Abstract
Male rats of WBN/Kob strain are one of the diabetic model animals and develop long‐lasting diabetic symptoms and some complications from about 40 weeks of age without any treatment. A single intravenous dose of alloxan, a non‐genotoxic diabetogenic chemical, frequently induced proliferative lesions of squamous epithelium in tongue, esophagus and forestomach of male and female WBN/Kob rats, and hastened the onset and acceleration of diabetic conditions. Histopathologically, proliferative changes of squamous cell of forestomach varied with the severity of hyperplasia in alloxan‐treated rats (100% of 31 males and 94.1% of 17 females) and progressed to SCC in approximately 20% of all rats. Metastasis to regional lymph nodes was also observed in two cases. Proliferative changes were most severe in the forestomach and were constantly accompanied with chronic suppurative inflammation of the mucosal epithelium with infection of filamentous fungi and/or bacterial colonies. In contrast, forestomach of the spontaneously diabetic male rats showed only slight hyperplasia of the mucosal epithelium confined to the limiting ridge in approximately 30% of the cases. All non‐diabetic female rats showed neither proliferative changes nor the inflammatory process in the mucosa. Immunohistochemically, COX‐2 and iNOS were positive in these chronic suppurative inflammatory lesions accompanied by proliferative squamous epithelium. From these results, it is suggested that chronic inflammatory processes play an important role in the pathogenesis of alloxan‐induced SCC. An experimental system of alloxan‐induced SCC might serve as a suitable model for the study of the inflammation‐related promotion of carcinogenesis. (Cancer Sci 2006; 97: 1023–1030)
Abbreviations:
- COX‐2
cyclooxygenase‐2
- iNOS
inducible nitric oxide synthase
- SCC
squamous cell carcinoma
- ssDNA
single‐stranded DNA
- TBST
50 mM Tris‐HCl buffer (pH 7.6) with 0.03% Tween20.
WBN/Kob rats are an inbred strain of Wistar rats and have been established as one of the diabetic model animals that spontaneously develop long‐lasting diabetic symptoms in aged males.( 1 ) The diabetic conditions are characterized by low‐level blood insulin,( 2 ) and glucosuria from approximately 40 weeks of age and development of various diabetic complications such as peripheral neuropathy,( 3 , 4 ) nephropathy( 5 ) and retinopathy( 6 ) in advanced age. Furthermore, these rats had been reported to be an animal model with successful induction of gastric cancer in the lesser curvature of stomach, a common occurrence site of human gastric carcinoma, by the treatment of carcinogens.( 7 ) However, no spontaneous proliferative lesions were described in the alimentary tracts even in the older rats of this strain.
In the serial studies for the analyses of pathogenesis of diabetic complications, male and female WBN/Kob rats were given a single dose of alloxan in order to accelerate the diabetic condition. A rat autopsy 50 weeks after treatment with alloxan presented a case of SCC in the forestomach.
Alloxan is one of the chemicals that induce loss of insulin‐producing islet β‐cells and cause a hypoinsulinemic condition and resultant diabetes mellitus in animals. This effect is thought to be mediated by a sequence of redox reactions involving the production of superoxide anion radicals in or near the β‐cells.( 8 , 9 , 10 ) As a neoplastic lesion caused by alloxan injection, β‐cell tumors leading to primary injury to the pancreatic islet have been reported in a few rats,( 11 ) but there were no reports of alloxan‐induced tumors in organs other than the pancreas. Alloxan is not mutagenic, judging from the result of the Ames test, and the genotoxicity is not recognized.( 12 ) Furthermore, to the best knowledge of the authors, there are no reports on carcinogenesis from a single dose of non‐genotoxic chemicals.
Histopathological studies were carried out to clarify characteristics and distribution of the proliferative change of alimentary tracts, especially focused on the proliferative changes including carcinoma in the forestomach induced by a single dose of alloxan to WBN/Kob rats. Furthermore, we attempted immunohistochemical analysis for appearance of COX‐2 and iNOS that is induced by inflammation and reported recently to be linked with carcinogenesis, as it was frequently accompanied with inflammation around the neoplastic tissue.
In addition, we investigated the presence or absence of direct gastric mucosal injury from alloxan after observations of acute changes within the short injection period.
Materials and Methods
Animals Male and female WBN/Kob rats were obtained from Shizuoka Laboratory Animal Center (Shizuoka, Japan). They were reared in a barrier‐sustained animal room maintained at a temperature of 24 ± 2°C and a relative humidity of 60 ± 20%, with 12 h light/dark cycles and ventilated at least 12 times/h by sterilized fresh air. All rats were housed and reared in aluminum mesh cages. To protect against infection, the cages were changed once or more each week. Rats were given a pellet diet (CRF‐1; Oriental Yeast, Tokyo, Japan) and chlorinated water ad libitum.
All procedures were in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Committee for Animal Experiments of Setsunan University and the Japanese Association for Laboratory Animal Science.
Glucosuria and glycemia monitoring Urinary glucose levels were measured semiquantitatively, using a urine test paper (Wako Pure Chemical Industries, Osaka, Japan) every day from day 1 to day 3 after dosing, once every week for 1 month after the first week, and once every month thereafter from the fresh urine obtained from alloxan‐treated rats. Blood glucose levels were also measured semiquantitatively by the glucose oxidase method (Glutest E; Sanwakagaku, Aichi, Japan) once every month from the fourth week after dosing, using blood samples from the tail vein. Urinary and blood glucose levels from untreated male WBN/Kob rats were measured once every month after 40 weeks of age. Samples of blood from the tail vein and fresh urine were collected from 13.00 to 16.00.
Experimental design
Long‐term test The experimental design is shown in Figure 1. A total of 85 rats were divided into four groups. Thirty‐one male and 17 female rats, aged 7–36 weeks, were given a single dose of alloxan (Sigma‐Aldrich Japan, Tokyo, Japan) through the tail vein at dosage levels of 50 and 40 mg/kg body weight, respectively. These concentrations were set up as a given dose which a rat survives for a long period of time after diabetes symptoms, and induces glucosuria continuously. Twenty‐four non‐treated male rats that were confirmed to show diabetic conditions, aged 100 weeks, and 13 female rats of the same age were used as controls.
Figure 1.
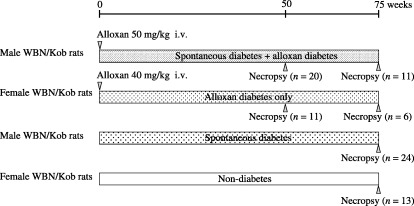
Experimental protocol, showing timeline of alloxan treatment in WBN/Kob rats with and without spontaneous diabetes.
Alloxan‐treated rats were killed for full pathologic examination at 50 or 75 weeks after dosing and non‐treated age‐matched rats were killed at 75–113 weeks.
Short‐term test To assess the early histopathological changes in the mucosa of forestomach and damages in the nuclear DNA of epithelial cells, a short‐term test was designed in which 40 male and 40 female rats (aged 7 weeks) were used. They were given a single dose of alloxan through the tail vein at a dosage level of 40 mg/kg body weight, and were killed at 0, 1, 3, 6, 12, 24, 72 and 120 h after dosing (n = 5 on each term). The concentrations was enough to given dose which a rat survives and induces glucosuria.
Histopathological analysis All rats were killed by exsanguination from the abdominal aorta under deep ether anesthesia at the end of each scheduled period, or when they began to show a moribund condition. The entire alimentary tract was immediately removed following autopsy. The organs of 64 rats were fixed by immersion in 10% phosphate‐buffered formalin solution immediately after necropsy, and the other rats were treated by cardiac perfusion with phosphate‐buffered 4% paraformaldehyde and 2% glutaraldehyde solution (pH 7.4) after being perfused with Ringer's solution. After perfusion, all organs were removed and immersed in 10% phosphate‐buffered neutral formalin like those in the other cases.
Fixed organs were trimmed, dehydrated by automated processor, and embedded in paraffin. Sections (4 µM thick) of tissue specimens were stained with hematoxylin–eosin for histopathological examination.
The severity of proliferative lesions in forestomach squamous epithelium was judged from the thickness of the mucosal epithelium and estimated to grade into five stages as follows: –, equivalent with control; +, slight change; + +, moderate change; + + +, severe change; + + + +, significantly severe change.
Immunohistochemical analysis The expressions of COX‐2 and iNOS were examined by immunohistochemistry for sections containing carcinomatous tissue. In addition, immunohistochemistry for ssDNA and Ki‐67 were examined in forestomach sections of rats from the short‐term test. Routinely processed formalin‐fixed paraffin‐embedded tissue specimens were cut into 4 µm thick sections. The sections were deparaffinized in xylene, and rehydrated through graded ethanol at room temperature. Rehydrated sections were autoclaved in 10 mM citrate buffer (pH 6.0) for 15 min at 121°C to Ki‐67 antigen activation, microwaved in 10 mM citrate buffer (pH 6.0) for 20 min to iNOS antigen activation and digested by pepsin for 20 min at 37°C to COX‐2 antigen activation.
TBST was used to prepare solutions and washes between various steps. Nonspecific endogenous peroxidase activity was blocked by exposure to 0.03% hydrogen peroxide in methanol for 5 min, and masking was conducted with 1% bovine serum albumin or 5% normal goat serum in phosphate‐buffered saline for 5 min at room temperature. Incubation was carried out with primary antibodies, such as anti‐ssDNA rabbit polyclonal antibodies (diluted 1:1000, A4506; DakoCytomation, Kyoto, Japan), anti‐Ki67 mouse monoclonal antibodies (Clone MIB‐5, diluted 1:100, M7248; DakoCytomation), anti‐COX‐2 mouse monoclonal antibodies (diluted 1:200, C22420; BD Transduction Laboratories, USA), and anti‐iNOS rabbit polyclonal antibodies (diluted 1:8000, 017–16001; Wako Pure Chemical Industries), overnight at 4°C. Color was developed using 3,3′‐diaminobenzidine, and counterstaining was carried out with Mayer's hematoxylin. The ssDNA expression was estimated by the number of positive cells in a section of tissue (the total length was 20–80 mm). Ki‐67 was estimated by Student's t‐test (significance level 5%). The expressions of COX‐2 and iNOS were estimated to grade into four stages as follows: negative, (–) and slight (+) to strong (+ + + +) expression.
Double stain for COX‐2/vimentin and COX‐2/ED‐1 by immunohistochemistry Double staining for COX‐2 and vimentin or COX‐2 and ED‐1 was carried out to confirm the location of COX‐2 expression. The sections for double staining were deparaffinized in xylene, and rehydrated through graded ethanol at room temperature. Rehydrated sections were washed with distilled water and TBST and digested by pepsin for 20 min at 37°C after rinsing by TBST. Nonspecific endogenous peroxidase activity was blocked by exposure to 0.03% hydrogen peroxide in methanol for 5 min, and masking was conducted with 10% normal goat serum in phosphate‐buffered saline for 5 min at room temperature. First, these sections were incubated with anti‐COX‐2 mouse monoclonal antibodies at 1:200, overnight at 4°C, and colored to brown by 3,3′‐diaminobenzidine. After washing in distilled water, they were immediately microwaved in 10 mM citrate buffer (pH 6.0) for 5 min twice. Second, microwaved sections were incubated with anti‐vimentin mouse monoclonal antibodies (1:100, M0725; DakoCytomation) or anti‐rat ED‐1 mouse monoclonal antibodies (1:500, MCA341; Serotec) for 30 min at room temperature. Finally, the incubated sections were reacted to alkaline phosphatase for 30 min at room temperature and colored to red by fuchsin solution (K0698; DakoCytomation). After washing, counterstaining was carried out with Mayer's hematoxylin.
Microbiological evaluation Special staining methods for the general pathogens such as Gram, Giemsa, Grocott and periodic acid Schiff were carried out on representative sections of forestomach containing microorganisms. Identification of microbes was also attempted by culture of the gastric mucosa from WBN/Kob rats 50 weeks after the dosing of alloxan. The animals were fasted for 16 h before killing by exsanguination from the abdominal aorta under ether anesthesia, and the epicardia and pyloric orifice were ligated. The entire stomach was immediately removed, and was injected with 3 mL of sterilized saline. Shaken and suspended gastric contents were extracted to a sterilized tube. The samples were diluted 10 times, inoculated into brain heart infusion agar (Eiken Chemical Co., Tokyo, Japan), nutrient agar, MacConkey agar and potato dextrose agar (Nissui Pharmaceutical Co., Tokyo, Japan) and cultivated at 35°C or 25°C.
Statistical analysis Fisher's exact test and the unpaired Student's t‐test was used for statistical analysis of the frequency data and the body weight data, respectively.
Results
Glucosuria, glycemia and general condition monitoring Severe hyperglycemia (>327 mg/dL) and glucosuria (>500 mg/dL) continued for approximately 30–75 weeks from the day after injection to the time of scheduled necropsy or death in all alloxan‐treated WBN/Kob rats. Nine male and three female rats died within 29–75 weeks of dosing. Moderate to severe hyperglycemia ( 200 mg/dL) and glucosuria (>500 mg/dL) were also observed in the control male WBN/Kob rats for 10–57 weeks from approximately 50 weeks of age.
The body weights of alloxan‐treated male and female rats decreased sharply within several days of injection, and their final body weights were significantly decreased in 77.8% and 52%, respectively, compared with non‐treated WBN/Kob rats. The last average body weights of the alloxan‐treated male rats, alloxan‐treated female rats, non‐treated male rats and non‐treated female rats at approximately 100 weeks of age were 298.6 g, 383.6 g, 142.2 g and 273.3 g, respectively. Moreover, the appearance of alloxan‐treated rats was remarkable for their emaciation and rough hair state, compared with the non‐treated WBN/Kob rats.
Histopathology Hyperplasia of mucosal squamous epithelium was detected in the forestomach of alloxan‐treated male and female rats (100% and 94.1%, respectively) and evolved to SCC in six males (19.4%) and four females (23.5%) (Table 1). The stratified epithelium under hyperplasia increased its height and cell layer with a thickening of the cornified layer. Connective tissue stalk was elongated according to the increased thickness of the epithelial layer, but papillary protrusion of mucosal surface was inconspicuous (Fig. 2a). Hyperplastic change was more prominent in greater curvature than lesser curvature and most prominent in the side of the limiting ridge. The degree of hyperplasia began to be milder in areas more remote from the limiting ridge in approximately 70% of the animals (Table 2). Furthermore, hyperplastic changes in each area were more severe in the cases that survived for longer periods from the alloxan treatment (Table 1). In the case with severe hyperplasia, proliferated squamous cells in the basal layer invaded into the lamina propria, and invaded squamous cells occasionally formed keratinous cysts in the submucosa (Fig. 2b,c).
Table 1.
Frequency of lesions in forestomach in WBN/Kob rats treated with a single dose of alloxan
| Weeks after treatment Sex | Alloxan‐treated group | Untreated group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < 40 | 50 (±10) | 70 (±10) | Total | ||||||||
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | ||
| Animals | n | 6 | 3 | 14 | 8 | 11 | 6 | 31 | 17 | 24 | 13 |
| Squamous cell hyperplasia | n (%) | 6 (100.0)** | 3 (100.0)**** | 14 (100.0)** | 8 (100.0)**** | 11 (100.0)** | 5 (83.3)**** | 31 (100.0)** | 16 (94.1)**** | 7 (29.2) | 0 (0.0) |
| – | n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (5.9) | 17 (70.8) | 13 (100.0) |
| + | n (%) | 0 (0.0) | 1 (33.3) | 2 (14.3) | 2 (25.0) | 2 (18.2) | 0 (0.0) | 4 (12.9) | 3 (17.6) | 5 (20.8) | 0 (0.0) |
| + + | n (%) | 3 (50.0) | 1 (33.3) | 5 (37.5) | 3 (37.5) | 1 (9.1) | 0 (0.0) | 9 (29.0) | 4 (23.5) | 2 (8.3) | 0 (0.0) |
| + + + | n (%) | 2 (33.3) | 1 (33.3) | 6 (42.9) | 2 (25.0) | 4 (36.4) | 2 (33.3) | 12 (38.7) | 5 (29.4) | 0 (0.0) | 0 (0.0) |
| + + + + | n (%) | 1 (16.7) | 0 (0.0) | 1 (7.1) | 1 (12.5) | 4 (36.4) | 3 (50.0) | 6 (19.4) | 4 (23.5) | 0 (0.0) | 0 (0.0) |
| SCC | n (%) | 1 (16.7) | 0 (0.0) | 2 (14.3) | 1 (12.5) | 3 (27.3)* | 3 (50.0)*** | 6 (19.4)* | 4 (23.5) | 0 (0.0) | 0 (0.0) |
| Adenosquamous carcinoma | n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 1 (3.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Metastasis to lymph node | n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 1 (16.7) | 1 (3.2) | 1 (5.9) | 0 (0.0) | 0 (0.0) |
| Accumulation of neutrophils | n (%) | 6 (100.0)** | 2 (66.7)*** | 13 (92.9)** | 6 (75.0)**** | 8 (72.7)* | 5 (83.3)**** | 27 (87.1)** | 13 (76.5)**** | 7 (29.2) | 0 (0.0) |
| Lymphocyte and plasma cell infiltration in submucosa | n (%) | 6 (100.0)** | 3 (100.0)**** | 14 (100.0)** | 8 (100.0)**** | 11 (100.0)** | 5 (83.3)**** | 31 (100.0)** | 16 (94.1)**** | 6 (25.0) | 0 (0.0) |
| Ulceration or erosion | n (%) | 3 (50.0) | 1 (33.3) | 13 (92.9)** | 5 (62.5)**** | 8 (72.7)** | 5 (83.3)**** | 24 (77.4)** | 11 (64.7)**** | 3 (12.5) | 0 (0.0) |
| Bacterial infection | n (%) | 4 (66.7) | 1 (33.3) | 14 (100.0)** | 8 (100.0)**** | 11 (100.0)** | 5 (83.3)**** | 29 (93.5)** | 14 (82.4)**** | 6 (25.0) | 0 (0.0) |
| Fungal infection | n (%) | 4 (66.7) | 1.(33.3) | 13 (92.9)** | 6 (75.0)**** | 8 (72.7)** | 4 (66.7)**** | 25 (80.6)** | 11 (64.7)**** | 3 (12.5) | 0 (0.0) |
P < 0.05,
P < 0.01 compared to male rats of untreated group;
P < 0.05,
P < 0.01 compared to female rats of untreated group. +, slight hyperplasia; + +, moderate hyperplasia; + + +, severe hyperplasia; + + + +, significantly severe hyperplasia.
Figure 2.
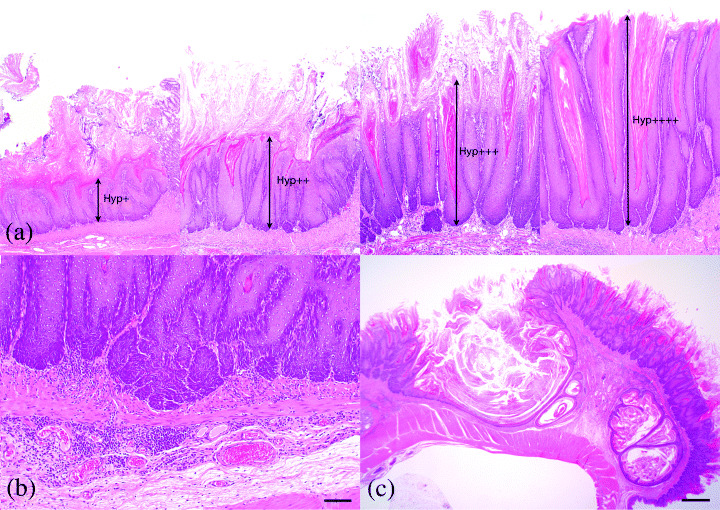
Hyperplasia of squamous cell epithelium in forestomach. (a) Hyperplastic epithelium of the forestomach increased its cell layer with hyperkeratosis, but papillary protruding into the lumen was not detected. Hyp+, slight hyperplasia; Hyp+ +, moderate hyperplasia; Hyp+ + +, severe hyperplasia; Hyp+ + + +, significantly severe hyperplasia. (b) Invagination of basal layer of squamous epithelium into the lamina propria (Bar: 50 µm). (c) Keratinous cyst formation in submucosa. The frequency of cyst formation increased with age (Bar: 1 m).
Table 2.
Proliferative change of squamous cells in forestomach of WBN/Kob rats treated with a single dose of alloxan
| Lesser curvature | Greater curvature | ||
|---|---|---|---|
| Observation of forestomach section | n | 47 | 45 |
| Lesion and the extent | |||
| Squamous cell hyperplasia | n (%) | 46 (97.9) | 40 (88.9) |
| – | n (%) | 1 (2.1) | 5 (11.1) |
| + | n (%) | 7 (14.9) | 8 (17.8) |
| + + | n (%) | 14 (29.8) | 14 (31.1) |
| + + + | n (%) | 19 (40.4) | 12 (26.7) |
| + + + + | n (%) | 6 (12.8) | 6 (13.3) |
| Invagination of squamous cells into submucosal tissus | n (%) | 21 (44.7) | 14 (31.1) |
| – | n (%) | 26 (55.3) | 31 (68.9) |
| + | n (%) | 4 (8.5) | 3 (6.7) |
| + + | n (%) | 3 (6.4) | 2 (4.4) |
| + + + | n (%) | 6 (12.8) | 3 (6.7) |
| + + + + | n (%) | 8 (17.0) | 6 (13.3) |
| SCC | n (%) | 7 (14.9) | 7 (15.6) |
| Region | |||
| Limiting ridge and environs | n (%) | 32 (68.1) | 32 (71.1) |
| Throughout forestomach | n (%) | 14 (29.8) | 13 (28.9) |
–, equivalent with control; +, slight change; + +, moderate change; + + +, severe change; + + + +, significantly severe change.
Approximately 20% of the animals had SCC in the forestomach. All cases were diagnosed as well‐differentiated SCC (Table 1, Fig. 3a,b), and one case also included adenosquamous cell carcinoma (Table 1, Fig. 3c). A mass of carcinomas developed in various areas. They were localized on only one side of the limiting ridge (20% of rats with SCC), or parts away from the limiting ridge in forestomach (50%) and extended all around the forestomach (30%). Tumor cells invaded surrounding tissue and reached the muscularis mucosa in 80% of rats with SCC and/or to the muscular layer in 20%. Metastasis to the regional lymphonodus was also observed in two cases (Table 1, Fig. 3d).
Figure 3.
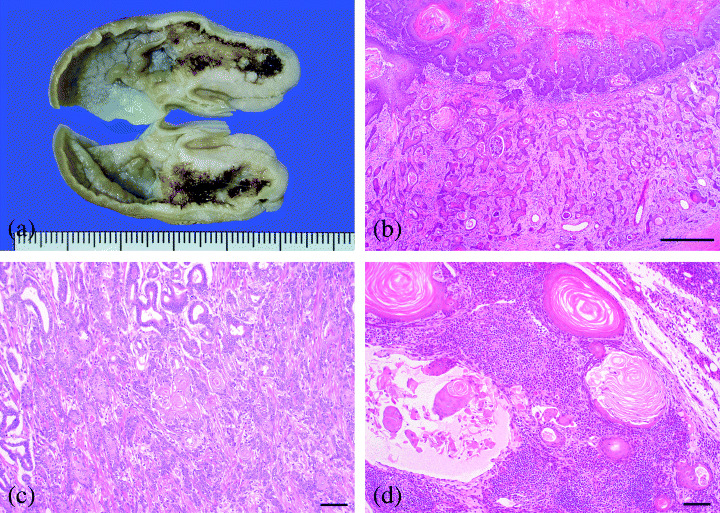
SCC in forestomach. (a) Gross findings of gastric mucosa after fixation. Note irregular thickening of gastric wall in lesser curvature. (b) SCC was well‐differentiated but invaded diffusely into submucosal layer (Bar: 500 µm). (c) Adenosquamous cell carcinoma with invasion to muscular and serosal layer (Bar: 100 µm). (d) Metastasis of SCC to the pancreaticoduodenal lymph node (Bar: 100 µm).
Accumulation of neutrophils and infiltration of lymphocytes were observed in 83.3% of the animals, invariably accompanied with proliferative changes and erosion or ulceration of the mucosal epithelium in forestomach (Table 1, Fig. 4a). Inflammatory cells mainly consisted of plasma cells and round mononuclear cells that infiltrated the underlying mucosal propria in 97.9% of rats (Table 1, Fig. 4b). Furthermore, filamentous fungi showing dimorphism such as yeast form and mycelial form (75.0%) and/or rod‐shaped bacterial (89.6%) colonies were also frequent in the superficial mucosal layer (Table 1).
Figure 4.
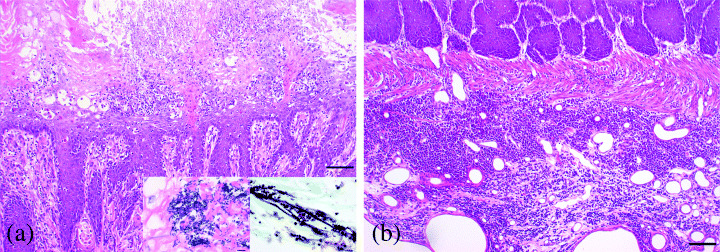
Acute and chronic inflammatory changes with bacterial or mycotic infection in the mucosa of forestomach. (a) Acute inflammatory change in superficial mucous membrane characterized by emigration of neutrophils in erosive and ulcerative epithelium and microbial infection (Bar: 100 µm). Inset: Left micrograph is Escherichia coli (Giemsa stain), right micrograph is Candida albicans (Grocott stain). (b) Chronic inflammatory change with severe infiltration of lymphocytes and plasma cells into the lamina propria and deep submucosal layer (Bar: 100 µm).
In contrast, forestomach of the control male rats showed only slight hyperplasia of the mucosal epithelium confined to the limiting ridge in 29.2% of cases. All non‐diabetic female rats showed neither proliferative nor inflammatory changes in the mucosa, nor microbe infection in the forestomach (Table 1).
In organs other than forestomach, the proliferative change of mucosal squamous epithelium accompanied by inflammation in alloxan‐treated male and female rats was observed in the central part of tongue (94.4% and 83.3%, respectively), hard palate (75.0% and 71.4%, respectively), gingiva (100.0% and 85.7%, respectively) and posterior parts of esophagus (88.2% and 90.0%, respectively). Some animals also had SCC in palate (one case), gingiva (two cases) and/or posterior parts of esophagus (one case).
Microbiological evaluation From the results of special staining for pathogens, these bacteria appeared to be large and/or small‐sized rod‐shaped Gram‐positive bacillus and Gram‐negative bacillus. By bacterial culture, they were identified as Lactobacillus sp., Bacillus licheniformis and Escherichia coli, respectively (Fig. 4a). Fungi showing dimorphism such as yeast and mycelial forms were identified as Candida albicans (Fig. 4a).
Immunohistochemical analysis COX‐2 was strongly positive in the cytoplasm of the spindle mesenchymal cells and/or mononuclear spherical cells that infiltrated the lamina propria near the lumen without any topographic relation to the neoplastic transformation in the alloxan‐treated rats (Fig. 5a,b). By the double staining immunohistochemistry for COX‐2 and vimentin or ED‐1, the cytoplasmic surfaces of spindle‐shaped mesenchymal cells showing positive for COX‐2 were also positive for vimentin, and mononuclear spherical cells were positive for ED‐1. From the results of double staining, COX‐2 expression was confirmed to be positive in the cytoplasm of fibroblasts and macrophages (Fig. 5c,d). The number of positive cells consistently increased in edematous or fibrotic lamina propria, but intensity and localization of expression in individual cells were comparable throughout the mucosal propria (Fig. 5a,b). In the epithelial layer adjacent to a purulent inflammatory area undergoing erosive or ulcerative changes, COX‐2 expression was also weakly positive in the cytoplasm of some epithelial cells (Fig. 5a). In contrast, all neoplastic epithelial cells were negative. In the control rats, COX‐2 was weakly positive for inflammatory cells infiltrated into the lamina propria at the side of the limiting ridge in the forestomach.
Figure 5.
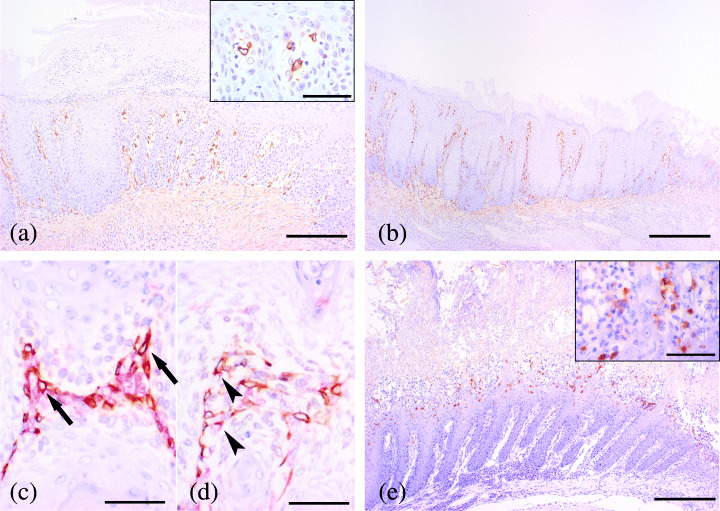
Immunohistochemistry for COX‐2 and iNOS. (a) COX‐2 expression in mucosal epithelial cells with suppurative inflammation (Bar: 200 µm). Inset: Large magnification of positive cells (Bar: 50 µm). (b) COX‐2 expression in mucosal epithelial cells without inflammation. The degree of overexpression was the same as in the non‐inflammatory area (Bar: 200 µm). (c) Double staining for COX‐2 and vimentin. Vimentin was dyed red in the membrane of fibroblast, and COX‐2 was dyed brown in cytoplasm. Double‐positive cells for COX‐2 and vimentin were considered to be fibroblasts. (d) Double staining for COX‐2 and ED‐1. ED‐1 was dyed red in the membrane of macrophage, and COX‐2 was dyed brown in cytoplasm. Double‐positive cells for COX‐2 and ED1 were considered to be macrophages. (e) iNOS was positive in cytoplasm of mononuclear inflammatory cells in suppurative inflammatory lesion (Bar: 200 µm). Inset: A large magnification of positive cells (Bar: 50 µm).
iNOS was positive for cytoplasm of inflammatory cells infiltrating into the epithelium of the suppurative inflammatory lesion in the alloxan‐treated rats, but was negative for epithelial cells, infiltrated cells and mesenchymal cells in neoplastic areas (Fig. 4e). In the control rats, iNOS expression was negative in all kinds of cells composing the forestomach.
Short‐term test Microscopically, no significant changes were observed in the forestomach mucosa of any rats in the alloxan‐treated and control groups. Time course changes were not detected until 120 h after dosing. Immunohistochemically, ssDNA and Ki‐67 expressions did not change with the time course in the nuclei of mucosal epithelial cells of forestomach in any alloxan‐treated rats.
Discussion
The present studies demonstrated that a single intravenous injection of alloxan caused proliferative lesions in squamous epithelium associated with inflammatory changes in the forestomach of WBN/Kob rats, and that lesions of the forestomach mucosa progress to SCC in some rats. Slight proliferative changes of forestomach mucosa were also detected in the non‐treated male WBN/Kob rats at advanced ages after a long‐lasting diabetic condition. Female WBN/Kob rats also experienced similar proliferative changes in the forestomach by a single intravenous treatment of alloxan, although no proliferative lesions were detected spontaneously, even in the older animals. These facts suggest that proliferative changes in forestomach mucosa are highly related to alloxan treatment or persistent diabetic conditions, although it is unclear whether such changes were direct effects of the alloxan injection or secondary factors owing to the long‐lasting diabetic condition.
In the forestomach of the rat, SCC has been induced by N‐nitroso compounds that are well known as genotoxic carcinogens, such as N‐methyl‐N′‐nitro‐N‐nitrosoguanidine and N‐methylnitrosourethane.( 13 ) SCC induced by these carcinogens are reported to follow a process of initially simple hyperplasia, subsequent papillary or tuberous hyperplasia and papilloma before the formation of carcinoma tissue.( 14 ) In the mucosal epithelia of rats in the present study, papillomatous proliferation or papilloma was not observed among the proliferating mucosa and directly progressed to invasive carcinoma with metastasis from the simple hyperplasia with increased cell layers. Therefore, the morphologic character of proliferative changes in various stages during the progress of alloxan‐induced SCC was clearly different from the carcinogenetic process by genotoxic chemicals. Morphological changes reflecting mucosal injury and immunohistochemical findings suggesting the increased rate of injured DNA were frequently seen shortly after treatment with a genotoxic chemical carcinogen such as N‐methylnitrosourea.( 15 ) However, no significant changes were noted in the forestomach mucosa a short time after the alloxan dosing. Moreover, unlike those seen in the forestomach shortly after treatment with genotoxic carcinogen, the positive rates of ssDNA and Ki‐67 were not increased.
In the rat forestomach, non‐genotoxic carcinogens such as butylated hydroxytoluene, butylated hydroxyanisole and caffeic acid have been reported to have carcinogenic potential.( 13 , 16 ) However, these compounds generally need long‐term exposure to show the promoter effect. SCC in this study was induced by only a single dose of alloxan. In addition, any pathological change is not formed until 120 h after treatment and the half‐life of alloxan is short.( 17 ) It is therefore unlikely that alloxan induced carcinomas by the promoter effect. Also, from the common understanding of carcinogenic mechanisms triggered by chemical compounds, secondary factors due to diabetic conditions are suspected to be deeply involved in the onset mechanism rather than being the direct effect of alloxan.
Recently, it has been frequently reported that diabetes mellitus is one of the risk factors for carcinogenesis.( 18 ) The carcinogenic mechanism in patients with diabetes mellitus remains unclear, and hyperinsulinemia is thought to increase the risk of developing neoplasms at several anatomically distinct sites. The relationship between an excess amount of insulin and cancer was supported by in vivo, animal and human epidemiologic studies.( 19 ) However, the WBN/Kob rats we used in this study are characterized by a low blood insulin level.( 2 )
Inflammatory changes were constantly observed in the forestomach with bacterial and fungal infections along with the proliferative epithelial cells. Although the clear cause of the exaggerated infectious sensitivity on the microbe in the forestomach is unknown in this study, it is well known that diabetic patients tend to induce the inflammation of the skin and internal organs by easily infectious conditions.( 20 ) Any inflammatory change was not formed until 120 h after alloxan treatment. Therefore, bacterial/fungal infection in this study is likely caused by the long‐standing diabetes condition. Recently, special attention has been focused on the inflammation‐related promotion of gastrointestinal cancer such as colorectal cancer, hepatic cancer and gastric cancer in humans,( 21 , 22 , 23 , 24 ) and it was proven that chronic inflammation causes malignant tumor. Many kinds of cytokines and free radicals generated by the inflammatory process are considered to involve the carcinogenesis of gastrointestinal mucosa. Among the many inflammation‐related changes, expression of COX‐2 and iNOS is a main target of investigators because of accumulating data demonstrating the overexpression of both inducible COX‐2 and iNOS in many epithelial neoplasias.( 25 )
Overexpression of COX‐2 is frequently detected in gastric cancer and colorectal cancer, and is deeply involved in tumorigenesis.( 26 , 27 ) Moreover, overexpression of COX‐2 was detected in fibroblasts or macrophages in benign colon tumors.( 28 ) COX‐2 overexpression with increased proliferating cell nuclear antigen‐positive cells was also described in hyperplastic epithelial lesions of the rat forestomach induced by butylated hydroxyanisole or caffeic acid treatment,( 13 ) suggesting that non‐genotoxic carcinogenesis is characterized by the induction of severe cell proliferation as well as toxicity‐dependent changes such as inflammation, erosion and ulceration in the forestomach during chemical treatment.( 29 ) In the present study, COX‐2 was positive for fibroblasts and macrophages in the lamina propria beneath the proliferating epithelial cells, but was negative in the epithelial cells of carcinoma cells, unlike those in colon cancer in humans.( 28 ) COX‐2 expression was weakly positive in epithelial cells adjacent to a purulent inflammatory area undergoing erosion and ulceration. Therefore, it is suggested that the COX‐2 expression was related to inflammation and proliferation in superficial mucous membrane in the present study.
iNOS expression has been observed in infiltrated inflammatory cells, vascular endothelium in inflammatory lesions or precancerous lesions of the alimentary tract.( 30 , 31 ) It is thought to play an important role in tumorigenesis by the acceleration of angiogenesis and vascular permeability.( 32 ) However, there was no apparent correlation between the sites of iNOS expression and neoplastic changes in the forestomach where the chronic inflammatory changes were severe in the underlying propria in our rats. The NO produced by iNOS was suggested to express an anti‐infective action due to the pathogenic microbe energy metabolism and inhibition of nucleic acid biosynthesis.( 33 ) Overexpressed iNOS in our rats was likely generated by the infection of fungus and/or bacterium, but its principal contribution to the neoplastic changes remains unclear. The infection of C. albicans is more important for carcinogenesis, because it reportedly has a promoter function in relation to the malignant tumor.( 34 )
This is the first report of successful induction of SCC by a single intravascular dose of the non‐genotoxic chemical, alloxan. This promising model might be useful for the study of inflammation‐related promotion of carcinogenesis, although further study is needed to clarify the mechanism of carcinogenesis.
References
- 1. Tsuchitani M, Saegusa T, Narama I et al. A new diabetic strain of rat (WBN/Kob). Lab Anim 1985; 19: 200–7. [DOI] [PubMed] [Google Scholar]
- 2. Nakama K, Shichinohe K, Kobayashi K et al. Spontaneous diabetes‐like syndrome in WBN/KOB rats. Acta Diabetol Lat 1985; 22: 335–42. [DOI] [PubMed] [Google Scholar]
- 3. Narama I, Kino I. Peripheral motor neuropathy in spontaneously diabetic WBN/Kob rats: a morphometric and electron microscopic study. Acta Neuropathol (Berl) 1989; 79: 52–60. [DOI] [PubMed] [Google Scholar]
- 4. Ozaki K, Miura K, Tsuchitani M et al. Peripheral neuropathy in the spontaneously diabetic WBN/Kob rat. Acta Neuropathol (Berl) 1996; 92: 603–7. [DOI] [PubMed] [Google Scholar]
- 5. Ishizaki M, Masuda Y, Fukuda Y et al. Renal lesions in a strain of spontaneously diabetic WBN/Kob rats. Acta Diabetol Lat 1987; 24: 27–35. [DOI] [PubMed] [Google Scholar]
- 6. Matsuura T, Horikiri K, Ozaki K et al. Proliferative retinal changes in diabetic rats (WBN/Kob). Lab Anim Sci 1999; 49: 565–9. [PubMed] [Google Scholar]
- 7. Kobori O, Gedigk P, Totovic V. Adenomatous changes and adenocarcinoma of glandular stomach in Wistar rats induced by N‐methyl‐N"‐nitro‐N‐nitrosoguanidine. An electron microscopic and histochemical study. Virchows Arch A Pathol Anat Histol 1977; 373: 37–54. [DOI] [PubMed] [Google Scholar]
- 8. Heikkila RE, Cabbat ES. Protection against alloxan‐induced diabetes in mice by the hydroxyl radical scavenger dimethylurea. Eur J Pharmacol 1978; 52: 57–60. [DOI] [PubMed] [Google Scholar]
- 9. Grankvist K, Marklund S, Taljedal IB. Superoxide dismutase is a prophylactic against alloxan diabetes. Nature 1981; 294: 158–60. [DOI] [PubMed] [Google Scholar]
- 10. Jorns A, Tiedge M, Lenzen S et al. Effect of superoxide dismutase, catalase, chelating agents, and free radical scavengers on the toxicity of alloxan to isolated pancreatic islets in vitro . Free Radic Biol Med 1999; 26: 1300–4. [DOI] [PubMed] [Google Scholar]
- 11. Yamagami T, Miwa A, Takasawa S et al. Induction of rat pancreatic B‐cell tumors by the combined administration of streptozotocin or alloxan and poly (adenosine diphosphate ribose) synthetase inhibitors. Cancer Res 1985; 45: 1845–9. [PubMed] [Google Scholar]
- 12. Zeiger E, Anderson B, Haworth S et al. Salmonella mutagenicity tests: V. Results from the testing of 311 chemicals. Environ Mol Mutagen 1992; 19 (Suppl. 21): 2–141. [DOI] [PubMed] [Google Scholar]
- 13. Kaneko M, Morimura K, Nishikawa T et al. Different genetic alterations in rat forestomach tumors induced by genotoxic and non‐genotoxic carcinogens. Carcinogenesis 2002; 23: 1729–35. [DOI] [PubMed] [Google Scholar]
- 14. Kroes R, Wester PW. Forestomach carcinogens: possible mechanisms of action. Food Chem Toxicol 1986; 24: 1083–9. [DOI] [PubMed] [Google Scholar]
- 15. Furihata C, Ikui E, Matsushima T. DNA damaging and cell proliferative activity of 1‐methyl‐1‐nitrosourea in rat glandular stomach mucosa. Mutat Res 1995; 348: 169–73. [DOI] [PubMed] [Google Scholar]
- 16. Hirose M, Masuda A, Imaida K et al. Induction of forestomach lesions in rats by oral administrations of naturally occurring antioxidants for 4 weeks. Jpn J Cancer Res 1987; 78: 317–21. [PubMed] [Google Scholar]
- 17. Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res 2001; 50: 537–46. [PubMed] [Google Scholar]
- 18. Strickler HD, Wylie‐Rosett J, Rohan T et al. The relation of type 2 diabetes and cancer. Diabetes Technol Ther 2001; 3: 263–74. [DOI] [PubMed] [Google Scholar]
- 19. Gupta K, Krishnaswamy G, Karnad A et al. Insulin: a novel factor in carcinogenesis. Am J Med Sci 2002; 323: 140–5. [DOI] [PubMed] [Google Scholar]
- 20. Sentochnik DE, Eliopoulos GM. Infection and diabetes. In: Kahn CR, Weir GC, eds. Joslin's Diabetes Mellitus. Philadelphia: Lea & Febiger, 1994. [Google Scholar]
- 21. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420: 860–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishikawa M, Nishiguchi S, Shiomi S et al. Somatic mutation of mitochondrial DNA in cancerous and noncancerous liver tissue in individuals with hepatocellular carcinoma. Cancer Res 2001; 61: 1843–5. [PubMed] [Google Scholar]
- 23. Ekbom A, Helmick C, Zack M et al. Ulcerative colitis and colorectal cancer. A population‐based study. N Engl J Med 1990; 323: 1228–33. [DOI] [PubMed] [Google Scholar]
- 24. Shimizu N, Ikehara Y, Inada K et al. Eradication diminishes enhancing effects of Helicobacter pylori infection on glandular stomach carcinogenesis in Mongolian gerbils. Cancer Res 2000; 60: 1512–4. [PubMed] [Google Scholar]
- 25. Marrogi A, Pass HI, Khan M et al. Human mesothelioma samples overexpress both cyclooxygenase‐2 (COX‐2) and inducible nitric oxide synthase (NOS2): in vitro antiproliferative effects of a COX‐2 inhibitor. Cancer Res 2000; 60: 3696–700. [PubMed] [Google Scholar]
- 26. Hu PJYuJ, Zeng ZR et al. Chemoprevention of gastric cancer by celecoxib in rats. Gut 2004; 53: 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reddy BS, Kawamori T, Lubet RA et al. Chemopreventive efficacy of sulindac sulfone against colon cancer depends on time of administration during carcinogenic process. Cancer Res 1999; 59: 3387–91. [PubMed] [Google Scholar]
- 28. Sano H, Kawahito Y, Wilder RL et al. Expression of cyclooxygenase‐1 and ‐2 in human colorectal cancer. Cancer Res 1995; 55: 3785–9. [PubMed] [Google Scholar]
- 29. Rodrigues C, Lok E, Nera E et al. Short‐term effects of various phenols and acids on the Fischer 344 male rat forestomach epithelium. Toxicology 1986; 38: 103–17. [DOI] [PubMed] [Google Scholar]
- 30. Mannick EE, Bravo LE, Zarama G et al. Inducible nitric oxide synthase, nitrotyrosine, and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res 1996; 56: 3238–43. [PubMed] [Google Scholar]
- 31. Singer II, Kawka DW, Scott S et al. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 1996; 111: 871–85. [DOI] [PubMed] [Google Scholar]
- 32. Ambs S, Merriam WG, Ogunfusika MO et al. p53 and vascular endothelial growth factor regulate tumor growth of NOS2‐expressing human carcinoma cells. Nat Med 1998; 4: 1371–6. [DOI] [PubMed] [Google Scholar]
- 33. Nathan CF, Hibbs JB Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 1991; 3: 65–70. [DOI] [PubMed] [Google Scholar]
- 34. O’Grady JF, Reade PC. Candida albicans as a promoter of oral mucosal neoplasia. Carcinogenesis 1992; 13: 783–6. [DOI] [PubMed] [Google Scholar]


