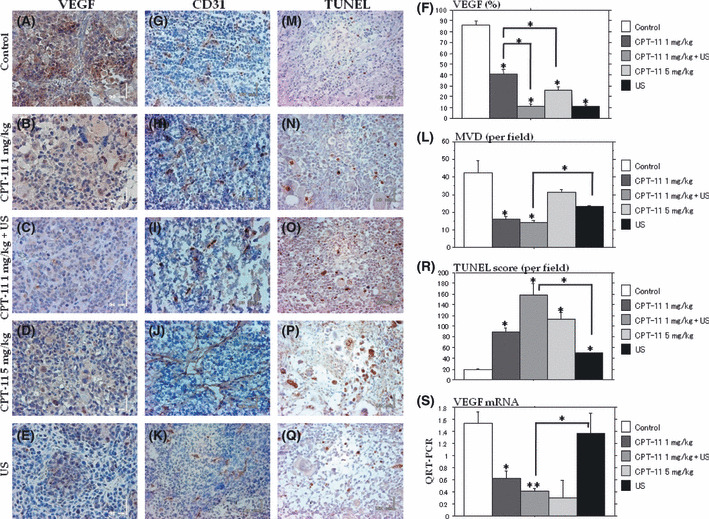
Quantitative assessments of vascular endothelial growth factor (VEGF), microvessel density (MVD) and TUNEL staining in the late regimen. Representative sections of the VEGF (A–E), MVD (G–K), and TUNEL staining (M–Q) in tumors treated with metronomic irinotecan alone, ultrasound (US) alone and a combination of metronomic 1 mg/kg irinotecan and US (VEGF, ×400; MVD, ×200; and TUNEL, ×200). The VEGF (F) and MVD (L) were significantly decreased in the combination treatment of metronomic 1 mg/kg irinotecan and US compared with the control. Apoptotic cells are significantly increased in the combination treatment group compared with each treatment alone (R). QRT‐PCR analysis of VEGF mRNA of the xenografts: the combination of metronomic irinotecan and US significantly inhibited the expression of VEGF mRNA (S).
Discussion
Uterine sarcomas, including carcinosarcoma, are among the most malignant human tumors( 1 ) and these tumors tend to show strong angiogenic activity.( 2 , 21 ) The standard treatments for these tumors, except in the early stages, have not yet been determined;( 22 ) thus, new therapeutic strategies must be immediately investigated. Previous studies have shown that US irradiation significantly enhances the antiangiogenic and antitumor effects of TNP‐470 in a preclinical model of uterine sarcoma.( 16 )
Metronomic chemotherapy is the frequent administration of cytotoxic agents at significantly smaller cumulative doses than the MTD.( 3 , 5 ) The current study evaluated two different dose levels of metronomic irinotecan (1 and 5 mg/kg; the cumulative doses decreased 90% and 60%, respectively, compared with the MTD dose) in two different settings, an early and a late regimen. The early regimen was initiated when tumor volume reached 50–100 mm3, simulating the treatment for relatively early stage tumors. The late regimen was initiated to 300–500 mm3 tumors, which simulates the therapy for advanced tumors. The previous preclinical and clinical studies suggest that metronomic irinotecan mainly acts through inhibition of VEGF expression and HIF‐α.( 23 , 24 ) Another possible mechanism is the induction of TSP‐1, an endogenous inhibitor of angiogenesis.( 11 ) The current data support these findings, since low‐dose SN38 resulted in a significant upregulation of TSP‐1 in vitro and the downregulation of VEGF in tumor cells, in vitro and in vivo. Moreover, the apoptotic cells significantly increased in the metronomic irinotecan alone treated xenografts. This might result from the frequent and prolonged periods of administration of irinotecan, and thus is attributable to the accumulation of the drug, rather than the single administration doses of irinotecan.
Recent studies show that low‐intensity US irradiation enhances the antitumor and antiangiogenic effects of chemotherapeutic agents,( 14 , 15 ) or the angiogenesis inhibitor.( 16 ) Ultrasound alone could inhibit VEGF secretion in tumor cells in vivo. The mechanism of this effect is unknown, but US might have some influence on the protein translation stage rather than the VEGF mRNA transcription stage, because the expression of VEGF mRNA was not inhibited in the in vivo study. Ultrasound alone significantly induced the TSP‐1 expression of tumor cells in vitro. Further study is therefore needed to evaluate these mechanisms. The combination therapy of metronomic irinotecan with US irradiation significantly inhibited the growth of FU‐MMT‐3 in vivo compared with each treatment alone, suggesting US had an accelerated effect on the metronomic treatment. The uptake of metronomic irinotecan might thus be enhanced in both tumor cell and endothelial cells by sonoporation (increased cellular membrane permeability), as suggested by previous studies.( 14 , 16 ) Moreover, this combination treatment was associated with elongation of the survival time with a more tolerable toxicity profile, which is the most important goal of antitumor therapy.
The CEP are thought to be mobilized from the bone marrow in response to growth factors such as VEGF, then migrate to sites of ongoing angiogenesis and differentiate into mature endothelial cells.( 4 , 18 , 25 ) Recent studies have shown that metronomic regimens have an inhibitory effect on the mobilization and viability of bone marrow‐derived CEP.( 18 , 26 ) The MTD cyclophosphamide regimens caused a sharp decline in CEP following therapy, but these cells quickly increased during the drug‐free period, whereas there was sustained suppression of CEP in response to metronomic cyclophosphamide.( 27 ) Metronomic irinotecan was significantly more effective in suppressing CEP than MTD irinotecan in the current study. The CEP significantly increased 1 week after beginning US treatment, but after 5 weeks the CEP significantly decreased. Further study is needed to explain this phenomenon, but previous studies showed low‐intensity US (0.03, 0.05 W/cm2) has an angiogenic effect, including an increase in VEGF.( 28 , 29 , 30 ) Therefore, 2 W/cm2 US might be expected to show a similar effect in the early term of the treatment. However, prolonged US treatment alone significantly inhibited VEGF, thus resulting in a decrease of MVD and CEP. Moreover, the combination of metronomic irinotecan and US irradiation caused a significant reduction of CEP compared with metronomic irinotecan alone.
In conclusion, these data suggest that the antitumor effect of metronomic irinotecan for uterine sarcoma was accelerated by low‐intensity US irradiation in vitro and in vivo by the suppression of angiogenesis. Therefore, this combination may contribute to the effective treatment of solid tumors. Assessment of the CEP level in peripheral blood may be a useful biomarker of the therapeutic response in metronomic regimens.
References
- 1. Acharya S, Hensley ML, Montag AC, Flemming GF. Rare uterine cancers. Lancet Oncol 2005; 6: 961–71. [DOI] [PubMed] [Google Scholar]
- 2. Emoto M, Charnock‐Jones DS, Licence DR et al. Localisation of the VEGF and angiopoietin genes in uterine carcinosarcoma. Gynecol Oncol 2004; 95: 474–82. [DOI] [PubMed] [Google Scholar]
- 3. Kerbel RS, Kamen BA. The anti‐angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 2004; 4: 423–36. [DOI] [PubMed] [Google Scholar]
- 4. Shaked Y, Henke E, Roodhart JM et al. Rapid chemotherapy‐induced acute endothelial progenitor cell mobilization: implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell 2008; 14: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Browder T, Butterfield CE, Kraling BM et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug‐resistant cancer. Cancer Res 2000; 60: 1878–86. [PubMed] [Google Scholar]
- 6. Drevs J, Fakler J, Eisele S et al. Antiangiogenic potency of various chemotherapeutic drugs for metronomic chemotherapy. Anticancer Res 2004; 24: 1759–63. [PubMed] [Google Scholar]
- 7. Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low‐dose metronomic chemotherapy. Proc Natl Acad Sci USA 2002; 100: 12917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Klement G, Huang P, Mayer B et al. Differences in the therapeutic indexes of combination metronomic chemotherapy and an anti‐VEGFR‐2 antibody in multidrug‐resistant human breast cancer xenografts. Clin Cancer Res 2002; 8: 221–32. [PubMed] [Google Scholar]
- 9. O’Leary J, Shapiro RL, Ren CJ, Chuang N, Cohen HW, Potmesil M. Antiangiogenic effects of camptothecin analogues 9‐amino‐20(s)‐camptothecin, topotecan, and CPT‐11 studied in the mouse cornea model. Clin Cancer Res 1999; 5: 181–7. [PubMed] [Google Scholar]
- 10. Kamiyama H, Takano S, Tsuboi K, Matsumura A. Anti‐angiogenic effects of SN38 (active metabolite of irinotecan): inhibition of hypoxia‐inducible factor‐1 alpha (HIF‐1α)/vascular endothelial growth factor (VEGF) expression of glioma and growth factor of endothelial cells. J Cancer Res Clin Oncol 2005; 131: 205–13. [DOI] [PubMed] [Google Scholar]
- 11. Allegrini G, Falcone A, Fioravanti A et al. A pharmacokinetic and pharmacodynamic study on metronomic irinotecan in metastatic colorectal cancer patients. Br J Cancer 2008; 98: 1312–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takano S, Kamiyama H, Mashiko R, Osuka S, Ishikawa E, Matsumura A. Metronomic treatment of malignant glioma xenografts with irinotecan (CPT‐11) inhibits angiogenesis and tumor growth. J Neurooncol 2010; 99: 177–85. [DOI] [PubMed] [Google Scholar]
- 13. Longo FW, Longo WE, Tomashefsky P, Lattimer JK, Rivin BD, Tannenbaum M. Interaction of ultrasound with neoplastic tissue. Local effect on subcutaneously implanted Furth‐Colombia rat Wilms’ tumor. Urology 1975; 6: 631–4. [DOI] [PubMed] [Google Scholar]
- 14. Feril LB Jr, Kondo T, Umemura S, Tachibana K, Manalo AH, Riesz P. Sound waves and antineoplastic drugs: the possibility of an enhanced combined anticancer therapy. J Med Ultrason 2002; 29: 173–87. [DOI] [PubMed] [Google Scholar]
- 15. Mitragotri S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov 2005; 4: 255–60. [DOI] [PubMed] [Google Scholar]
- 16. Emoto M, Tachibana K, Iwasaki H, Kawarabayashi T. Antitumor effect of TNP‐470, an angiogenesis inhibitor, combined with ultrasound irradiation for human uterine sarcoma xenografts evaluated using contrast color Doppler ultrasound. Cancer Sci 2007; 98: 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monestiroli S, Mancuso P, Burlini A, et al. Kinetics and viability of circulating endothelial cells as a surrogate marker in an animal model of human lymphoma. Cancer Res 2001; 61: 4341–4. [PubMed] [Google Scholar]
- 18. Beaudry P, Force J, Naumov GN, et al. Differential effects of vascular endothelial growth factor receptor‐2 inhibitor ZD6474 on circulating endothelial progenitors and mature circulating endothelial cells: Implications for use as a surrogate marker of antiangiogenic activity. Clin Cancer Res 2005; 11: 3514–22. [DOI] [PubMed] [Google Scholar]
- 19. Emoto M, Iwasaki H, Oshima K, Kikuchi M, Kaneko Y, Kawarabayashi T. Characteristics of rhabdomyosarcoma cell lines derived from uterine carcinosarcomas. Virchows Arch 1997; 431: 249–56. [DOI] [PubMed] [Google Scholar]
- 20. Bocci G, Falcone A, Fioravanti A et al. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br J Cancer 2008; 98: 1610–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cimbaluk D, Rotmensch J, Scudiere J, Gown A, Bitterman P. Uterine carcinosarcoma: immunohistochemical studies on tissue microarrays with focus on potential therapeutic targets. Gynecol Oncol 2006; 105: 138–44. [DOI] [PubMed] [Google Scholar]
- 22. Nam JH, Park JY. Update on treatment of uterine sarcoma. Current opinion in obstetrics and gynecology. Curr Opin Obstet Gynecol 2010; 22: 36–42. [DOI] [PubMed] [Google Scholar]
- 23. Rapisarda A, Uranchimeg B, Scudiero DA et al. Identification of small molecule inhibitors of hypoxia‐inducible factor 1 transcriptional activation pathway. Cancer Res 2002; 62: 4316–24. [PubMed] [Google Scholar]
- 24. Pencreach E, Guerin E, Nicolet C et al. Marked activity of irinotecan and rapamycin combination toward colon cancer cells in vivo and in vitro is mediated through cooperative modulation of the mammalian target of rapamycin/hypoxia‐inducible factor‐1a axis. Clin Cancer Res 2009; 15: 1297–307. [DOI] [PubMed] [Google Scholar]
- 25. Nowak K, Rafat N, Belle S et al. Circulating endothelial progenitor cells are increased in human lung cancer and correlate with stage of disease. Eur J Cardiothorac Surg 2010; 37: 758–63. [DOI] [PubMed] [Google Scholar]
- 26. Kamat AA, Kim TJ, Landen CN Jr et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res 2007; 67: 281–8. [DOI] [PubMed] [Google Scholar]
- 27. Bertolini F, Paul S, Mancuso P et al. Maximum tolerable dose and low‐dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells. Cancer Res 2003; 63: 4342–6. [PubMed] [Google Scholar]
- 28. Barzelai S, Sharabani‐Yosef O, Holbova R et al. Low‐intensity ultrasound induces angiogenesis in rat hind‐limb ischemia. Ultrasound Med Biol 2006; 32: 139–45. [DOI] [PubMed] [Google Scholar]
- 29. Rawool NM, Goldberg BB, Forsberg F, Winder AA, Hume E. Power Doppler assessment of vascular changes during fracture treatment with low‐intensity ultrasound. J Ultrasound Med 2003; 22: 145–53. [DOI] [PubMed] [Google Scholar]
- 30. Ramli R, Reher P, Harris M, Meghji S. The effect of ultrasound on angiogenesis: an in vivo study using the chick chorioallontoic membrane. Int J Oral Maxillofac Implants 2009; 24: 591–6. [PubMed] [Google Scholar]


