Abstract
Tumor‐related angiogenesis is likely to be a potential target for the treatment of cancer. One key to develop this angiostatic strategy would be to find useful angiogenesis inhibitors. Here we report the effects of radicicol, a microbial angiogenesis inhibitor that we previously identified using the chorioallantoic membrane assay, and its novel analog, 14,16‐dipalmitoyl‐radicicol, on tumor angiogenesis and growth. As expected for agents containing a penolic hydroxyl group, systemic administration of radicicol had little or no effect on neovascularization triggered by a M5076 mouse tumor cell line or a RMT‐1 rat mammary carcinoma cell line established from autochthonous rat mammary tumors induced by 7,12‐dimethylbenz[a]anthracene in a mouse dorsal air sac assay system. The agent did not show growth‐inhibitory activity against either transplantable M5076 tumors or autochthonous 7,12‐dimethylbenz[a]anthracene‐induced rat mammary tumors. In contrast, 14,16‐dipalmitoyl‐radicicol potently suppressed tumor angiogenesis and growth in these experimental models. Furthermore, the analog significantly prolonged the survival rate of M5076‐implanted mice. Although not stronger than radicicol, it dose‐dependently inhibited embryonic angiogenesis in the chorioallantoic membrane assay, the dose required for half‐maximal inhibition (ID50) value being 23 µg (27 nmol) per egg, and showed concentration‐dependent antiproliferative activity against microvascular endothelial cells in vitro. These data suggest that 14,16‐dipalmitoyl‐radicicol is a promising antitumor agent with antiangiogenic activity. (Cancer Sci 2007; 98: 219–225)
It is widely accepted that angiogenesis is a key factor for growth, invasion and metastasis of malignant tumors,( 1 , 2 , 3 , 4 , 5 , 6 ) all of which are major causes of cancer mortality. This implies that angiogenesis is a potential target for the treatment of cancer. Thus, if a useful antiangiogenic agent were developed, it could serve as a therapeutic drug for improving cancer treatment. Several inhibitors of angiogenesis are in clinical trials, including bevacizumab, an agent that interferes with activators of angiogenesis and a neutralizing antibody against vascular endothelial growth factor (VEGF), which is one of the most potent angiogenesis activators.( 4 , 5 , 6 , 7 , 8 , 9 , 10 ) Bevacizumab (or Avastin), in combination with 5‐fluorouracil‐based chemotherapy regiments, is now used to treat patients with metastatic colorectal cancer in the USA and Europe. Antiangiogenic agents with low molecular weight have been examined for their efficacy in animal studies and in humans, including 2‐methoxyestradiol and analogs of thalidomide.
We previously found that radicicol, a microbial cell differentiation modulator, is a powerful angiogenesis inhibitor in the chorioallantoic membrane (CAM) model on the basis of two assumptions.( 11 ) One assumption is based on the previous findings that microorganisms produce useful bioactive substances such as antibiotics and enzyme inhibitors. This implies to us that microbial metabolites could provide an appealing treasury of effective angiogenesis inhibitors,( 12 ) although compounds that are not derived from microorganisms might show antiangiogenic activity.( 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 ) In fact several research groups, including ours, have shown that a number of seemingly unrelated classes of small molecules derived from microorganisms interfere with angiogenesis in in vitro and in vivo experimental models.( 23 , 24 , 25 , 26 , 27 ) Our microbial inhibitors of angiogenesis include herbimycin, eponemycin, cytogenin and rhizoxin.( 28 , 29 , 30 , 31 ) The second assumption is that cell differentiation modulators could have potent antiangiogenic effects, because cell differentiation is a key event in angiogenesis.( 32 ) The validity of this assumption might be supported by the observation that modulators of cell differentiation, including synthetic compounds like retinoids and vitamin D3 analogs and microbial substances like depudecin, suppress the formation of new blood vessels.( 33 , 34 , 35 )
The aims of the present study were to assess and compare the antiangiogenic and antitumor effects of radicicol and its novel analog 14,16‐dipalmitoyl‐radicicol when administered systemically. We examined their antiangiogenic activity using a mouse dorsal air sac assay and CAM assay. Their antitumor activities were examined in solid tumor growth models involving transplantable mouse M5076 tumors or autochthonous rat mammary tumors induced by 7,12‐dimethylbenz[a]anthracene (DMBA).
Materials and Methods
Materials. Radicicol was prepared by Sankyo Co. (Tokyo, Japan), and 14,16‐dipalmitoyl‐radicicol was originally synthesized by Medical Chemistry Research Laboratories of Sankyo Co., as described below. Their chemical structures are shown in Fig. 1a. For in vivo experiments, radicicol and its analog were suspended in physiological saline containing 5% polyoxyethylated (60 mol) hydrogenated castor oil (HCO60; Nikko Chemicals, Tokyo, Japan) and 5%N,N‐dimethylacetamide (DMA; Wako Pure Chemical Industries, Osaka, Japan), and administered intraperitoneally. For in vitro experiments, radicicol and the analog were dissolved in dimethylsulfoxide and a 1:1 mixture of ethanol (EtOH) and DMA, respectively.
Figure 1.

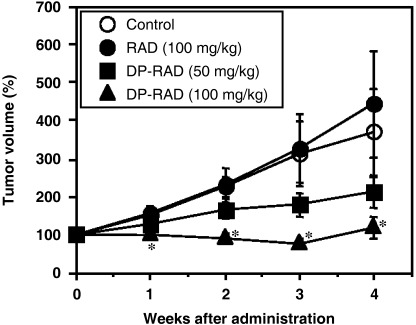
(a) Chemical structures of radicicol (RAD) and 14,16‐dipalmitoyl‐radicicol (DP‐RAD). (b) Antitumor activity of RAD and DP‐RAD against the early stage of transplantable M5076 tumors. Control, RAD and DP‐RAD were administered intraperitoneally four times every 4 days from day 6 after tumor cell inoculation. Points are mean ± SD for seven mice. *P < 0.05; **P < 0.001 compared to the vehicle‐treated control group.
Synthesis of 14,16‐dipalmitoyl‐radicicol. To a stirred solution of radicicol (1.6 g, 4.4 mmol) in benzene (37 mL) at 0°C were added pyridine (2.2 g, 28 mmol) and 4‐dimethylaminopyridine (54 mg, 0.44 mmol), and the solution was stirred for 20 min. To the solution was added palmitoyl chloride (1.3 g, 4.7 mmol) in benzene (20 mL) dropwise at 0°C, and the reaction was stirred for 1 h. The solution was poured into ice water and extracted with ethylacetate (EtOAc). The organic layer was washed with 5% NaHCO3, water and brine, dried over MgSO4, and evaporated to dryness. The residue was purified by flash column chromatography on a silica gel (hexane : EtOAc, 1 : 1) to give 1.5 g of the title compound 1H‐NMR (270 MHz, CDCl3): σ 7.52 (1H, dd, J = 16.0, 10.3 Hz), 7.01 (1H, s), 6.14 (1H, dd, J = 10.7, 10.3 Hz), 6.06 (1H, d, J = 16.1 Hz), 5.78 (1H, dd, J = 10.7, 3.9 Hz), 5.41–5.39 (1H, m), 3.91, 4.03 (2H, AB‐q, J = 16.1 Hz), 3.52 (1H, m), 3.02–2.99 (1H, m), 2.58 (2H, t, J = 7.3 Hz), 2.49 (2H, t, J = 6.8 Hz), 2.45–2.37 (1H, m), 1.79–1.63 (3H, m), 1.53 (3H d, J = 4.8 Hz), 1.42–1.21 (50H, m), 0.88 (6H, t, J = 6.3 Hz); IR (nujor) v 1759, 1722 cm−1.
Animals. Female Crlj:CDI (ICR) mice (7–9 weeks old), female C57BL/6 mice (6 weeks old) and female Sprague‐Dawley rats (7 weeks old) were purchased from Charles River Japan (Kanagawa, Japan). They were housed for 1 week in an air‐ and light‐controlled room maintained at 25 ± 1°C, and given free access to a standard laboratory rodent chow and water. Animals were treated according to the ethical guidelines for Animal Experiments of the Tokyo Metropolitan Institute of Medical Science. The Animal Experiments Committee of the Tokyo Metropolitan Institute of Medical Science approved the experimental protocol.
Cells. M5076 mouse tumor cells were obtained from Dr Tashiro (Cancer Chemotherapy Center, Tokyo, Japan), and maintained by serial intraperitoneal passage in syngeneic mice. The RMT‐1 rat mammary tumor cell line, which was established from DMBA‐induced rat mammary tumors, was maintained in a mixture (1:1) of Ham F‐12 and Dulbecco's modified Eagle's medium (HD) supplemented with 10% fetal bovine serum (FBS; Moregate, Melbourne, Australia). Human dermal microvascular endothelial cells (HDMEC) were obtained from Cell Systems (Kirkland, Washington, DC, USA), and were maintained in gelatin‐coated dishes containing MCDM‐131 supplemented with 10% FBS, 10 µg/mL endothelial cell growth supplement (Upstate Biotechnology, Lake Placid, NY, USA), 10 ng/mL epidermal growth factor (Sigma, St Louis, MO, USA) and 10 µg/mL heparin (Sigma). Cells were cultured at 37°C in a humidified 5% CO2 incubator.
Tumors. Mammary tumors were induced in female Sprague‐Dawley rats at 8 weeks of age by means of a single intragastric ingestion of DMBA (100 mg/kg), as described previously.( 36 ) After 8–12 weeks, rats bearing mammary tumors of approximately 10 mm in diameter were used for antitumor experiments. M5075 tumors were established by inoculation of M5076 tumor cells into the flank of adult C57BL/6 mice.
Mouse dorsal air sac assay. Agents were examined for their effects on tumor‐triggered angiogenesis in the mouse dorsal air sac assay, as described previously.( 30 , 31 ) The tumor cell number in a Millipore chamber (Tokyo, Japan) was 2.5 × 107 and 4 × 106 cells for M5076 and RMT‐1 cells, respectively. Angiogenesis indexes 0, 1, 2 and 3 represented neovessel numbers of 0, 1, 2 and 3 or more, respectively.
Antitumor experiments. Antitumor activity against transplantable murine M5076 tumors. M5076 cells (1 × 106 cells/0.1 mL) were inoculated subcutaneously into the right flank of C57BL/6 mice and compounds were injected using various administration schedules from 6 or 10 days after tumor cell inoculation. The tumor volume was determined every 3 days by direct measurement with calipers and calculated using the formula (width2[mm2] × length [mm]/2).
Antitumor activity against autochthonous rat mammary tumors induced by DMBA. Rats bearing DMBA‐induced mammary tumors of approximately 10‐mm diameter were given intraperitoneal injections of various doses of agents every 4 days, up to six times. The tumor volume was determined every 7 days by direct measurement with calipers and calculated using the formula (width2[mm2] × length [mm]/2).
Life‐prolonging effects in M5076 tumors. M5076 cells (1 × 106 cells/0.1 mL) were inoculated subcutaneously into the right flank of C57BL/6 mice and compounds were injected using various administration schedules from 10 days after tumor cell inoculation. Survival time was monitored daily until termination of the study on day 52.
CAM assay. According to a previous method,( 32 , 37 ) the effects of the agents on embryonic angiogenesis were examined using the 5‐day‐old CAM. The CAM were treated with various doses of radicicol and 14,16‐dipalmitoyl‐radicicol at 37°C for 2 days in a humidified egg incubator. When the avascular zone in the treated CAM was 3 mm or more in diameter, the antiangiogenic response was assessed as effective.
In vitro antiproliferative assay. As described previously,( 37 ) the HDMEC (1 × 104 cells/well) were cultured in gelatinized 24‐multiwell dishes containing 1 mL of MCDM‐131 supplemented with 10% FBS, 10 µg/mL endothelial cell growth supplement, 10 ng/mL epidermal growth factor and 10 µg/mL heparin in the presence of various concentrations of radicicol or its dipalmitoylated analog. RMT‐1 cells (1 × 104 cells/well) were cultured in 12‐multiwell dishes containing 1 mL of HD supplemented with 10% FBS in the presence of various concentrations of radicicol or its dipalmitoylated analog. After 72 h of culture, the cells were trypsinized and counted using a Coulter counter (Z1, Coulter Japan, Tokyo, Japan).
Statistics. Statistical analysis was carried out using StatView 4.51 software (Abacus Concepts, Berkeley, CA, USA). Data on the mouse dorsal air sac assay were analyzed by means of the Mann–Whitney U‐test. Data on the CAM assay were analyzed using Fisher's exact probability test. The results of the other experiments were analyzed using Bonferroni–Dunn's multiple range test. A P‐value < 0.05 was considered significant for all analyses.
Results
Preliminary experiments showed that systemic administration of radicicol had neither antiangiogenic activity nor antitumor activity in the models used. This might be, at least in part, due to poor bioavailability of systemically administered radicicol, because it is well known that when compounds like radicicol containing a phenolic hydroxyl group are administered systemically, they are often easily inactivated by conjugation with sulfate or glucronide via this group, then eliminated from the body.( 38 , 39 ) We therefore used the radicicol analog 14,16‐dipalmitoyl‐radicicol, whose hydroxyl groups were modified, for examining its antiangiogenic and antitumor activities.
Antitumor activity in transplantable M5076 mouse solid tumors. The antitumor activities of radicicol and its analog 14,16‐dipalmitoyl‐radicicol were examined in subcutaneous M5076‐implanted mice. This tumor was generally used for determining the efficacy of antiangiogenic as well as conventional chemotherapeutic agents because it seemed sensitive to different types of antiangiogenic agents.( 40 , 41 , 42 ) The treatment was started on day 6 after inoculation of M5076 cells into the flank of adult C57BL/6 mice; at that time tumor volume was approximately 100 cm3. The agents were administered intraperitoneally four times every 4 days. The dipalmitoylated analog showed dose‐dependent antitumor activity against transplantable M5076 solid tumors, whereas the parent compound, radicicol, failed to show such an activity against these tumors (Fig. 1b). Radicicol at a dose of 100 mg/kg is equivalent to a dose of 230 mg/kg of 14,16‐dipalmitoyl‐radicicol on a molar basis. The analog caused significant growth inhibition of M5076 tumors in a dose‐dependent manner, compared to the control group (P < 0.05), and almost complete growth inhibition of M5076 tumors occurred at the highest dose (200 mg/kg) of the analog examined.
Furthermore, treatment with the analog began on day 10 after M5076 tumor cell inoculation, at which time the tumor volume was approximately 300 cm3. In this case mice bearing M5076 tumors received one or two cycles of treatment with the analog. One cycle of treatment was characterised by dipalmitoyled radicicol administration (at 200 mg/kg, intraperitoneally) three times every 4 days. A remarkable therapeutic effect was observed in the groups receiving one‐ and two‐cycle treatment (Fig. 2a). Interestingly, tumor growth inhibition depended on the treatment schedule; tumor growth stopped entirely during treatment but recommenced some days after treatment was discontinued.
Figure 2.
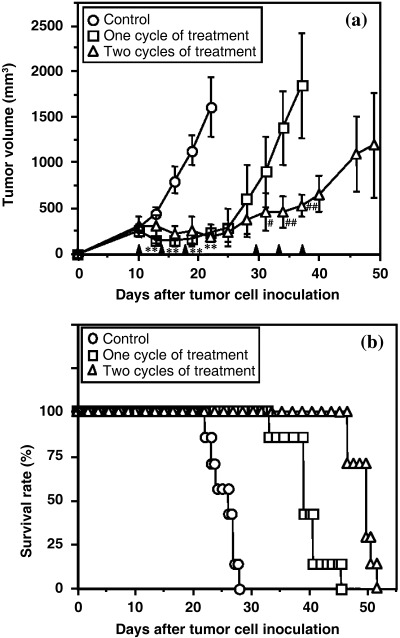
(a) Antitumor activity of dipalmitoyl‐radicicol (DP‐RAD) against the advanced stage of transplantable M5076 tumors. Mice bearing the advanced stage of M5076 tumors received control, one cycle or two cycles of treatment with DP‐RAD (200 mg/kg, three times every four days, intraperitoneally). Points are mean ± SD for five ot seven mice. **P < 0.001 compared to the vehicle‐treated control group. # P < 0.05, ## P < 0.001 compared to one‐cycle treatment group. (b) Survival of mice bearing the advanced stage of transplantable M5076 tumors after one or two cycles of treatment with DP‐RAD (200 mg/kg, three times every 4 days, intraperitoneally).
With respect to survival time of M5076 tumor‐bearing mice, significant efficacy of 14,16‐dipalmitoyl‐radicicol was observed at two treatment schedules (P < 0.001). One cycle of treatment with the dipalmitoylated radicicol prolonged the mean survival time of M5076 tumour‐bearing mice to 60% over the control mice (mean survival time ± SD, 24.1 ± 2.3 days) (Fig. 2b). Furthermore, at two cycles of treatment the mean survival time (48.3 ± 2.0 days) was extended by 100% over that of control mice.
Antitumor activity in autochthonous rat mammary carcinomas. The antitumor effects of radicicol and 14,16‐dipalmitoyl‐radicicol were also examined in autochthonous DMBA‐induced rat mammary carcinomas, which had relatively potent angiogenic activity compared with other solid tumors such as mouse mammary carcinomas and human gliomas,( 43 ) and was sensitive to various angiogenesis inhibitors, including medroxyprogesterone acetate( 36 ) and 22‐oxa‐1α,25‐dihydroxyvitamin D3.( 44 ) Dose‐dependent growth inhibition of these mammary tumors was observed in the groups treated with the analog, although the parent compound (radicicol) at a dose of 100 mg did not affect the growth of these mammary tumors, as shown in Fig. 3. The dipalmitoylated radicicol at 100 mg/kg showed significant growth‐inhibitory activity against DMBA‐induced rat mammary tumors at all days observed, compared to the control (P < 0.05). There was a trend for 50 mg/kg of the radicicol analog to suppress tumor growth, although this did not reach statistical significance (P > 0.05).
Figure 3.
Antitumor activity of radicicol (RAD) and dipalmitoyl‐radicicol (DP‐RAD) against autochthonous rat mammary tumors. Rats bearing mammary carcinomas of approximately 10 mm in diameter received intraperitoneal injection of RAD or DP‐RAD six times every 4 days. Tumor volume was expressed as a percentage of the starting volume (100%). Points are mean ± SD for 14–25 tumors in six or seven rats. *P < 0.05 compared to the vehicle‐treated control group.
Antiangiogenic activity in the mouse dorsal air sac assay. Radicicol and 14,16‐dipalmitoyl‐radicicol were examined for their effects on angiogenesis induced by M5076 cells in the mouse dorsal air sac assay. Figure 4a represents the observations obtained in typical experiments. In a negative control group, in which phosphate‐buffered saline (PBS)‐containing chambers were implanted, and which received the vehicle alone, there was little or no formation of neovessels (Fig. 4aA). In a positive control group, which had had M5076 cell‐containing chambers implanted and was given the vehicle alone, there was drastic induction of new blood vessel formation, characterized by zigzag lines (Fig. 4aB). When administered intraperitoneally, the dipalmitoylated radicicol strongly prevented angiogenesis induced by M5076 cells (Fig. 4aD), whereas such an effect was not observed in the group treated with radicicol (Fig. 4aC). The angiogenesis index (±SD) was 0.25 ± 0.43, 2.75 ± 0.43, 3.00 ± 0.00 and 0.50 ± 0.50 for groups A, B, C and D, respectively (Fig. 4b). There were significant differences in the angiogenesis index between groups A and B, and between groups B and D, whereas the difference in angiogenesis index was not significant between groups A and D.
Figure 4.

(a) Effect of radicicol and dipalmitoyl‐radicicol on M5076 cell‐induced angiogenesis in the mouse dorsal air sac assay system. (A) Mice with an implanted chamber containing phosphate‐buffered saline (PBS) were treated with the vehicle (i.e. negative control group); mice with an implanted chamber containing M5076 cells (2.5 × 107) were administered the vehicle (B) (i.e. positive control), radicicol (100 mg/kg) and dipalmitoyl‐radicicol (200 mg/kg) on days 0 and 4 after the implantation of chambers. Four mice per group were killed on day 5 to assess the antiangiogenic effects. (b) Inhibition of M5076 cell‐induced angiogenesis by dipalmitoryl‐radicicol, but not radicicol. Groups A and B received chambers containing PBS and M5076 cells, respectively, and each group was treated with the vehicle. Groups C and D received M5076 cell‐containing chambers, and were treated with radicicol (100 mg/kg) and dipalmitoyl‐radicicol (200 mg/kg), respectively, on days 0 and 4 after the implantation of chambers. Four mice per group were killed on day 5 to assess the antiangiogenic effects. *P < 0.05 between the indicated groups.
The antiangiogenic activity of radicicol and its analog were also examined in the RMT‐1 mammary tumor cell‐implanted chambers using the mouse dorsal air sac assay mentioned above (data not shown). In a negative control group, which had been implanted with PBS‐containing chambers and treated with the vehicle, little or no formation of neovessels occurred. In a positive control group, which had been implanted with RMT‐1 cell‐containing chambers and treated with the vehicle, drastic induction new blood vessel formation was observed. Significant and strong suppression of angiogenesis induced by RMT‐1 cells was observed in the group treated with the dipalmitoylated analog of radicicol, whereas such an effect was not seen in the group treated with radicicol.
Antiangiogenic activity in the CAM assay. We next asked whether 14,16‐dipalmitoyl‐radicicol exhibited antiangiogenic activity in the CAM assay. The parent compound radicicol at a dose of 500 ng (1.4 nmol) per egg inhibited 5‐day‐old CAM angiogenesis by 75% (Fig. 5aB). The inhibition degree was consistent with a previous result with the ID50 of 540 pmol/egg.( 6 ) Although weaker than the parent compound, its dipalmitoylated analog (100 µg/egg) also inhibited embryonic angiogenesis (Fig. 5aC). This inhibition was dose dependent, the ID50 value being 23 µg (27 nmol) per egg (Fig. 5b), and therefore 20‐fold higher than that of the parent on a molar basis.
Figure 5.

(a) Effect of radicicol and dipalmitoyl‐radicicol on 5‐day‐old chorioallantoic membrane (CAM) angiogenesis. The CAM angiogenesis assay was carried out with an empty methylcellulose pellet alone (i.e. control) (A), a methylcellulose pellet containing radicicol (0.5 µg per egg) (B) or dipalmitoyled radicicol (100 µg per egg) (C). The results shown are for a representative experiment. Arrowheads indicate an avascular zone. (b) Dose‐dependent inhibition of 5‐day‐old CAM angiogenesis by dipalmitoyl‐radicicol. The points indicate the frequencies (%) of avascular zones showing an antiangiogenic response. The values in parentheses are the numbers of CAM examined. *P < 0.05; **P < 0.001 compared to the value for control CAM (n = 25) not showing an avascular zone.
Antiproliferative activities of radicicol and its analog in vitro. Our previous study showed the antiproliferative activity of radicicol against vascular endothelial cells, and suggested the possible involvement of this inhibitory activity in the inhibition of embryonic angiogenesis by radicicol.( 11 ) We therefore examined whether 14,16‐dipalmitoyl‐radicicol could affect the proliferation of vascular endothelial cells in vitro (Fig. 6). The radicicol analog also suppressed microvascular endothelial cell proliferation. This suppression was concentration dependent, and the IC50 value was 140 nM, thereby 10‐fold higher than that of radicicol (IC50 = 14 nM) on a molar basis. In addition, the effects of radicicol and its analog on the RMT‐1 rat mammary tumor cell growth were examined in vitro (Fig. 6). Although not stronger, they also inhibited the mammary tumor cell growth in a concentration‐dependent manner, their IC50 values being 50 and 500 nM for radicicol and the analog, respectively.
Figure 6.

Antiproliferative activity of radicicol (RAD) and dipalmitoyl‐radicicol (DP‐RAD) against microvascular endothelial cells and RMT‐1 rat mammary tumor cells in vitro. The proliferation of human dermal microvascular endothelial cells (HDMEC) or RMT‐1 cells was determined in the presence of various concentration of RAD or DP‐RAD. Values are expressed as a percentage of control vehicle alone. Points are mean ± SD (n = 3 for HDMEC; n = 4 for RMT‐1 cells).
Discussion
It is well known that differentiation (or dedifferentiation) of angiogenic endothelial cells is a key step during the formation of new blood vessels.( 45 , 46 ) On the basis of this finding we have speculated that modulation of angiogenic endothelial cells leads to the blockade of angiogenic responses.( 12 , 32 ) A study along this line has been beginning to yield interesting findings. Namely, that natural and synthetic retinoids that show cell differentiation‐modulating activity were found to exert antiangiogenic action in the CAM assay system, with synthetic retinoid Re 80 showing the strongest activity (ID50 = 6.3 pmol/egg).( 32 , 33 ) One should note that the rank order of the antiangiogenic potency of these retinoids correlated well with that of their cell differentiation potency. Such a correlation was observed in the experiments involving vitamin D3 and its analogs.( 34 ) We also speculated that microbial metabolites could be an appealing treasury of useful angiogenesis inhibitors.( 12 ) This was based on the findings that microorganisms produce useful materials, including antibiotics and inhibitors of enzymes. More recent study has revealed that microorganisms also produce bioprobes used in studies on the mechanism of mammalian cell cycles.( 47 ) Our microorganism‐derived inhibitors of angiogenesis include herbimycin, eponemycin, cytogenin, lactacystin, depudecin and rhizoxin. Other groups have reported that microorganisms produce different angiogenesis inhibitors, including fumagillin,( 27 ) thiolutin,( 24 ) ICM0301s,( 48 ) epoxyquinol A,( 25 ) trichostatin A( 26 ) and dehydroxymethylepoxyquinomicin.( 23 ) Collectively, we assumed that microbial metabolites with cell‐differentiation activity, like radicicol, could also affect angiogenesis. This was based on the finding that radicicol was identified as a microbial inhibitor of angiogenesis in the CAM assay system. However, preliminary experiments showed that systemic administration of radicicol prevented neither tumor cell‐induced angiogenesis nor tumor growth.( 49 ) This indicated that radicicol had poor bioavailability or was unstable in vivo. Therefore, in the present study we assessed and compared the antiangiogenic and antitumor effects of radicicol and its novel analog 14,16‐dipalmitoyl‐radicicol when administered systemically. The present in vivo experiments involving transplantable M5076 tumors revealed that 14,16‐dipalmitoyl‐radicicol, but not radicicol, exhibited antitumor activity and inhibitory effects on tumor‐related angiogenesis. Such a relationship between antitumor and antiangiogenesis was observed in experiments involving autochthonous DMBA‐induced rat mammary tumors. In addition, its analog significantly increased the survival time of mice bearing M5076 solid tumors. In these experiments loss of bodyweight in the treated group was not observed, although the bodyweight gain was somewhat less compared to that of the controls (data not shown). Thus, dipalmitoylation resulted in improved activity of radicicol for suppressing tumor angiogenesis and solid tumor growth in vivo without unacceptable toxic side effects.
Independently, another group reported the unstable property of radicicol and its lack of antitumor activity in vivo, and then synthesized derivatives of the compound with more stable and potent biological activities (such as in vivo antitumor activity) than its parent compound. Such derivatives involve O‐carbamoylmethyloxime and hydroxime.( 50 , 51 , 52 , 53 , 54 ) These derivatives showed stronger antitumor activity than the parent in both in vitro and in vivo experiments. KF25706, a hydroxime derivative of radicicol, at a dose of 100 mg/kg twice daily for five consecutive intravenous injections caused 67–94% growth inhibition against several human carcinoma xenografts transplanted into nude mice, whereas the same treatment administered once daily resulted in 22–51% growth inhibition against such animal models.( 50 ) Growth inhibition of 64–98% caused by KF58333, a O‐carbamoylmethyloxime derivative of radicicol, was observed in human carcinoma cells transplanted into nude mice at a dose of 50 mg/kg once daily for five consecutive intravenous injections. The present study demonstrated that the intraperitoneal administration of 14,16‐dipalmitoyl‐radicicol, at doses of 50, 100 and 200 mg/kg four times every 4 days, inhibited M5076 tumor growth by 44, 71 and 90%, respectively, and that 58–69% inhibition of RMT‐1 tumor cell growth occurred at intraperitoneal doses of 50–100 mg/kg every 4 days, six times. Overall, our synthetic radicicol analog is likely to show almost the same antitumor activity as its oxime derivatives, although it seems hard to compare directly the potency of antitumor activity of each because there are some differences in experimental conditions. In addition, KF58333 was found to inhibit tumor‐related angiogenesis in vivo using histochemical analysis of microvascular area.( 51 ) The group indicated possible involvement of antiangiogenic activity of radicicol derivatives in antitumor activity against human cancer xenografts implanted in athymic nude mice. (51) On the other hand, the present study demonstrated that our dipalmitoyl‐radicicol had more potent inhibitory effects on solid tumor growth and tumor‐related angiogenesis in vivo than the parent, whereas the dipalmitoylated form had weaker inhibitory activity against human microvascular endothelial cell proliferation in vitro and 5‐day‐old CAM angiogenesis than the parent. Such a phenomenon was observed in the case of palmitoylation of rhizoxin, a microbial antitubulin inhibitor.( 55 ) Interestingly, rhizoxin was also found to exhibit antiangiogenic activity in both the CAM and mouse dorsal air sac assays.( 31 )
The present mouse dorsal air sac assay demonstrated that systemic administration of dipalmitoyl‐radicicol, but not radicicol itself, could exhibit antiangiogenic activity, whereas topical treatment with radicicol resulted in suppression of angiogenesis in the present and previous CAM assay. The present in vitro antiproliferative assay system showed that dipalmitoyl‐radicicol, though not with stronger activity than the parent, affected vascular endothelial cell growth, an important event in the process of angiogenesis. Overall, one might assume that dipalmitoyl‐radicicol itself has antiangiogenic ability. To verify this assumption we used the CAM assay system, which involves all of the endothelial cell functions related to in vivo angiogenesis. As a result, this assay system revealed that both dipalmitoyl‐radicicol and radicicol have antiangiogenic activity, although there was a remarkable difference in antiangiogenic potency between the two compounds. It is likely that the CAM could not provide sufficient enzymes that convert a test sample into active metabolite with antiangiogenic activity as retinyl acetate, vitamin D3 and cytogenin had little or no inhibitory activity against angiogenesis on the 5‐day‐old CAM of growing chick embryos.( 30 , 32 , 34 ) In addition, it seems reasonable to consider that dipalmitoyl‐radicicol itself shows an inhibitory action on embryonic angiogenesis. However, the possibility that dipalmitoyl‐radicicol without antiangiogenic activity could be converted into radicicol on the CAM treated, which would in turn affect CAM angiogenesis, cannot be excluded conclusively because we have no direct evidence of the local concentration of radicicol in CAM at present. Alternatively, it might be possible that the inhibitory effect of dipalmitoyl‐radicicol on embryonic angiogenesis involves the combined antiangiogenic activity of dipalmitoyl‐radicicol and radicicol, a hydrolysis product of the dipalmitoylated form. Furthermore 14,16‐dipalmitoyl‐radicicol might serve as a prodrug when administered systematically.
As mentioned above, the present in vitro antiproliferative activity assay revealed that 14,16‐dipalmitoyl‐radicicol also inhibited microvascular endothelial cell growth although it showed weaker inhibitory activity than the parent compound. Thus, it might be possible that the inhibitory activity against endothelial cells is involved in the antiangiogenic activity of the radicicol analog in the present study. It remains unclear whether 14,16‐dipalmitoyl‐radicicol and its parent compound could affect other angiogenic steps, including endothelial cell migration and tube formation. In addition, their effects on the secretion of VEGF from tumor cells should be examined because KF58333 was found to inhibit VEGF secretion.( 51 ) Thus, further studies on these points are needed.
The present study has indicated that the in vivo antiangiogenic activity of our radicicol analog is involved in its antitumor activity, although the mechanism of its antiangiogenic action remains to be clarified. Previous studies have shown that radicicol and its analogs have different biological properties, which are likely to be responsible for their antitumor activity, including inhibition of p60 v‐src protein kinase( 56 ) and depletion of Hsp90‐binding signaling molecules.( 50 ) Furthermore, the possibility that the antiproliferative activity of our radicicol analog and the previously reported derivatives of radicicol against tumor cells may contribute to their antitumor activity in vivo cannot be excluded conclusively.
Acknowledgments
This work was supported in part by Grant‐in‐Aid for Scientific Research on Priority Area 17035068 from The Ministry of Education, Culture, Sports, Science and Technology of Japan. We thank Medical Chemistry Research Laboratories of Sankyo Co. for the supply of 14,16‐dipalmitoyl‐radicicol.
References
- 1. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med 1995; 1: 27–31. [DOI] [PubMed] [Google Scholar]
- 2. Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64. [DOI] [PubMed] [Google Scholar]
- 3. Risau W. Mechanism of angiogenesis. Nature 1997; 386: 671–4. [DOI] [PubMed] [Google Scholar]
- 4. Kuwano M, Uchiumi T, Hayakawa H et al. The basic and clinical implications of ABC transporters, Y‐box‐binding protein‐1 (YB‐1) and angiogenesis‐related factors in human malignancies. Cancer Sci 2003; 94: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shibuya M. Vascular endothelial growth factor receptor‐2: its unique signaling and specific ligand, VEGF‐E. Cancer Sci 2003; 94: 751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akiyama S, Furukawa T, Sumizawa T et al. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci 2004; 95: 851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yano S, Nishioka Y, Goto H, Sone S. Molecular mechanisms of angiogenesis in non‐small cell lung cancer, and therapeutics targeting related molecules. Cancer Sci 2004; 95: 479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42. [DOI] [PubMed] [Google Scholar]
- 9. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005; 438: 967–74. [DOI] [PubMed] [Google Scholar]
- 10. Folkman J. Angiogenesis. Annu Rev Med 2006; 57: 1–18. [DOI] [PubMed] [Google Scholar]
- 11. Oikawa T, Ito H, Ashino H et al. Radicicol, a microbial cell differentiation modulator, inhibits in vivo angiogenesis. Eur J Pharmacol 1993; 241: 221–7. [DOI] [PubMed] [Google Scholar]
- 12. Oikawa T. Strategies to find novel angiogenesis inhibitors as potential therapeutic agents for cancer. Curr Med Chem 1995; 1: 406–17. [Google Scholar]
- 13. Ueda Y, Yamagishi T, Samata K et al. A novel low molecular weight antagonist of vascular endothelial growth factor receptor binding: VGA1155. Mol Cancer Ther 2003; 2: 1105–11. [PubMed] [Google Scholar]
- 14. Aoki S, Cho SH, Ono M et al. Bastadin 6, a spongean brominated tyrosine derivative, inhibits tumor angiogenesis by inducing selective apoptosis to endothelial cells. Anticancer Drugs 2006; 17: 269–78. [DOI] [PubMed] [Google Scholar]
- 15. Hirata H, Uehara H, Izumi K, Naito S, Kuwano M, Ono M. Direct inhibition of EGF receptor activation in vascular endothelial cells by gefitinib (‘Iressa’, ZD1839). Cancer Sci 2004; 95: 614–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arakawa K, Endo Y, Kimura M et al. Multifunctional anti‐angiogenic activity of the cyclic peroxide ANO‐2 with antitumor activity. Int J Cancer 2002; 100: 220–7. [DOI] [PubMed] [Google Scholar]
- 17. Uchimiya H, Furukawa T, Okamoto M et al. Suppression of thymidine phosphorylase‐mediated angiogenesis and tumor growth by 2‐deoxy‐l‐ribose. Cancer Res 2002; 62: 2834–9. [PubMed] [Google Scholar]
- 18. Watanabe K, Hasegawa Y, Yamashita H et al. Vasohibin as an endothelium‐derived negative feedback regulator of angiogenesis. J Clin Invest 2004; 114: 898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toi M, Bando H, Ramachandran C et al. Preliminary studies on the anti‐angiogenic potential of pomegranate fractions in vitro and in vivo . Angiogenesis 2003; 6: 121–8. [DOI] [PubMed] [Google Scholar]
- 20. O’Reilly MS, Boehm T, Shing Y et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88: 277–85. [DOI] [PubMed] [Google Scholar]
- 21. O’Reilly MS, Holmgren L, Shing Y et al. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell 1994; 79: 315–28. [DOI] [PubMed] [Google Scholar]
- 22. Matsumoto K, Nakamura T. NK4 (HGF‐antagonist/angiogenesis inhibitor) in cancer biology and therapeutics. Cancer Sci 2003; 94: 321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsumoto G, Namekawa J, Muta M et al. Targeting of nuclear factor κB pathways by dehydroxymethylepoxyquinomicin, a novel inhibitor of breast carcinomas: antitumor and antiangiogenic potential in vivo . Clin Cancer Res 2005; 11: 1287–93. [PubMed] [Google Scholar]
- 24. Minamiguchi K, Kumagai H, Masuda T, Kawada M, Ishizuka M, Takeuchi T. Thiolutin, an inhibitor of HUVEC adhesion to vitronectin, reduces paxillin in HUVECs and suppresses tumor cell‐induced angiogenesis. Int J Cancer 2001; 93: 307–16. [DOI] [PubMed] [Google Scholar]
- 25. Kakeya H, Onose R, Koshino H et al. Epoxyquinol A, a highly functionalized pentaketide dimer with antiangiogenic activity isolated from fungal metabolites. J Am Chem Soc 2002; 124: 3496–7. [DOI] [PubMed] [Google Scholar]
- 26. Kim MS, Kwon HJ, Lee YM et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med 2001; 7: 437–43. [DOI] [PubMed] [Google Scholar]
- 27. Ingber D, Fujita T, Kishimoto S et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature 1990; 348: 555–7. [DOI] [PubMed] [Google Scholar]
- 28. Oikawa T, Hirotani K, Shimamura M, Ashino‐Fuse H, Iwaguchi T. Powerful antiangiogenic activity of herbimycin A (named angiostatic antibiotic). J Antibiot (Tokyo) 1989; 42: 1202–4. [DOI] [PubMed] [Google Scholar]
- 29. Oikawa T, Hasegawa M, Shimamura M, Ashino H, Murota S, Morita I. Eponemycin, a novel antibiotic, is a highly powerful angiogenesis inhibitor. Biochem Biophys Res Commun 1991; 181: 1070–6. [DOI] [PubMed] [Google Scholar]
- 30. Oikawa T, Sasaki M, Inose M et al. Effects of cytogenin, a novel microbial product, on embryonic and tumor cell‐induced angiogenic responses in vivo . Anticancer Res 1997; 17: 1881–6. [PubMed] [Google Scholar]
- 31. Onozawa C, Shimamura M, Iwasaki S, Oikawa T. Inhibition of angiogenesis by rhizoxin, a microbial metabolite containing two epoxide groups. Jpn J Cancer Res 1997; 88: 1125–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oikawa T, Hirotani K, Nakamura O, Shudo K, Hiragun A, Iwaguchi T. A highly potent antiangiogenic activity of retinoids. Cancer Lett 1989; 48: 157–62. [DOI] [PubMed] [Google Scholar]
- 33. Oikawa T, Okayasu I, Ashino H, Morita I, Murota S, Shudo K. Three novel synthetic retinoids, Re 80, Am 580 and Am 80, all exhibit anti‐angiogenic activity in vivo . Eur J Pharmacol 1993; 249: 113–16. [DOI] [PubMed] [Google Scholar]
- 34. Oikawa T, Hirotani K, Ogasawara H et al. Inhibition of angiogenesis by vitamin D3 analogues. Eur J Pharmacol 1990; 178: 247–50. [DOI] [PubMed] [Google Scholar]
- 35. Oikawa T, Onozawa C, Inose M, Sasaki M. Depudecin, a microbial metabolite containing two epoxide groups, exhibits anti‐angiogenic activity in vivo . Biol Pharm Bull 1995; 18: 1305–7. [DOI] [PubMed] [Google Scholar]
- 36. Oikawa T, Hiragun A, Yoshida Y, Ashino‐Fuse H, Tominaga T, Iwaguchi T. Angiogenic activity of rat mammary carcinomas induced by 7,12‐dimethylbenz[a]anthracene and its inhibition by medroxyprogesterone acetate: possible involvement of antiangiogenic action of medroxyprogesterone acetate in its tumor growth inhibition. Cancer Lett 1988; 43: 85–92. [DOI] [PubMed] [Google Scholar]
- 37. Oikawa T, Murakami K, Sano M, Shibata J, Wierzba K, Yamada Y. A potential use of a synthetic retinoid TAC‐101 as an orally active agent that blocks angiogenesis in liver metastases of human stomach cancer cells. Jpn J Cancer Res 2001; 92: 1225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004; 79: 727–47. [DOI] [PubMed] [Google Scholar]
- 39. Shangari N, Chan TS, O’Brien PJ. Sulfation and glucuronidation of phenols: implications in coenyzme Q metabolism. Meth Enzymol 2005; 400: 342–59. [DOI] [PubMed] [Google Scholar]
- 40. Folkman J, Langer R, Linhardt RJ, Haudenschild C, Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science 1983; 221: 719–25. [DOI] [PubMed] [Google Scholar]
- 41. Tanaka NG, Sakamoto N, Inoue K et al. Antitumor effects of an antiangiogenic polysaccharide from an Arthrobacter species with or without a steroid. Cancer Res 1989; 49: 6727–30. [PubMed] [Google Scholar]
- 42. Yamaoka M, Yamamoto T, Masaki T, Ikeyama S, Sudo K, Fujita T. Inhibition of tumor growth and metastasis of rodent tumors by the angiogenesis inhibitor O‐(chloroacetyl‐carbamoyl) fumagillol (TNP‐470; AGM‐1470). Cancer Res 1993; 53: 4262–7. [PubMed] [Google Scholar]
- 43. Oikawa T, Matsuzawa A, Iwaguchi T. Progression from hormone dependence to autonomy and angiogenesis in mouse mammary tumours. Br J Cancer 1986; 54: 91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oikawa T, Yoshida Y, Shimamura M, Ashino‐Fuse H, Iwaguchi T, Tominaga T. Antitumor effect of 22‐oxa‐1 α,25‐dihydroxyvitamin D3, a potent angiogenesis inhibitor, on rat mammary tumors induced by 7,12‐dimethylbenz[a]anthracene. Anticancer Drugs 1991; 2: 475–80. [DOI] [PubMed] [Google Scholar]
- 45. Folkman J. Tumor angiogenesis. Adv Cancer Res 1985; 43: 175–203. [DOI] [PubMed] [Google Scholar]
- 46. Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res 2001; 49: 507–21. [DOI] [PubMed] [Google Scholar]
- 47. Osada H. Development and application of bioprobes for mammalian cell cycle analyses. Curr Med Chem 2003; 10: 727–32. [DOI] [PubMed] [Google Scholar]
- 48. Kumagai H, Someno T, Dobashi K, Isshiki K, Ishizuka M, Ikeda D. ICM0301s, new angiogenesis inhibitors from Aspergillus sp. F‐1491. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot (Tokyo) 2004; 57: 97–103. [DOI] [PubMed] [Google Scholar]
- 49. Oikawa T. Control of angiogenesis by microbial products. In: Fan T‐PD, Kohn EC, eds. The New Angiotherapy. Totowa: Humana Press, 2001; 329–55. [Google Scholar]
- 50. Soga S, Neckers LM, Schulte TW et al. KF25706, a novel oxime derivative of radicicol, exhibits in vivo antitumor activity via selective depletion of Hsp90 binding signaling molecules. Cancer Res 1999; 59: 2931–8. [PubMed] [Google Scholar]
- 51. Kurebayashi J, Otsuki T, Kurosumi M, Soga S, Akinaga S, Sonoo H. A radicicol derivative, KF58333, inhibits expression of hypoxia‐inducible factor‐1α and vascular endothelial growth factor, angiogenesis and growth of human breast cancer xenografts. Jpn J Cancer Res 2001; 92: 1342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soga S, Sharma SV, Shiotsu Y et al. Stereospecific antitumor activity of radicicol oxime derivatives. Cancer Chemother Pharmacol 2001; 48: 435–45. [DOI] [PubMed] [Google Scholar]
- 53. Agatsuma T, Ogawa H, Akasaka K et al. Halohydrin and oxime derivatives of radicicol: synthesis and antitumor activities. Bioorg Med Chem 2002; 10: 3445–54. [DOI] [PubMed] [Google Scholar]
- 54. Ikuina Y, Amishiro N, Miyata M et al. Synthesis and antitumor activity of novel O‐carbamoylmethyloxime derivatives of radicicol. J Med Chem 2003; 46: 2534–41. [DOI] [PubMed] [Google Scholar]
- 55. Takatori T, Koizumi T, Tokui T, Mitsuhashi Y, Shiraishi A, Tsuruo T. Intracellular activation and cytotoxic action of RS‐1541 against cultured human tumor cells. Cancer Chemother Pharmacol 1995; 35: 283–90. [DOI] [PubMed] [Google Scholar]
- 56. Kwon HJ, Yoshida M, Fukui Y, Horinouchi S, Beppu T. Potent and specific inhibition of p60v‐src protein kinase both in vivo and in vitro by radicicol. Cancer Res 1992; 52: 6926–30. [PubMed] [Google Scholar]


