Abstract
Our recent study showed that a novel member of bone morphogenetic protein (BMP) family, BMP‐10, was decreased in prostate cancer. In the present study, we investigated the implication of BMP‐10 in breast cancer, particularly the relation of its expression with clinical aspects. The expression of BMP‐10 was examined in a cohort of human breast cancer specimens (normal, n = 23; cancer, n = 97), using both quantitative real‐time PCR and immunohistochemical staining. The full‐length human BMP‐10 was cloned into a mammalian expression plasmid vector and then transfected into breast cancer cells. The effect on growth, cell matrix adhesion, motility, and invasion of MDA‐MB‐231 cells by BMP‐10 was then investigated using in vitro growth assays. Immunohistochemical staining and quantitative real‐time PCR revealed a decreased expression of BMP‐10 in breast cancer. Further analysis of BMP‐10 transcript level against the clinical aspect demonstrated that the decreased BMP‐10 expression correlated with disease progression, bone metastasis, and poor prognosis. The disease‐free survival of the patients with a higher level of BMP‐10 was 132.8 (95% CI, 122.0–143.5) months, significantly longer compared to 93.7 (95% CI, 60.3–127.2) months for patients with a lower level of BMP‐10 expression (P = 0.043). The overexpression of BMP‐10 has broad inhibitory effects on the in vitro growth, invasion, and motility of breast cancer cells. Taken together, BMP‐10 can inhibit the cell growth of breast cancer cells, and decreased BMP‐10 expression correlates to poor prognosis and disease progression, particularly the lymphatic and bone metastasis. Bone morphogenetic protein‐10 (BMP‐10) may function as a tumor suppressor in breast cancer. (Cancer Sci 2010)
Breast cancer is the most common cancer in women in the UK and USA.( 1 , 2 ) The life‐threatening complications in breast cancer patients are metastases. The leading metastatic site of this disease is bone, dominantly osteolytic lesions, which result in severe bone pain, fracture, spinal cord compression, and hypercalcemia. It is critical to understand the reason for the predisposition of breast cancer to metastasize to bone, which may provide novel approaches to prevent and treat the disease‐specific bone lesions. In the past decade, a group of proteins which play pivotal roles in regulating formation of bone and cartilage, namely bone morphogenetic proteins (BMPs), have been investigated intensively in cancer due to their potential link to malignant bone lesions.
BMPs are members of the transforming growth factor‐β (TGF‐β) superfamily. To date, more than 20 BMPs have been identified in humans. BMPs play critical roles in fetal and postnatal development, and also the homeostasis of various tissues and organs. BMPs have been indicated in development and progression of several malignancies, including breast cancer, prostate cancer, lung cancer, colorectal cancer, melanoma, osteosarcoma, etc.( 3 )
Aberrations in BMPs expression have been indicated in breast cancer. Decreased expression of BMP‐2, BMP‐7, Growth and Differentiation Factor 9a (GDF 9a), and BMP‐15 have been seen in primary breast cancer, which correlate with poor prognosis.( 4 , 5 , 6 ) Most interestingly, the decreased BMP‐7 expression in primary breast tumors associate with bone metastasis. This is also supported by experimental data from an in vivo bone metastasis model which showed an inhibitory role for BMP‐7 in bone metastasis from breast cancer.( 6 ) In contrast to these observations, the elevated expression of some BMPs has also been implicated in breast cancer, such as BMP‐2, BMP‐4, BMP‐5, and BMP‐7.( 7 , 8 , 9 , 10 , 11 ) Some investigations also showed a plausible pattern of BMPs expression in breast cancer. Particularly, both decreased and increased expression of BMP‐7 in primary breast tumors have been implicated in the disease specific bone metastasis.( 6 , 11 ) Apart from the aberrant expression of BMPs in breast, perturbed expression of BMP receptors and downstream signaling were also indicated in the development and progression of breast cancer, particularly the disease‐specific bone metastasis.( 12 , 13 , 14 )
The mouse BMP‐10 gene was first cloned in 1999, and it is mostly abundant in the trabeculae of the embryonic heart.( 15 ) Bone morphogenetic protein‐10 (BMP‐10)‐deficient mice die in the uterus between E9.5 and E10.5 due to a defect in cardiogenesis.( 16 ) This suggests that BMP‐10 plays an important role in the trabeculation of the embryonic heart. It has been demonstrated that the Activin receptor‐like kinase 1 (ALK1), Bone morphogenetic protein receptor IA, ALK3 (BMPR‐IA (ALK3)), and BMPR‐IB (ALK6) are candidate type I receptors for BMP‐10, and BMPR‐II and Activin A receptor, type IIA (ActR‐IIA) are the candidate type II receptors for the protein.( 17 , 18 ) Our recent study has shown that this protein is decreased in prostate cancer, particularly in the higher grade tumors. Experimental data have also suggested that BMP‐10 could suppress the growth, invasion, and migration of prostate cancer cells through a Smad‐independent pathway.( 19 )
Despite these observations of BMP‐10 in prostate cancer, its role in breast cancer remains unknown. In the present study, the expression of BMP‐10 was examined in a cohort of breast cancer samples. Breast cancer cells were forced to express this molecule, in order to establish the functional role of BMP‐10 in breast cancer using in vitro function tests.
Materials and Methods
Materials. Human breast cancer cell line MDA‐MB‐231 was obtained from the ECACC (European Collection of Animal Cell Culture, Salisbury, UK). The cells were routinely maintained in DMEM‐F12 medium supplemented with 10% fetal calf serum and antibiotics. Polyclonal rabbit antihuman‐BMP‐10 was purchased from Orbigen (San Diego, CA, USA). Unless stated, other materials and reagents were purchased from Sigma‐Aldrich (Poole, England, UK).
Breast tissue samples collection. Breast cancer tissues (n = 97) and normal background tissues (n = 23) were collected immediately after surgery and stored at −80°C until use. The clinical follow‐up was routinely performed after surgery. The median follow‐up period was 120 months (June 2004). Details of histology were obtained from pathology reports and confirmed by a consultant pathologist (A.D.J.) (Table 1).
Table 1.
Patients’ clinicopathological information
| n | |
|---|---|
| Node status | |
| Negative | 52 |
| Positive | 45 |
| Grade | |
| 1 | 14 |
| 2 | 34 |
| 3 | 48 |
| Histology | |
| Ductal | 76 |
| Lobular | 11 |
| Medullary | 2 |
| Tubular | 2 |
| Mucinous | 1 |
| Others | 5 |
| TNM staging | |
| 1 | 52 |
| 2 | 32 |
| 3 | 5 |
| 4 | 4 |
| Clinical outcome | |
| Disease free | 69 |
| With metastasis | 5 |
| With local recurrence | 3 |
| Died of breast cancer | 15 |
| Died of unrelated diseases | 5 |
The number of samples in each group is shown.
RNA extraction, reverse transcription–PCR, and quantitative PCR. Frozen sections of tissues were cut at a thickness of 5–10 μm and were kept for immunohistochemistry and routine histology. Total RNA extraction from frozen tissues and culture cells was performed using a standard RNA isolation kit.
Reverse transcription was carried out using 0.5 μg of total RNA for each 20‐μL RT reaction. Conventional PCR was performed with specific primers for BMP‐10. Amplification conditions were as follows: 94°C for 5 min, followed by 40 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min, and the final extension for 7 min at 72°C. The level of BMP‐10 transcripts from the above‐prepared cDNA was also determined using a real‐time quantitative PCR, based on the Amplifluor technology, modified from a method reported previously.( 20 ) Briefly, pairs of PCR primers (Table 2) were similarly designed using the Beacon Designer software, but an additional sequence was added to the antisense primer. This is known as the Z sequence (5′‐actgaacctgaccgtaca‐3′) which is complementary to the universal Z probe (Intergen, Oxford, UK). The reaction was carried out using the following: Hot‐start Q‐master mix (Abgene, Epsom, UK), 10 pmol of specific forward primer, 1 pmol reverse primer which has the Z sequence, 10 pmol of FAM‐tagged probe (Intergen), and cDNA from 50 ng of RNA. The reaction was carried out using IcyclerIQ (Bio‐Rad, Surrey, UK), which is equipped with an optic unit that allows real‐time detection of 96 reactions, under the following conditions: 94°C for 12 min and 80 cycles of 94°C for 15 s, 55°C for 40 s, and 72°C for 20 s. The levels of the BMP‐10 transcript are shown here as number of the transcript copies per 50 ng RNA, generated from an internal standard, that was simultaneously amplified during the same quantitative real‐time PCR, with full details described previously.
Table 2.
Primer sequences
| Forward | Reverse | PCR products (bps) | Annealing temperature (°C) | |
|---|---|---|---|---|
| hBMP‐10 | 5′‐CTGCCAACATCATTAGGAGT | 5′‐ACTGAACCTGACCGTACA ATGGACACATTGAAGAGGAG | 108 | 55 |
| hBMP‐10 expression | 5′‐ATGGGCTCTCTGGTCCTG | 5′‐CTATCTACAGCCACATTCGGAGA | 1275 | 58 |
| hGAPDH | 5′‐AGCTTGTCATCAATGGAAAT | 5′‐CTTCACCACCTTCTTGATGT | 593 | 55 |
| CK19 | 5′‐CAGGTCCGAGGTTACTGAC | 5′‐ACTGAACCTGACCGTACA CCGTTTCTGCCAGTGTGTCTTC | 107 | 55 |
| hβ‐actin | 5′‐ATGATATCGCCGCGCTCG | 5′‐CGCTCGGTGAGGATCTTCA | 580 | 55 |
The prefix “h” indicates human. BMP‐10, bone morphogenetic protein‐10; CK19, cytokeratin.
Immunohistochemical staining of BMP‐10. Frozen sections of breast tumors and background tissues were cut at a thickness of 6 μm. Immunohistochemistry was performed using anti‐BMP‐10 antibody and a Vectastain Universal Elite ABC kit (Vector Laboratories, Peterborough, UK).
Western blot analysis. Cells were lysed in HEPES‐buffered Ca2+, Mg2+‐free Hanks’ solution (HCMF) buffer containing 1% Triton, 0.1% SDS, 2 mM CaCl2, 100 μg/mL phenylmethylsulfonyl fluoride, 1 μg/mL leupeptin, and 1 μg/mL aprotinin for 30 min before clarification at 13 000 g for 10 min. Protein concentrations were measured using a DC Protein Assay kit (Bio‐Rad), and were quantified by using a spectrophotometer (ELx800; Bio‐Tek, Potton, UK). Equal amounts of protein from each cell sample (10 or 25 μg/lane) were loaded onto a 10% polyacrylamide gel. After electrophoresis, proteins were blotted onto nitrocellulose sheets and blocked in 10% skimmed milk for 60 min before being probed with the anti‐BMP‐10 antibody and peroxidase‐conjugated secondary antibodies. Protein bands were visualized using the Supersignal West Dura system (Pierce Biotechnology, Rockford, IL, USA), and photographed using a UVITech imager (UVITech, Cambridge, UK).
In vitro cell growth assay. A standard procedure was used as previously described.( 21 ) Cells were plated into a 96‐well plate (2500 cells/well). Cell growth was assessed after 1, 3, and 5 days. Crystal violet was used to stain cells, and absorbance was determined at a wavelength of 540 nm using a spectrophotometer.
In vitro invasion assay. According to a standard procedure,( 22 ) Transwell inserts with 8‐μm pore size were coated with 50 μg Matrigel (BD Matrigel Basement Membrane Matrix, BD Biosciences, Oxford, UK) and air dried. After rehydration, 20 000 cells were added to each well. After 96 h, cells that had migrated through the matrix to the other side of the insert were fixed, stained, and then counted under a microscope.
In vitro motility assay using Cytodex‐2 beads. We followed the protocol described by Rosen.( 23 , 24 ) 106 cells were incubated with 100 μL of Cytodex‐2 beads in 10 mL DMEM overnight. After washing, 100 μL of beads/cells were transferred into each well of a 24‐well plate. After 4 h of incubation, migrated cells were then stained and counted. Three independent experiments were performed.
Cell–matrix adhesion assay. This procedure has been previously described.( 22 ) Forty thousand cells were added to each well of 96‐well plates pre‐coated with Matrigel (5 μg/well). After 40 min of incubation, non‐adherent cells were washed off using BSS buffer. The remaining adhered cells were fixed, stained, and then counted.
Tumor growth in an athymic mice model. Female athymic nude mice (4–8 weeks old; CD1; Charles River Laboratories, Inc., Kent, UK) were subcutaneously (s.c.) injected with the MDA‐MB‐231 breast cancer cells (1 × 106) in Matrigel (2.5 mg/mL). MDA‐MB‐231 breast cancer cells had been genetically modified to express BMP‐10 (MDA‐MB‐231 BMP‐10) or contained the empty plasmid vector to act as the control group (MDA‐MB‐231 pEF). The mice were kept in sterilized, filtered cages in 12‐h dark/12‐h light standardized environmental conditions approved by the local ethical committee. They were weighed twice weekly in accordance with Home Office regulations. Tumor size was measured twice a week using digital calipers and calculated as mm3 = 0.512 × width2 × length. These experimental procedures were done over a 4‐week period (experimental end‐point).
Statistical analysis. Statistical analysis was performed using the Minitab (Minitab Ltd., Coventry, UK) statistical software package (version 14). Non‐normally distributed data was assessed using the Mann–Whitney test, while the two sample t‐test was used for normally distributed data. Kaplan–Meier survival analysis, Pearson correlation, and Cox hazardous proportion analysis were performed using SPSS statistical software (version 11; SPSS. Chicago, IL, USA). Differences were considered to be statistically significant at P < 0.05.
Results
The expression of BMP‐10 in breast cancer. The expression of BMP‐10 was examined in breast cancer cell lines and the cohort of breast cancer. The expression of BMP‐10 mRNA in breast cancer cell lines was determined using RT‐PCR. It was not detectable in most breast cancer cell lines examined except BT‐482 and MDA‐MB‐463, in comparison with normal breast tissue and placenta which were both positive for BMP‐10 expression (Fig. 1a). Bone morphogenetic protein‐10 (BMP‐10) transcript was also examined in the breast cancer tumors using quantitative PCR. A decreasing level of BMP‐10 expression was revealed in breast tumors (290 ± 240 copies/50 ng RNA), compared to the normal background tissues (1930 ± 1400 copies/50 ng RNA, P = 0.27) (Fig. 1b). Immunochemical staining further confirmed the expression of BMP‐10 in human breast tissue, which showed BMP‐10 staining in the cytoplasm of normal mammary epithelia. In consistence with its transcript level, BMP‐10 staining was weaker or absent in breast cancer (Fig. 1c).
Figure 1.
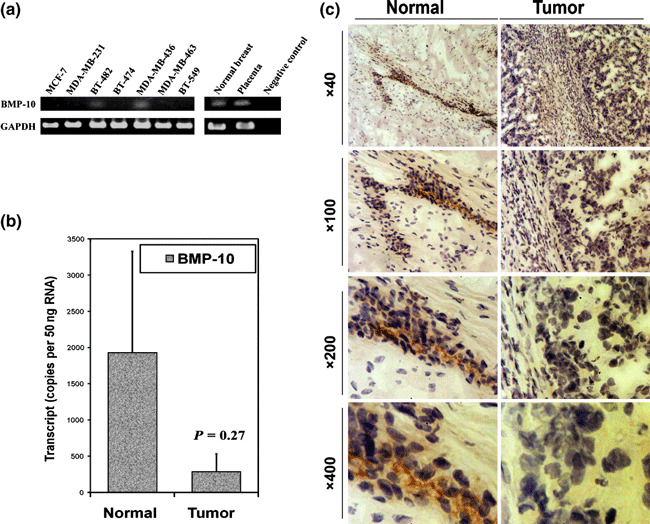
Expression of bone morphogenetic protein‐10 (BMP‐10) in breast cancer. (a) The expression of BMP‐10 mRNA in breast cancer cell lines using RT‐PCR. (b) The BMP‐10 transcript level is decreased in human breast cancer using quantitative PCR. (c) Immunohistochemical staining revealed a decreased staining of BMP‐10 in breast cancer compared to normal background tissue.
Bone morphogenetic protein‐10 (BMP‐10) and histological type, grade, lymph node involvement, and TNM staging. To assess the relation of BMP‐10 expression to disease progression, BMP‐10 transcript levels in the breast cancer samples were analyzed against important pathological statuses, such as histological type, grade, node status, and TNM staging (Table 3). According to the histological type, a much higher level of BMP‐10 transcripts (914 ± 391) was seen in ductal breast cancer, which is the most common type of breast cancer (P < 0.05) compared to lobular (0.0145 ± 0.0141) and other types of breast cancer (0.006 ± 0.006) (Fig. 2a). As sample number was very small (≤2), the statistical results of some histological types such as mucinous, medullary, and tubular are not shown. In relation to the histological grade of tumor cells, BMP‐10 expression tended to be decreased in the poorly differentiated tumor cells including grade 2 (24.2 ± 24.1) and grade 3 (569 ± 388) (P = 0.087, P = 0.17), compared with grade 1 (2953 ± 1585) (Fig. 2b). With regard to lymph node status, a relatively higher level of BMP‐10 transcript was seen in tumor samples with lymph node involvement, (1104 ± 537, P = 0.25) compared to that in tumor with no lymph node involvement (366 ± 335) (Fig. 2c). With regard to TNM stages, BMP‐10 was expressed at a relatively lower level in advanced breast cancer (31.2 ± 30.2 in patients with TNM4, P = 0.18) compared with early stage breast cancer (490 ± 339, TNM1) (Fig. 2d).
Table 3.
Summary of quantitative PCR results
| BMP‐10 transcripts (Copies/50 ng RNA) | P‐value | |
|---|---|---|
| Histological type | ||
| Ductal | 914 ± 391 | |
| Lobular | 0.0145 ± 0.014 | 0.022 |
| Others | 0.006 ± 0.006 | 0.022 |
| Histological grade | ||
| Grade 1 | 2953 ± 1585 | |
| Grade 2 | 24.2 ± 24.1 | 0.087 |
| Grade 3 | 569 ± 388 | 0.17 |
| Estrogen receptor (ER) status | ||
| ERα (−) | 635 ± 360 | |
| ERα (+) | 572 ± 522 | 0.92 |
| ERβ (−) | 540 ± 298 | |
| ERβ (+) | 826 ± 809 | 0.74 |
| TNM staging | ||
| TNM1 | 490 ± 339 | |
| TNM2 | 1365 ± 751 | 0.29 |
| TNM3 | 56.6 ± 56.6 | 0.21 |
| TNM4 | 31.2 ± 30.2 | 0.18 |
| Lymph node involvement | ||
| Lymph node (−) | 1104 ± 537 | |
| Lymph node (+) | 366 ± 335 | 0.25 |
| Nottingham Prognostic Index (NPI) | ||
| NPI1 (<3.4) | 1104 ± 537 | |
| NPI2 (3.4–5.4) | 515 ± 470 | 0.41 |
| NPI3 (>5.4) | 1 ± 0.7 | 0.045 |
| Clinical outcome | ||
| Disease free | 988 ± 430 | |
| Metastasis | 228 ± 160 | 0.10 |
| Local recurrence | 0.0097 ± 0.0097 | 0.025 |
| Died of breast cancer | 18.4 ± 18.1 | 0.027 |
| Poor prognosis | 61.5 ± 38.7 | 0.035 |
| Bone metastasis | 127 ± 93 | 0.054 |
Bold, P‐value ≤ 0.05.
Figure 2.
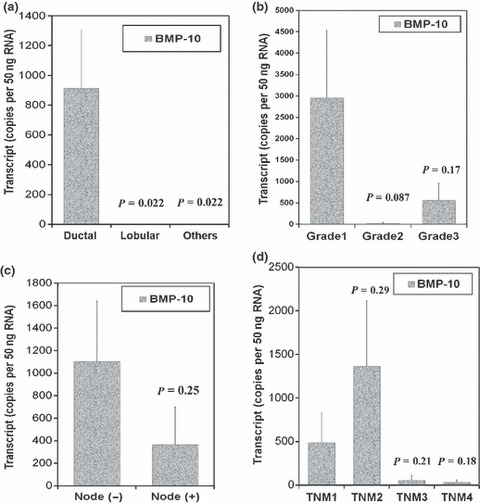
Bone morphogenetic protein‐10 (BMP‐10) and histological type, tumor grade, nodal status, and TNM stage. (a) Decreased levels of BMP‐10 transcripts were seen in lobular and other histological types of breast cancer compared to ductal breast cancer. (b) Bone morphogenetic protein‐10 (BMP‐10) transcripts were reduced in moderate‐ and poorly differentiated cancer cells compared to well‐differentiated tumor cells. (c) Decreased BMP‐10 expression was associated with lymphatic metastasis. (d) Lower levels of BMP‐10 transcripts were seen in the advanced breast cancer, including TNM3 and TNM4.
Estrogen receptor (ER) status is very important in both the assessment and management of the disease. The expression level of BMP‐10 was also analyzed against ER status. No difference was noted in BMP‐10 transcripts levels of patients with various ER statuses, including ERα/β positive and negative (Fig. 3). Furthermore, we failed to demonstrate a significant correlation between the transcript levels of BMP‐10 and human epidermal growth factor receptor 2 (HER‐2) (coefficient = 0.038, by Pearson correlation).
Figure 3.
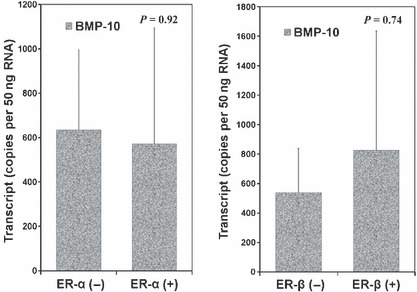
Bone morphogenetic protein‐10 (BMP‐10) transcript level and estrogen receptor (ER) status.
Prognostic relevance of BMP‐10 in breast cancer. The prognostic potential of BMP‐10 expression was firstly examined in accordance with patients’ Nottingham Prognostic Index (NPI). The NPI1 group (NPI score <3.4; n = 48) represented patients with good prognosis, the NPI2 group (NPI score 3.4–5.4; n = 32) represented patients with moderate prognosis, while patients of the NPI3 group (NPI score >5.4; n = 13) had a poor prognosis. Statistical analysis showed BMP‐10 expression was reduced in patients with moderate prognosis (NPI1), while patients with poor prognosis (NPI3) had significantly decreased levels of BMP‐10 transcripts, (P = 0.045) when compared with patients with good prognosis (NPI1) (Fig. 4a).
Figure 4.
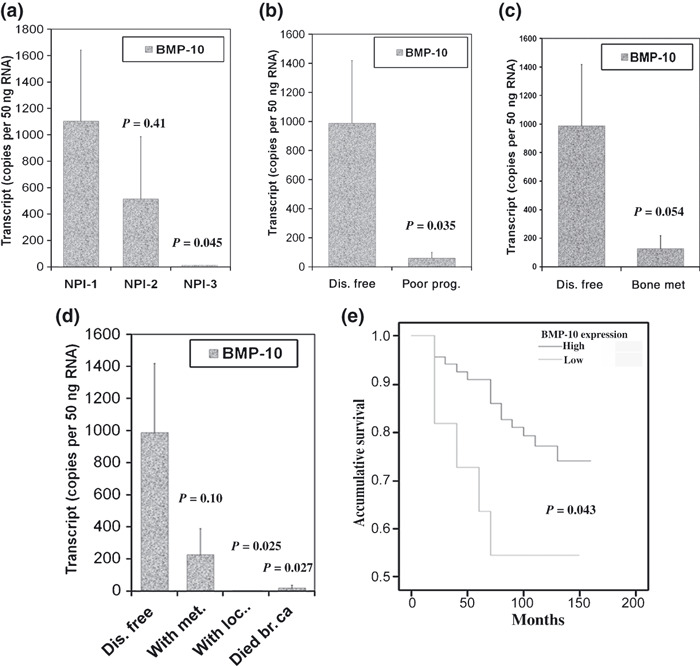
BMP‐10 and clinical outcomes and prognosis. (a) Bone morphogenetic protein‐10 (BMP‐10) and Nottingham prognostic index (NPI). (b) Expression of BMP‐10 is decreased in patients with poor prognosis compared to disease‐free patients. (c) Decreased BMP‐10 expression in primary tumor is associated with bone metastasis. (d) BMP‐10 and clinical outcomes. Expression of BMP‐10 is significantly decreased in patients with local recurrence or death due to breast cancer compared to disease‐free patients. (e) Higher level of BMP‐10 expression in primary breast tumors is correlated with longer disease‐free survival.
Regarding clinical outcomes, patients fell into the following categories: remaining disease free, with metastasis, with local recurrence, and death due to breast cancer after a median 120 months’ follow‐up. The BMP‐10 transcript level was reduced in the other three groups compared to the group of patients who remained disease free (Fig. 4d). In particular, patients with local recurrence or those who died from breast cancer had significantly decreased levels of BMP‐10 transcripts, P = 0.025, or P = 0.027 in comparison with disease‐free patients. Therefore, patients with poor prognosis, including those with metastasis, local recurrence, and death due to breast cancer had significantly lower levels of BMP‐10 transcripts compared to disease‐free patients (P = 0.035) (Fig. 4b). Patients with bone metastasis had a lower expression level of BMP‐10 (127 ± 93) compared to disease‐free patients (988 ± 430, P = 0.054) (Fig. 4c). Bone morphogenetic protein‐10 (BMP‐10) expression also tended to be lower in tumors with distant metastases (including bone metastases and other distant metastases) (228 ± 160, P = 0.10, compared to disease‐free patients). The expression of BMP‐7 was previously examined in the same breast cancer cohort.( 4 ) However, the correlation between BMP‐10 and BMP‐7 was not significant (coefficient = 0.023, by Pearson correlation).
Kaplan–Meier survival analysis showed that patients with higher expression levels of BMP‐10 had longer disease‐free survival: 132.8 (95% CI, 122.0–143.5) months versus 93.7 (95% CI, 60.3–127.2) months for patients with a lower BMP‐10 transcript level (P = 0.043) (Fig. 4e). However, patients with a higher level of BMP‐10 did not tend to be better in overall survival: 134.1 (95% CI, 123.5–144.6) months versus 120.0 (89.9–150.1) months in patients with lower expression of BMP‐10. Using Cox’s multivariate analysis for nodal status, TNM stage, tumor grade, ER status, HER‐2, and BMP‐10, it was also found that BMP‐10 is a significant independent prognostic factor (P = 0.024).
Bone morphogenetic protein‐10 (BMP‐10) inhibited in vitro growth and aggressiveness of breast cancer cells. To evaluate the biological function of BMP‐10 in breast cancer cells, forced overexpression was performed in a breast cancer cell line which does not express this molecule. Overexpression was confirmed using both RT‐PCR and western blot analysis (Fig. 5). A reduction of in vitro growth was seen in MDA‐MB‐231BMP−10exp cells. The absorbance of MDA‐MB‐231BMP−10exp cells at day 5 was 1.40 ± 0.19, P < 0.01, compared with the absorbance of MDA‐MB‐231WT (2.17 ± 0.11) and MDA‐MB‐231pEF/His cells (1.83 ± 0.17) (Fig. 6a).
Figure 5.
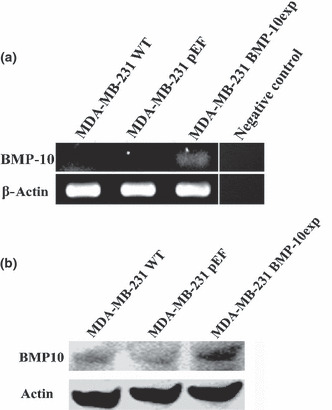
Forced expression of bone morphogenetic protein‐10 (BMP‐10) in breast cancer cells. (a) Overexpression of BMP‐10 was seen in MDA‐MB‐231BMP−10exp cells using RT‐PCR, compared to both MDA‐MB‐231WT and MDA‐MB‐231pEF cells. (b) Overexpression of BMP‐10 protein in MDA‐MB‐231BMP−10exp cells was also verified using western blot analysis, in comparison with both controls.
Figure 6.
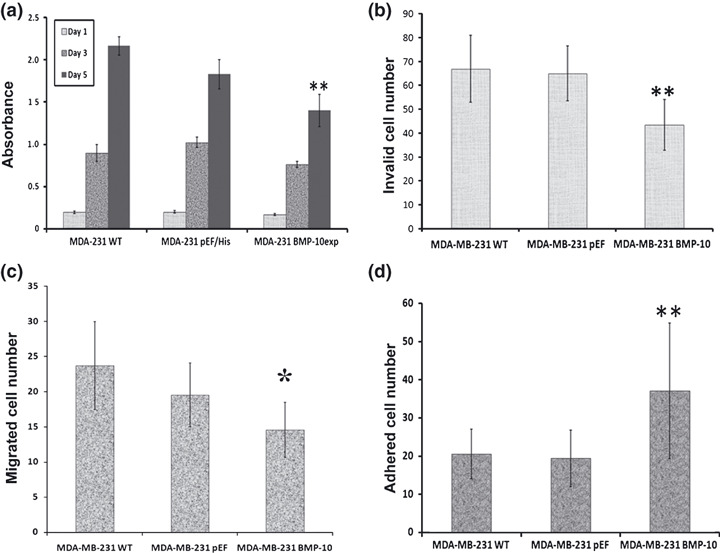
The effects on biological functions of breast cancer cells by bone morphogenetic protein‐10 (BMP‐10) overexpression. BMP‐10 inhibits in vitro growth breast cancer cells (a); and reduces invasion of breast cancer cells (b). (c) Overexpression of BMP‐10 results in a reduction of cell motility. (d) Cell–matrix adhesion of breast cancer cells was enhanced by BMP‐10. Shown are representative results of three independent experiments of each function assay. **P < 0.01, *P < 0.05.
Invasiveness is a crucial capacity enabling the dissemination of tumor cells. BMP‐10 could inhibit this aggressive ability of breast cancer cells. The invaded cell number of MDA‐MB‐231BMP−10exp was 43.4 ± 10.6, P < 0.01, compared to both MDA‐MB‐231WT (66.9 ± 14.0) and MDA‐MB‐231pEF/His (65.0 ± 11.5) cells (Fig. 6b). BMP‐10 could also reduce the motility of MDA‐MB‐231 cells. The Cytodex‐beads motility assay showed a decrease in the migrated cell number in MDA‐MB‐231 BMP−10exp (14.6 ± 3.9), P < 0.01, compared to MDA‐MB‐231WT (23.7 ± 6.3), and P = 0.048 in comparison with MDA‐MB‐231pEF/His cells (19.6.0 ± 4.5).
The overexpression of BMP‐10 in MDA‐MB‐231 cells could promote their adhesion. The adhered cell number of MDA‐MB‐231BMP−10exp was 121.3 ± 9.4, P < 0.01, compared to both MDA‐MB‐231WT (56.6 ± 2.2) and MDA‐MB‐231pEF/His (78.3.0 ± 3.3) cells.
Bone morphogenetic protein‐10 (BMP‐10) reduced in vivo tumor growth of breast cancer. To further examine the inhibitory role of BMP‐10 in breast cancer, MDA‐MB‐231BMP10exp and MDA‐MB‐231pEF/His cells were s.c. injected in athymic nude mice respectively. The overexpression of BMP‐10 could reduce the tumor development in vivo. A significant difference was seen at Day 22 after injection between the MDA‐MB‐231 BMP‐10 overexpression and control groups (P = 0.028) (Fig. 7).
Figure 7.
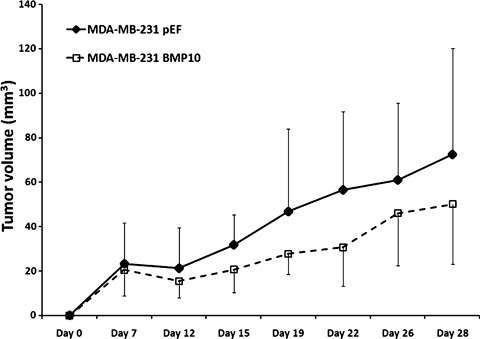
Influence of bone morphogenetic protein‐10 (BMP‐10) overexpression on in vivo tumor growth.
Discussion
Bone morphogenetic protein‐10 (BMP‐10), a novel member of the BMP family, has been shown to be a key factor in development of trabeculae of embryonic heart. We recently examined the role of BMP‐10 in prostate cancer, in which its expression was decreased, and found that BMP‐10 could inhibit the growth, cell–matrix adhesion, and migration of prostate cancer cells through a Smad‐independent pathway.( 19 ) In the current study, we first noted that BMP‐10 expression was decreased in breast cancer which was associated with disease progression and poor prognosis, and also highlighted its anticancer potential.
Aberrations in the expression and signaling of certain BMPs have been indicated in breast cancer. For example, decreased expression of BMP‐3, GDF‐9a, and BMP‐15 have been seen in breast cancer, and were associated with disease progression and poor prognosis.( 4 , 5 ) In contrast, increased expression of BMP‐4 and BMP‐5 have been indicated in breast cancer.( 10 ) Meanwhile, both increased and decreased expression of certain BMPs and receptors, such as BMP‐2, BMP‐6, BMP‐7 and BMPR‐IB, has been demonstrated in breast cancer with a potential link to disease progression and bone metastasis.( 7 , 10 , 11 , 12 , 13 , 25 ) This suggests that BMPs can be divided into subgroups according to their functions in breast cancer, such as inhibiting and promoting. Certain BMPs may also play different roles according to the development and progression of the disease. In the current study, we first examined the expression of BMP‐10 at the mRNA and protein level in breast cancer tissues. Decreased BMP‐10 expression was seen in breast cancer compared to normal background tissues. In line with the observation in human breast tissues, the expression of BMP‐10 mRNA was also lower or undetectable compared to normal breast tissue and placenta. Therefore, this is first study to identify the expression of BMP‐10 in human breast tissue and decreased BMP‐10 expression in breast cancer.
We further analyzed the quantity of BMP‐10 transcripts in breast cancer samples against the corresponding clinical data. The decreased BMP‐10 expression correlated with disease progression and prognosis. A relatively higher level of BMP‐10 transcripts was seen in ductal breast cancer, compared to in lobular, medullar, and other histological types. According to TNM staging, the BMP‐10 transcript level was lower in the advanced breast cancer. The decreased expression of BMP‐10 also correlated with lymphatic metastasis, and was also seen in patients with bone metastasis. Bone morphogenetic proteins (BMPs) may play diverse roles in bone metastasis from breast cancer. For example, BMP‐7 can inhibit the growth of mammary tumors at both the primary site and in bone in vivo.( 6 ) On the other hand, BMP‐2 can promote the spread of breast cancer cells in an in vivo bone metastasis model.( 26 ) Bone morphogenetic proteins (BMPs) are widely involved in the regulation of cellular functions of breast cancer cells, ranging from cell growth and death, cell migration, invasion, and epithelial–mesenchymal transition (EMT).( 27 ) The response of breast cancer cells to BMPs may differ according to the different BMPs applied, and the phenotypic profile of BMP receptors and relevant downstream molecules in the cancer cells which have been examined. For example, BMP‐2 and BMP‐6 inhibit the proliferation of breast cancer cells,( 28 , 29 ) while BMP‐7 could promote proliferation of MDA‐MB‐231 and BT‐474 cells, but showed an inhibitory effect on the other breast cancer cell lines tested.( 30 ) The current study suggests that BMP‐10 is an inhibitory factor for the dissemination of breast cancer cells, particularly during lymphatic metastasis and bone metastasis.
The expression of BMP‐10 was associated with patients’ clinical outcomes, which showed a higher level in disease‐free patients after a 10‐year follow‐up, and a decreased level in patients with local recurrence, metastasis, or death due to breast cancer. Kaplan–Meier survival analysis enhanced this correlation, which indicated that a higher level of BMP‐10 transcript was associated with longer disease‐free survival. This link between decreased BMP‐10 expression and poor prognosis was also supported by analysis against patients’ NPI scores, and suggests that BMP‐10 may be a prognostic factor in breast cancer.
The involvement of estrogen in the development and progression of breast cancer is well established. Prolonged exposure to estrogen, such as early menarche, late menopause, and nulliparity, has been considered as a high risk of breast carcinoma development. The effect of estrogen is mediated through two estrogen receptors, ERα (ESR1) and ERβ (ESR2). Estrogen receptor (ER) statuses are correlated with the prognosis of breast cancer patients, and are also a key indicator for selecting hormone therapy. Bone morphogenetic proteins (BMPs) have some interaction with estrogen signaling in breast cancer, either through the interaction of intracellular signaling or via regulation of the expression of the other.( 31 , 32 ) In particular, there are certain links between BMP expression and ER status. For example, increased BMP‐7 expression in breast cancer is associated with the level of expression of estrogen receptor.( 33 ) In contrast BMP‐6 expression is suppressed in ER‐negative breast tumors through promoter hypermethylation.( 34 ) In the current study, we also analyzed BMP‐10 transcript levels against ER status. There is no obvious link between BMP‐10 expression and ER status. This suggests that the decreased expression of BMP‐10 is not due to the phenotypic shift of ER. Apart from ER status, the present study did not find a significant correlation between BMP‐10 and HER‐2 gene transcripts.
In line with the correlations between decreased BMP‐10 expression and disease progression and poor prognosis, the overexpression of BMP‐10 in breast cancer cells could inhibit in vitro growth, invasiveness, and motility. These capacities are essential for tumor cells to disseminate and settle down at secondary sites.
In summary, the current study indicated a decreased expression of BMP‐10 in breast primary tumors, which was associated with disease progression and poor prognosis. The experimental overexpression of BMP‐10 in breast cancer cells consis‐tently highlighted its inhibitory effect during disease progression, in particular, disseminations of tumor cells to lymph node and bone.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
The authors would like to thank Anthony Douglas‐Jones for his assistance in histological diagnosis and the support of Cancer Research Wales.
References
- 1. CancerStats . CancerStats Incidence UK. Cancer Research UK. 2005.
- 2. Jemal A, Siegel R, Ward E et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. [DOI] [PubMed] [Google Scholar]
- 3. Ye L, Lewis‐Russell JM, Kyanaston HG, Jiang WG. Bone morphogenetic proteins and their receptor signaling in prostate cancer. Histol Histopathol 2007; 22: 1129–47. [DOI] [PubMed] [Google Scholar]
- 4. Davies SR, Watkins G, Douglas‐Jones A, Mansel RE, Jiang WG. Bone morphogenetic proteins 1 to 7 in human breast cancer, expression pattern and clinical/prognostic relevance. J Exp Ther Oncol 2008; 7: 327–38. [PubMed] [Google Scholar]
- 5. Hanavadi S, Martin TA, Watkins G, Mansel RE, Jiang WG. The role of growth differentiation factor‐9 (GDF‐9) and its analog, GDF‐9b/BMP‐15, in human breast cancer. Ann Surg Oncol 2007; 14: 2159–66. [DOI] [PubMed] [Google Scholar]
- 6. Buijs JT, Henriquez NV, Van Overveld PG et al. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res 2007; 67: 8742–51. [DOI] [PubMed] [Google Scholar]
- 7. Raida M, Clement JH, Ameri K, Han C, Leek RD, Harris AL. Expression of bone morphogenetic protein 2 in breast cancer cells inhibits hypoxic cell death. Int J Oncol 2005; 26: 1465–70. [PubMed] [Google Scholar]
- 8. Bobinac D, Maric I, Zoricic S et al. Expression of bone morphogenetic proteins in human metastatic prostate and breast cancer. Croat Med J 2005; 46: 389–96. [PubMed] [Google Scholar]
- 9. Alarmo EL, Rauta J, Kauraniemi P, Karhu R, Kuukasjarvi T, Kallioniemi A. Bone morphogenetic protein 7 is widely overexpressed in primary breast cancer. Genes Chromosomes Cancer 2006; 45: 411–9. [DOI] [PubMed] [Google Scholar]
- 10. Alarmo EL, Kuukasjarvi T, Karhu R, Kallioniemi A. A comprehensive expression survey of bone morphogenetic proteins in breast cancer highlights the importance of BMP4 and BMP7. Breast Cancer Res Treat 2007; 103: 239–46. [DOI] [PubMed] [Google Scholar]
- 11. Alarmo EL, Korhonen T, Kuukasjarvi T, Huhtala H, Holli K, Kallioniemi A. Bone morphogenetic protein 7 expression associates with bone metastasis in breast carcinomas. Ann Oncol 2008; 19: 308–14. [DOI] [PubMed] [Google Scholar]
- 12. Helms MW, Packeisen J, August C et al. First evidence supporting a potential role for the BMP/SMAD pathway in the progression of oestrogen receptor‐positive breast cancer. J Pathol 2005; 206: 366–76. [DOI] [PubMed] [Google Scholar]
- 13. Bokobza SM, Ye L, Kynaston HE, Mansel RE, Jiang WG. Reduced expression of BMPR‐IB correlates with poor prognosis and increased proliferation of breast cancer cells. Cancer Genomics Proteomics 2009; 6: 101–8. [PubMed] [Google Scholar]
- 14. Katsuno Y, Hanyu A, Kanda H et al. Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 2008; 27: 6322–33. [DOI] [PubMed] [Google Scholar]
- 15. Neuhaus H, Rosen V, Thies RS. Heart specific expression of mouse BMP‐10 a novel member of the TGF‐beta superfamily. Mech Dev 1999; 80: 181–4. [DOI] [PubMed] [Google Scholar]
- 16. Chen H, Shi S, Acosta L et al. BMP10 is essential for maintaining cardiac growth during murine cardiogenesis. Development 2004; 131: 2219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mazerbourg S, Sangkuhl K, Luo CW, Sudo S, Klein C, Hsueh AJ. Identification of receptors and signaling pathways for orphan bone morphogenetic protein/growth differentiation factor ligands based on genomic analyses. J Biol Chem 2005; 280: 32122–32. [DOI] [PubMed] [Google Scholar]
- 18. David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor‐like kinase 1 (ALK1) in endothelial cells. Blood 2007; 109: 1953–61. [DOI] [PubMed] [Google Scholar]
- 19. Ye L, Kynaston H, Jiang WG. Bone morphogenetic protein‐10 suppresses the growth and aggressiveness of prostate cancer cells through a Smad independent pathway. J Urol 2009; 181: 2749–59. [DOI] [PubMed] [Google Scholar]
- 20. Jiang WG, Martin TA, Lewis‐Russell JM, Douglas‐Jones A, Ye L, Mansel RE. Eplin‐alpha expression in human breast cancer, the impact on cellular migration and clinical outcome. Mol Cancer 2008; 7: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang WG, Davies G, Martin TA et al. Targeting matrilysin and its impact on tumor growth in vivo: the potential implications in breast cancer therapy. Clin Cancer Res 2005; 11: 6012–9. [DOI] [PubMed] [Google Scholar]
- 22. Jiang WG, Hiscox S, Hallett MB, Scott C, Horrobin DF, Puntis MC. Inhibition of hepatocyte growth factor‐induced motility and in vitro invasion of human colon cancer cells by gamma‐linolenic acid. Br J Cancer 1995; 71: 744–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosen EM, Meromsky L, Setter E, Vinter DW, Goldberg ID. Smooth muscle‐derived factor stimulates mobility of human tumor cells. Invasion Metastasis 1990; 10: 49–64. [PubMed] [Google Scholar]
- 24. Ye L, Lewis‐Russell JM, Kynaston H, Jiang WG. Endogenous bone morphogenetic protein‐7 controls the motility of prostate cancer cells through regulation of bone morphogenetic protein antagonists. J Urol 2007; 178: 1086–91. [DOI] [PubMed] [Google Scholar]
- 25. Clement JH, Sanger J, Hoffken K. Expression of bone morphogenetic protein 6 in normal mammary tissue and breast cancer cell lines and its regulation by epidermal growth factor. Int J Cancer 1999; 80: 250–6. [DOI] [PubMed] [Google Scholar]
- 26. Moreau JE, Anderson K, Mauney JR, Nguyen T, Kaplan DL, Rosenblatt M. Tissue‐engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res 2007; 67: 10304–8. [DOI] [PubMed] [Google Scholar]
- 27. Ye L, Bokobza SM, Jiang WG. Bone morphogenetic proteins in development and progression of breast cancer and therapeutic potential (review). Int J Mol Med 2009; 24: 591–7. [DOI] [PubMed] [Google Scholar]
- 28. Du J, Yang S, Wang Z et al. Bone morphogenetic protein 6 inhibit stress‐induced breast cancer cells apoptosis via both Smad and p38 pathways. J Cell Biochem 2008; 103: 1584–97. [DOI] [PubMed] [Google Scholar]
- 29. Arnold SF, Tims E, McGrath BE. Identification of bone morphogenetic proteins and their receptors in human breast cancer cell lines: importance of BMP2. Cytokine 1999; 11: 1031–7. [DOI] [PubMed] [Google Scholar]
- 30. Alarmo EL, Parssinen J, Ketolainen JM, Savinainen K, Karhu R, Kallioniemi A. BMP7 influences proliferation, migration, and invasion of breast cancer cells. Cancer Lett 2009; 275: 35–43. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi M, Otsuka F, Miyoshi T et al. Bone morphogenetic protein 6 (BMP6) and BMP7 inhibit estrogen‐induced proliferation of breast cancer cells by suppressing p38 mitogen‐activated protein kinase activation. J Endocrinol 2008; 199: 445–55. [DOI] [PubMed] [Google Scholar]
- 32. Yamamoto T, Saatcioglu F, Matsuda T. Cross‐talk between bone morphogenic proteins and estrogen receptor signaling. Endocrinology 2002; 143: 2635–42. [DOI] [PubMed] [Google Scholar]
- 33. Schwalbe M, Sanger J, Eggers R et al. Differential expression and regulation of bone morphogenetic protein 7 in breast cancer. Int J Oncol 2003; 23: 89–95. [PubMed] [Google Scholar]
- 34. Zhang M, Wang Q, Yuan W et al. Epigenetic regulation of bone morphogenetic protein‐6 gene expression in breast cancer cells. J Steroid Biochem Mol Biol 2007; 105: 91–7. [DOI] [PubMed] [Google Scholar]


